Abstract
The feasibility of using Flinders Technology Associates filter papers (FTA® cards) to collect allantoic fluid and chicken tissue samples for Newcastle disease virus (NDV) molecular detection was evaluated. Trizol® RNA extraction and one-step reverse transcriptase-polymerase chain reaction (RT-PCR) were used. FTA® cards allowed NDV identification from allantoic fluid with a titre of 105.8 median embryo lethal doses/ml. The inactivated virus remained stable on the cards for 15 days. NDV was detected from FTA® imprints of the trachea, lung, caecal tonsil and cloacal faeces of experimentally infected birds. RT-PCR detection from FTA® cards was confirmed by homologous frozen-tissue RT-PCR and virus isolation. Direct nucleotide sequence of the amplified F gene allowed prediction of NDV virulence. No virus isolation was possible from the FTA® inactivated samples, indicating viral inactivation upon contact. The FTA® cards are suitable for collecting and transporting NDV-positive samples, providing a reliable source of RNA for molecular characterization and a hazard-free sample.
Utilisation du papier filtre FTA® pour la détection moléculaire du virus de la maladie de Newcastle
La faisabilité de l'utilisation des cartes FTA® pour récolter le liquide allantoïdien (AF) et les échantillons de tissu de poulet pour la détection moléculaire du virus de la maladie de Newcastle (NDV) a été évaluée. L'extraction de l'ARN par le Trizol® et la réaction en une étape de transcription inverse et d'amplification en chaîne par polymérase (RT-PCR) ont été utilisées. Les cartes FTA® ont permis l'identification du NDV à partir du AF avec un titre de 105.8 doses létales 50 pour l'embryon/ml. Le virus inactivé est resté stable sur les cartes pendant 15 jours. Le NDV a été détecté sur FTA à partir des empreintes de la trachée, des poumons, des amygdales cæcales et des fèces cloacales de poulets infectés expérimentalement. La détection par RT-PCR à partir de cartes FTA® été confirmée par la RT-PCR sur un mélange de tissu congelé homologue et par l'isolement du virus. La séquence nucléotidique directe du gène F amplifié a permis la prédiction de la virulence du NDV. Aucun isolement de virus n'a été possible à partir des échantillons inactivés des cartes FTA®, indiquant l'inactivation viral par contact. Les cartes FTA® conviennent pour la collecte et le transport des échantillons positifs en NDV, en fournissant une source fiable d'ARN pour la caractérisation moléculaire et un échantillon ne présentant aucun risque.
Verwendung von FTA®-Filterpapier zum molekularen Nachweis des Virus der Newcastle Krankheit
Es wurde die Verwendbarkeit von FTA®-Karten für die Entnahme von Allantoisflüssigkeit und Hühnergewebeproben zum molekularen Nachweis des Virus der Newcastle Krankheit (NKV) untersucht. Dazu wurde eine Trizol®-RNS-Extraktion und eine einstufige Reverse Transkriptase-Polymerasekettenreaktion (RT-PCR) durchgeführt. Die FTA®-Karten ermöglichten die NKV-Identifizierung aus der Allantoisflüssigkeit mit einem Titer von 105,8 Embryo letalen Dosen50/ml. Inaktiviertes Virus blieb auf der Karte über 15 Tage lang stabil. NKV wurde in FTA®-Abstrichen von Trachea, Lunge, Zäkaltonsillen und Kloake experimentell infizierter Hühner nachgewiesen. Der RT-PCR-Nachweis aus den FTA®-Karten konnte durch RT-PCR und Virusisolation aus entsprechenden, eingefrorenen Gewebeproben bestätigt werden. Die direkte Sequenzierung des amplifizierten Gens erlaubte die Feststellung der NKV-Virulenz. Aus den inaktivierten FTA®-Proben konnte kein Virus isoliert werden, was auf eine Virusinaktivierung unmittelbar nach dem Kontakt hinweist. Die FTA®-Karte ist für die Entnahme und den Transport NKV-positiver Proben geeignet und stellt somit eine sichere RNS-Quelle zur molekularen Charakterisierung und eine gefahrlose Probe bereit.
Uso del papel de filtro de FTA® para la detección molecular del virus de la enfermedad de Newcastle
Se evaluó la viabilidad de las targetas de FTA® para la toma de muestras de tejidos de aves y de líquido alantoideo (AF) para la detección molecular del virus de la enfermedad de Newcastle (NDV). Se utilizaron la extracción de RNA mediante Trizol® y la transcriptasa reversa y reacción en cadena de la polimerasa (RT-PCR) en un solo paso. Las targetas de FTA® permitieron la identificación de NDV a partir de AF con un título de 105.8 dosis letales medias en embrión /ml. El virus inactivado permaneció estable en las targetas durante 15 días. Se detectó NDV a partir de improntas de tráquea, pulmón, tonsila cecal y heces de la cloaca de aves infectadas experimentalmente. La detección mediante RT-PCR a partir de targetas FTA® se confirmó mediante RT-PCR de tejidos homólogos congelados y aislamiento vírico. La secuenciación nucleotídica directa del gen F amplificado permitió predecir la virulencia de NDV. No se pudo aislar virus a partir de las muestras inactivadas en FTA®, lo cual indicó la inactivación del virus tras el contacto. Las targetas FTA® son adecuadas para la toma y transporte de muestras positivas de NDV, proporcionando una fuente de RNA fiable y libre de peligrosidad para la caracterización molecular.
Introduction
Newcastle disease virus (NDV) is widely distributed and is considered a major concern to the poultry industry (Villegas, Citation1998a; Alexander, Citation2001). The virus belongs to the family Paramyxoviridae, subfamily Paramyxovirinae, and is a member of the genus Avulavirus (Mayo, Citation2002). The molecular basis for NDV pathogenicity has been shown to be highly dependent on the amino acid sequence of the fusion (F) protein cleavage site (Aldous & Alexander, Citation2001), which has been used as a molecular marker of virulence (OIE, Citation2004; Panda et al., Citation2004). Nevertheless, strong evidence has arisen that other factors such as the haemagglutinin neuraminidase protein (de Leeuw et al, Citation2005) and the V protein (Park et al., Citation2003; Zeng et al., Citation2004) may contribute to NDV virulence.
Conditions for importation of infectious agents by the US Department of Agriculture require that these organisms must be inactivated by chemicals, such as phenol or formalin, before being transported (Snyder, Citation2002). An alternative and safe way of transportation of inactivated microorganisms is represented by the Flinders Technology Associates filter paper (FTA® card), which is a chemically treated filter paper designed for the collection and room-temperature storage of biological samples for subsequent analysis (Natarajan et al., Citation2000; Rogers & Burgoyne, Citation2000; Moscoso et al., Citation2004; Smith & Burgoyne, Citation2004). The FTA® cards have been used for multiple molecular studies such as DNA processing from human or wildlife samples (Raina & Dogra, Citation2002; Smith & Burgoyne, Citation2004) and recently have become a very interesting approach for the detection of poultry microorganisms, such as Mycoplasmas and infectious bronchitis virus (Moscoso et al., Citation2004 Citation2005).
The reverse transcriptase-polymerase chain reaction (RT-PCR) procedure has been established as a reliable tool for NDV detection in allantoic fluid (AF) and in poultry vaccines (Stäuber et al., Citation1995; Farsang et al., Citation2003). The detection of NDV in fresh faeces and tissues by RT-PCR has also been described (Gohm et al., Citation2000; Aldous & Alexander, Citation2001). Molecular detection and characterization of NDV is not commonly performed on chemically inactivated samples due to reports of RNA modifications and problems in nucleic acid extraction, which compromise the yield of high-quality DNA or RNA (Coombs et al., Citation1999; Masuda et al., Citation1999). A virus-inactivation process able to ensure high-quality RNA for molecular pathotyping would be an improvement in field sampling and shipping of NDV for diagnosis means. In this study, the feasibility of using FTA® cards for sampling, inactivation and virus detection from AF and tissue samples by RT-PCR was assessed.
Materials and Methods.
Virus
LaSota NDV strain vaccine (Merial Select, Inc., Gainesville, Georgia. USA) was propagated by inoculation into embryonating chicken eggs, as previously described (Senne, Citation1998). The AF was collected and tested using rapid plate haemagglutination of 5% chicken red blood cells (Alexander, Citation1998). Virus titration was performed as previously described (Villegas, Citation1998b). The titre obtained was 108.8 median egg lethal doses (ELD50)/ml. The AF stock virus was stored at −80°C until needed.
RNA extraction and amplification
Following the manufacturer's recommendations, two RNA extraction procedures and two amplification protocols were used to determine the best extraction/amplification method for FTA® detection of NDV. (A) High Pure RNA isolation kit (Roche Diagnostics Co., Indianapolis, Indiana, USA) + one-step RT-PCR (Titan Kit; Roche Diagnostics Co). (B) Trizol® (Life Technologies Inc., Grand Island, USA) + one-step RT-PCR. (C) High Pure RNA isolation kit + two-step RT-PCR (SuperScript III/Failsafe®). (D) Trizol® + two-step RT-PCR.
Degenerate primers designed to amplify a region of the F gene that includes its cleavage site were used (NDV-F328, 5′-TGGTGAITCTATCCGIAGG-3′; NDV-R581, 5′-CTGCCACTGCTAGTTGIGATATACC-3′) (Seal et al., Citation1995). RT-PCR tests were carried out in a My Cycler thermocycler (BIO-RAD, Hercules, USA) with incubation for 45 min at 48°C for reverse transcription heating at 94°C for 2 min and 40 cycles of denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec and polymerization at 68°C for 60 sec, with a final elongation step of 7 min at 68°C. The amplification products were analysed by electrophoresis on a 1.5% agarose gel stained with ethidium bromide with a concentration of 0.5 µg/ml.
Sensitivity and stability of the FTA®/RT-PCR system
Serial 10-fold dilutions up to 10−9 were made from the initial AF stock (108.8 EID50/ml) to evaluate the detection sensitivity of FTA® cards for NDV from positive AF. For each one of the nine dilutions and the undiluted AF, 50 µl were applied to the four matrix circles present in the FTA® cards. After 24 h, 25 punches were taken from one matrix circle of each card, using a 2-mm puncher (Harris Micro-Punch™; Fisher Scientific, Pittsburgh, USA) in order to recover the surface covered by the 50 µl added. RT-PCR reactions were run for each sample to determine the highest dilution where viral RNA was detectable. RT-PCR reactions (50 µl) of the same AF dilutions applied to the cards were run parallel as a control. Further evaluation of the sensitivity was performed by decreasing the number of punches, using 25, 20, 15, 10, 5 and 1. Virus identification by RT-PCR was attempted on days 1, 7, 14 and 30 after sample collection from cards stored at room temperature (approximately 25°C) to evaluate the stability of viral RNA on the FTA® cards by looking for amplification efficiency over time, as judged visually on ethidium bromide stained gels.
Organ selection for FTA®/RT-PCR detection of NDV and virus isolation
Ten 7-day-old specific pathogen free (SPF) chicks (Merial Select, Inc.) were used to select the best-suited tissues for RT-PCR detection of NDV from FTA® cards. Seven birds were inoculated conjunctival-orally with 100 µl stock virus (108.8 ELD50/ml). The remaining three birds were used as controls and were inoculated with 100 µl phosphate-buffered saline. Starting on day 1 post-inoculation (p.i.), one chicken was killed humanely every day for 7 days. One control bird was killed on days 1, 4 and 7. From each bird, faecal samples and tracheal swabs were smeared on the cards using sterile cotton-tipped applicators. Tissue samples (approximately 1 cm2) from the heart, kidney, trachea, spleen, proventriculus, brain, lung and caecal tonsils were collected from the birds following previously recommended protocols (Gohm et al., Citation2000). An imprint was made by gently pressing the tissue against the provided matrix area of the FTA® cards, as described by Higgins et al. (Citation2000). The remaining tissues were stored at −80°C until processing. After 24 h, RT-PCR was performed on both card and frozen tissue samples. To confirm the RT-PCR results obtained from FTA® cards, virus isolation was attempted from samples obtained on day 2 p.i. (Alexander, Citation1998).
In vivo experiment
Forty-four SPF chickens hatched and reared in isolation. At 3 weeks of age, four chickens were bled for antibody titre determination by enzyme-linked immunosorbent assay (ELISA), using FlockCheck® Newcastle disease antibody test (IDEXX, Maine, USA), were then killed and the tissues collected as described later. The remaining 40 birds were separated into two groups of 20 chickens each. One group was inoculated conjunctival-orally with 100 µl stock NDV virus (108.8 ELD50/ml). After 2 days, the non-inoculated group was wing banded and added to the inoculated group as contact birds. Two inoculated birds were killed daily until day 6, then on days 8, 10 and 12 p.i. Two contact birds were killed on the same days as the inoculated animals, starting on day 4, until day 12. From each bird, trachea, lung, caecal tonsil and cloacal faecal samples were collected and applied to the cards for RT-PCR analysis. Prior to killing, experimentally infected birds were bled to measure their antibody levels or exposure to the virus using ELISA.
Sequencing of the F gene
RT-PCR amplified fragments containing the 250 base pair (bp) portion of the F gene were purified with the QIAquick gel extraction kit (Qiagen, Valencia, California, USA) using the manufacturers’ recommendations. Sequencing reactions were performed with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California, USA) as described by the manufacturer. Sequencing reactions were run in an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). Sequences were analysed with the aid of the DNAStar software (DNAStar, Inc., Madison, Wisconsin, USA).
Virus inactivation by FTA® cards
AF and trachea FTA® samples positive for NDV imprints were allowed to elute in FTA® purification reagent (Whatman International, Ltd., UK). One hundred microlitres of this solution were inoculated in the chorioallantoic sac of 9-day-old SPF embryos. After 5 days, the AF was collected and tested using the rapid plate haemagglutination test with 5% chicken red blood cells (Alexander, Citation1998). Virus detection by RT-PCR was also attempted from the collected AF.
Results
RNA extraction and amplification
The overall average RNA yield obtained from the different dilutions using Trizol ® extraction was 58.9 µg/ml and for the High Pure RNA kit procedure was 20.1 µg/ml. The RT-PCR amplification after Trizol® treatments was more sensitive (a 10-fold difference in detection) than the treatments using the High Pure RNA kit. The use of FTA® cards for NDV detection from allantoic fluid and the comparison between different RNA extraction and amplification methods are presented in .
Table 1. Use of FTA® cards for NDV detection from allantoic fluid and comparison between different RNA extraction and amplification methods
Sensitivity and stability of the FTA®/RT-PCR system
The lowest concentration at which nucleic acid amplification from the FTA® cards occurred was 105.8 ELD50/ml. The detection level of NDV nucleic acids was always higher when AF prior to its inactivation was used as study material in comparison with the level of detection observed for the FTA® cards (1 log10 difference). The effect of decreasing the amount of RNA template on RT-PCR detection of NDV (by decreasing the number of punches from the FTA® cards) was observed as a weakening of the amplification signal, judged visually on ethidium bromide stained gels. RT-PCR allowed the detection of NDV nucleic acids from the cards even when only one punch was used. The stability of viral RNA on FTA® cards was measured by performing RT-PCR on 10-fold serial dilutions of the card inactivated AF at days 1, 7, 14 and 30 after collection. The detection sensitivity decreased one log10 by day 14.
Organ selection for FTA®/RT-PCR detection of NDV and virus isolation
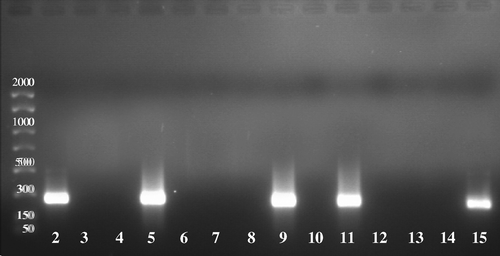
RT-PCR-positive results were obtained in the organ selection experiment from the trachea, lung, caecal tonsils and cloacal faeces (). The trachea was positive in all infected birds up to day 6 p.i., caecal tonsils tested positive on days 6 and 7 p.i., and cloacal faeces samples were positive only on days 1 and 2 p.i. No amplification was obtained from control birds at any time point. Samples of the heart, kidney, spleen, proventriculus and brain, or tracheal swabs from experimentally infected birds, gave negative results to RT-PCR analysis at all time points. Meanwhile, RT-PCR procedures performed on homologous frozen tissues were positive for the trachea, lung, caecal tonsils and cloacal faeces at the same time points as the card-inactivated samples. Negative tissues were negative for both the frozen tissues and FTA® card inactivated samples. Virus isolation attempted on the frozen samples from day 2 p.i. was positive for the trachea, lung and faeces, confirming the presence of the virus detected by the RT-PCR procedure.
Figure 1. Gel electrophoresis of RT-PCR of FTA® card tissue imprints from experimentally inoculated and control birds. Lane 1, DNA PCR marker (Amresco®, Ohio, USA); lane 2, cloacal faeces; lane 3, heart; lane 4, kidney; lane 5, trachea; lane 6, spleen; lane 7, proventriculus; lane 8, brain; lane 9, lung; lane 10, tracheal swab; lane 11, caecal tonsil; lane 12, control bird trachea; lane 13, control bird lung; lane 14, control bird caecal tonsil; lane 15, positive control (AF).
In vivo experiment
The RT-PCR results for the inoculated and contact birds are presented in . Trachea and faeces showed the first positive RT-PCR results (day 1 p.i.). The trachea remained positive longer (6 days p.i.). In the contact animals, the first positive signals were found in the trachea on day 3 after exposure. Molecular detection was not possible from FTA® inactivated tracheal swabs. No single organ was consistently positive for all the days tested. No clinical signs were observed in the inoculated or contact birds. Pre-inoculation serum samples were all negative for NDV antibody, and seroconversion of the inoculated animals was observed 6 days p.i. The contact birds seroconverted 7 days after exposure. The highest titres were recorded on day 12 p.i. with an ELISA geometric mean titre of 11 995 for the inoculated birds and 1553 for the contact group.
Table 2. Detection of NDV in selected FTA® card samples after in-vivo inoculation with the LaSota strain of NDV
Sequencing of the F gene
After analysis of the sequenced data, no differences were observed in the alignment of the amplified nucleotides obtained from the FTA® cards when compared with the uninactivated AF samples RT-PCR products (data not shown). Sequencing of the 250 bp portion of the NDV F gene allowed the prediction of the amino acid sequence at the F0 cleavage site, which as expected was found to correspond to a lentogenic virus.
Inactivation of NDV on FTA® cards
The AF obtained from embryos inoculated with a FTA® card eluate failed to haemagglutinate chicken red blood cells and was negative for NDV/RT-PCR detection. No amplicons were observed from the FTA® card inactivated fluid after RT-PCR analysis. These results indicate complete inactivation of the NDV on contact with the FTA® cards.
Discussion
Tests using live virus, such as the mean death time and intracerebral and intravenous pathogenicity indices, are biological tests required to determine the isolates’ pathotypes to confirm an outbreak of Newcastle disease (Alexander, Citation1998; OIE, Citation2004). Meanwhile, the molecular approach for NDV identification and pathotyping using direct sequencing of the F protein gene cleavage site is accepted as a pathotyping procedure and has been used for surveillance of NDV (Seal et al., Citation1995; Marin et al., Citation1996; OIE, Citation2004; Panda et al., Citation2004; Zeng et al., Citation2004). RT-PCR and direct nucleotide sequencing are not available in some countries or regions, so samples need be transported in a safe way to laboratories with those capabilities, following high standards of biosecurity during shipping (Snyder, Citation2002). We report the feasibility of using FTA® inactivated NDV isolates for diagnosis and molecular pathotyping avoiding the risks of handling and processing live viruses.
A comparison between four different extraction/amplification procedures was made to select the most suitable protocol for NDV identification on FTA® cards. No difference on detection sensitivity was observed when one-step or two-step RT-PCR were used. The one-step RT-PCR is a simpler method, so it was selected to be used in combination with the Trizol® extraction protocol, which yielded higher RNA levels than the High Pure RNA isolation kit. This agrees with previous reports recommending the use of Trizol® over other RNA extraction procedures (Wex et al., Citation2003).
The FTA® cards for NDV sampling and inactivation coupled with RT-PCR, allowed the detection of virus from AF with a titre of 105.8 ELD50/ml (a 10 − 3 dilution of the 108.8 ELD50/ml viral stock). Higher sensitivity of the RT-PCR test for AF and NDV live vaccines (detection of titres as low as 5×102 ELD50) has been previously reported (Stäuber et al., Citation1995; Gohm et al., Citation2000). These results differ from the 104.8 ELD50/ml reported here for the control samples (direct RNA extraction from AF). A lower amount (50 µl) of initial template used in this trial to match the AF sampled on the cards when compared with previous studies may explain the RT-PCR decrease in sensitivity. Further differences in sensitivity observed in the RT-PCR for the FTA® inactivated fluids when compared with the control samples may be due to some detrimental effect of FTA® inactivation on the viral RNA, as previously reported for other chemical inactivated samples (Coombs et al., Citation1999; Masuda et al., Citation1999).
The RT-PCR identification of NDV in stored FTA® cards was possible after 30 days, although a decrease in sensitivity was observed after 14 days of storage at room temperature. The decrease in sensitivity over time has been previously explained as a consequence of RNA denaturation by formation of nicks on the RNA strands (Rogers & Burgoyne, Citation2000; Dobbs et al., Citation2002; Moscoso et al., Citation2005).
The nucleic acids of the NDV inactivated on FTA® cards from experimentally and contact-infected chickens were detected by RT-PCR, as early as 1 day p.i. in experimentally infected chickens, but serological detection was not possible until day 6 p.i. These results emphasize the importance of molecular detection as a diagnostic tool in NDV surveillance (Aldous & Alexander, Citation2001). The trachea was the most suitable organ for NDV detection from the FTA® cards imprints, but no amplification from FTA® inactivated tracheal swabs was possible, although tracheal and cloacal swabs are commonly recommended for virus isolation during field outbreaks (Alexander, Citation1998 Citation2001). This represents the drawback that, for NDV detection from FTA® cards, the birds would need to have been killed in order to collect the sample. The failure to identify the virus in the trachea swabs may be related to the amount of viral RNA present in the swab, when compared with the amount of virus obtained from a tissue imprint, where epithelial cells actively targeted by virus replication remain over the FTA® cards matrix.
The organ selection for FTA® sampling might be affected by NDV strain tropism, age and physical conditions of the birds, and even immunological aspects such as maternal antibodies or the presence of immunosuppressive factors (Alexander, Citation1998 Citation2001; Villegas, Citation1998a). In this trial, SPF chickens were experimentally infected and therefore no interference of maternal antibodies with the virus pathogenesis was expected; and the LaSota strain used has a high rate of replication in the respiratory tract, which may explain the prevalence of the virus in the trachea (Alexander, Citation1998).
Amplification of a specific cDNA segment has been reported from inactivated oil-adjuvanted NDV vaccines without prior treatment (Stäuber et al., Citation1995). Nevertheless, inactivated samples are not commonly used in NDV molecular detection and pathogenicity studies due to the difficulties generated by the chemical inactivation procedures, which can impair the quality of the nucleic acids, jeopardizing the reliability of the tests (Coombs et al., Citation1999; Masuda et al., Citation1999). The sensitivity of the RT-PCR detection from the FTA® cards inactivated tissues proved to be the same as that obtained by RT-PCR from frozen tissues and for virus isolation. Therefore, we have demonstrated the feasibility of using FTA® cards for direct sampling and inactivation of tissues and faeces from NDV-infected animals for RT-PCR detection.
Nucleotide sequencing of the amplified 250 bp segment of the F gene allowed molecular pathotyping of the inactivated NDV, representing a useful tool for the surveillance of the disease in areas where molecular or biological pathotyping of the field isolates is not possible. These results are in agreement with previous reports on molecular pathotyping of NDV (Seal et al., Citation1995; Marin et al., Citation1996; Panda et al., Citation2004).
The RT-PCR procedures failed to identify viral RNA from the embryos inoculated with a FTA® elute, which means no virus re-isolation from the cards was possible. This result agrees with previous publications, where the complete inactivation of the microorganisms by the FTA® card was reported (Raina & Dogra, Citation2002; Smith & Burgoyne, Citation2004; Moscoso et al., Citation2005). The FTA® card sampling and inactivation procedure used in this trial can provide an improvement in NDV analysis protocols. It allows virus identification and molecular pathotyping direct from the bird, avoiding time consuming virus isolation and amplification steps, simplifying the field work and diminishing the risk of handling live viruses.
Acknowledgments
The authors will like to thank to Dr Mark Jackwood and Dr Stanley Kleven, University of Georgia, Poultry Diagnostic and Research Center, for reviewing this manuscript and for their valuable suggestions.
References
- Aldous , E. and Alexander , D. 2001 . Detection and differentiation of Newcastle disease virus (avian paramixovirus type 1) . Avian Pathology , 30 : 117 – 128 .
- Alexander , D.J. 1998 . “ Newcastle disease virus and other avian paramyxoviruses ” . In A Laboratory Manual for Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. 235 – 240 . Kennett Square, PA : The American Association of Avian Pathologists .
- Alexander , D. 2001 . Gordon memorial lecture. Newcastle disease . British Poultry Science , 42 : 5 – 22 .
- Coombs , N. , Gough , A. and Primrose , J. 1999 . Optimization of DNA and RNA extraction from archival formalin-fixed tissue . Nucleic Acids Research , 27 : 2223 – 2226 .
- de Leeuw , O. , Koch , G. , Hartong , L. , Ravenshort , N. and Peeters , B. 2005 . Virulence of Newcastle disease virus is determined by the cleavege site of the fusion protein and by both the stem region and globular head of the haemagglutinin-nuraminidase protein . Journal of General Virology , 86 : 1759 – 1769 .
- Dobbs , L. , Madigan , M. , Carter , A. and Earls , L. 2002 . Use of FTA gene guard filter paper for the storage and transportation of tumor cells for molecular testing . Archives of Pathology and Laboratory Medicine , 126 : 56 – 63 .
- Farsang , A. , Wehmann , E. , Soós , T. and Lomniczi , B. 2003 . Positive identification of Newcastle disease virus vaccine strains and detection of contamination in vaccine batches by restriction site analysis of the matrix protein gene . Journal of Veterinary Medicine , 50 : 311 – 315 .
- Gohm , D. , Thür , B. and Hofmann , M. 2000 . Detection of Newcastle disease virus in organs and faeces of experimentally infected chickens using RT-PCR . Avian Pathology , 29 : 143 – 152 .
- Higgins , J. , Hubalek , Z. , Halouzka , J. , Elkins , K. , Sjostedt , A. , Shipley , M. and Ibrahim , M. 2000 . Detection of Francisella tularensis in infected mammals and vectors using a probe based polymerase chain reaction . American Journal of Tropical Medicine and Hygiene , 62 : 310 – 318 .
- Marin , M. , Villegas , P. , Bennett , J.D. and Seal , B.S. 1996 . Virus characterization and sequence of the fusion protein gene cleavage site of recent Newcastle disease virus field isolates from the southeastern United States and Puerto Rico . Avian Diseases , 40 : 382 – 390 .
- Masuda , N. , Ohnishi , T. , Kawamoto , S. , Monden , M. and Okubo , K. 1999 . Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples . Nucleic Acids Research , 27 : 4436 – 4443 .
- Mayo , M. 2002 . Virus taxonomy—Houston 2002 . Archives of Virology , 147 : 1071 – 1076 .
- Moscoso , H. , Thayer , S.G. , Hofacre , C.L. and Kleven , S.H. 2004 . Inactivation, storage, and PCR detection of mycoplasma on FTA filter paper . Avian Diseases , 48 : 841 – 850 .
- Moscoso , H. , Raybon , O. , Thayer , S.G. and Hofacre , C.L. 2005 . Molecular detection and serotyping of infectious bronchitis virus from FTA filter paper . Avian Diseases , 49 : 24 – 29 .
- Natarajan , P. , Trinh , T. , Mertz , L. , Goldsboroug , M. and Fox , D. 2000 . Paper-based archiving of mammalian and plant samples for RNA analysis . Biotechniques , 29 : 1328 – 1333 .
- OIE ( 2004 ). Newcastle disease . In OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (pp. 161 – 169 ). Paris : Office International des Epizooties .
- Panda , A. , Huang , Z. , Elankumaran , S. , Rockeman , D. and Samal , S. 2004 . Role of fusion protein cleavage site in the virulence of Newcastle disease virus . Microbiology Pathology , 36 : 1 – 10 .
- Park , M. , Shaw , M. , Munoz-Jordan , J. , Cros , J. , Nakaya , T. , Bouvier , N. , Palese , P. , Garcia-Sastre , A. and Blaser , C. 2003 . Newcastle disease virus (NDV)-based assay demonstrates interferon antagonist activity for the NDV V protein and Nipah virus V, W, and C proteins . Journal of Virology , 77 : 1501 – 1511 .
- Raina , A. and Dogra , T.D. 2002 . Application of DNA fingerprinting in medicolegal practice . Journal of Indian Medical Association , 100 : 688 – 694 .
- Rogers , C. and Burgoyne , L. 2000 . Reverse transcription of an RNA genome from databasing paper (FTA®) . Biotechnology and Applied Biochemistry , 31 : 219 – 224 .
- Seal , B.S. , King , D.J. and &. Bennett , J.D. 1995 . Characterization of Newcastle disease virus isolates by reverse transcription PCR coupled to direct nucleotide sequencing and development of sequence database for pathotypes prediction and molecular epidemiological analysis . Journal of Clinical Microbiology , 33 : 2624 – 2630 .
- Senne , D.A. 1998 . “ Virus propagation in embryonated eggs ” . In A Laboratory Manual for Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. 235 – 240 . Kennett Square, PA : The American Association of Avian Pathologists .
- Smith , L.M. and Burgoyne , L.A. 2004 . Collecting, archiving and processing DNA from wildlife samples using FTA data basing paper . BMC Ecology , 4 : 4 – 9 .
- Snyder , J. 2002 . Packaging and shipping of infectious substances . Clinical Microbiology Newsletter , 12 : 89 – 92 .
- Staüber , N. , Brechtbuhl , K. , Bruckner , L. and Hofmann , M. 1995 . Detection of Newcastle disease virus in poultry vaccines using the polymerase chain reaction and direct sequencing of amplified cDNA . Vaccine , 13 : 360 – 364 .
- Villegas , P. 1998a . Viral diseases of the respiratory system . Poultry Science , 77 : 1143 – 1145 .
- Villegas , P. 1998b . “ Titration of biological suspensions ” . In A Laboratory Manual for Isolation and Identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. 248 – 253 . Kennett Square, PA : The American Association of Avian Pathologists .
- Wex , T. , Treiber , G. , Lendeckel , U. and Malfertheiner , P. 2003 . A two step method for the extraction of high-quality RNA from endoscopic biopsies . Clinical Chemistry and Laboratory Medicine , 41 : 1033 – 1037 .
- Zeng , J. , Fournier , P. and Schirrmacher , V. 2004 . High cell surface expression of Newcastle disease virus proteins via replicon vectors demonstrates syncytia forming activity of F and fusion promotion activity of HN molecules . International Journal of Oncology , 25 : 293 – 302 .