Abstract
Broiler chicks with maternal antibodies to Newcastle disease virus (NDV) but none to avian metapneumovirus (APV) were divided into six groups. One group was kept as an unvaccinated control group. Three of the other groups were vaccinated at 1 day old with live APV vaccine or one of two live NDV vaccines (VG/GA or HB1). The remaining two groups received the APV vaccine in combination with either of the two NDV vaccines at 1 day old. At intervals after vaccination for up to 42 days, distribution of the viruses in the tissues was monitored, together with humoral antibody responses. Few NDV isolations were made from any NDV-vaccinated chicks, probably due to the presence of NDV maternal antibodies. In both dual-vaccinated groups, APV persisted longer (up to 21 days post vaccination (d.p.v.)) than in the single vaccinates (up to 14 d.p.v.). After 14 d.p.v., antibody titres against APV in both dual-vaccinated groups remained higher than the single APV vaccinates. For NDV haemagglutination inhibition antibodies, similar titres were found in the single and dual NDV VG/GA vaccinates. However, for chickens dually vaccinated with NDV HB1 and APV, the haemagglutination inhibition titres were significantly higher at 21 and 28 d.p.v. than the single HB1 vaccinates. These differences reflect the fact that NDV haemagglutination inhibition titres may depend on the NDV vaccine used.
Des poulets de chair avec des anticorps maternels anti virus de la maladie de Newcastle (NDV) mais sans anticorps anti métapneumovirus aviaire (APV) ont été répartis en six groupes. Un groupe non vacciné a constitué le groupe témoin. Trois des autres groupes ont été vaccinés, à l'âge d'un jour, avec un vaccin vivant de l'AP ou avec un des deux vaccins vivants de la ND (VG/GA or HB1). Les deux groupes restants ont reçu, à l'âge d'un jour, le vaccin de l'AP en association avec l'un ou l'autre des deux vaccins de la ND. A différents intervalles après la vaccination et jusqu'à l'âge de 42 jours, la distribution des virus dans les tissus ainsi que les réponses humorales en anticorps ont été étudiées. Seuls, quelques réisolements de NDV ont pu être réalisés à partir des poussins vaccinés contre la ND, probablement dus à la présence d'anticorps maternels anti NDV. Dans les groupes ayant reçu les deux vaccins, l'APV a persisté plus longtemps [jusqu'à 21 jours après la vaccination (d.p.v.)] que dans le groupe n'ayant reçu qu'une vaccination (jusqu'à 14 jours d.p.v.). Après 14 jours d.p.v., les titres des anticorps anti APV dans les deux groupes ayant reçu les deux vaccinations sont restés plus élevés que dans les groupes ayant reçu le vaccin de l'AP seul. En ce qui concerne les anticorps inhibant l'hémagglutination (HI), des titres similaires ont été observés chez les animaux ayant reçu le vaccin de la ND VG/GA seul ou en association. Cependant pour les poulets ayant reçu le vaccin de la ND HB1 et le vaccin de l'AP, les titres des anticorps HI ont été significativement supérieurs à 21 et 28 d.p.v. à ceux des poulets ayant reçu le vaccin HB1 seul. Ces différences reflètent le fait que les titres en anticorps HI anti NDV peuvent dépendre du vaccin de la ND utilisé.
Broilerküken mit maternalen Antikörpern gegen das Virus der Newcastle-Krankheit (NKV), aber nicht gegen das aviäre Metapneumovirus (APV), wurden in sechs Gruppen unterteilt. Eine Gruppe war die nicht vakzinierte Kontrollgruppe. Drei der anderen Gruppen wurden am ersten Lebenstag entweder mit der APV-Lebendvakzine oder einer der zwei NKV-Vakzinen (VG/GA oder HB1) geimpft. Die verbliebenen zwei Gruppen erhielten am ersten Lebenstag die APV-Vakzine in Kombination mit einer der beiden NKV-Vakzinen. In bestimmten Abständen wurde bis 42 Tage nach der Vakzination die Virusverteilung in den Geweben zusammen mit der humoralen Antikörperbildung überprüft. Aus den NKV-vakzinierten Küken wurden wahrscheinlich wegen des Vorhandenseins NKV-spezifischer maternaler Antikörper nur wenige NKV-Isolate gewonnen. In den zweifach vakzinierten Gruppen persistierte APV länger [bis zu 21 Tage nach der Vakzination (d.p.v.)] als in der einfach vakzinierten Gruppe (bis zu 14 d.p.v.). Ab 14 d.p.v. blieben die Antikörpertiter gegen APV in beiden zweifach vakzinierten Gruppen höher als in der nur mit APV geimpften Gruppe. Hinsichtlich der hämagglutinationshemmenden (HAH)-Antikörper gegen NKV in den NKV-VG/GA-Gruppen wurden bei den einfach und zweifach vakzinierten Tieren ähnliche Titer ermittelt. Die Küken jedoch, die zweifach mit NKV-HB1 und APV geimpft worden waren, wiesen am 21. und 28. d.p.v. signifikant höhere HAH-Titer auf als die nur mit HB1 vakzinierten Tiere. Diese Unterschiede reproduzieren die Erkenntnis, dass die Höhe der NKV-HAH-Titer von der verwendeten NKV-Vakzine abhängen.
Pollos de engorde con anticuerpos maternales frente al virus de la enfermedad de Newcastle (NDV) pero no frente a metapneumovirus aviar (APV) se dividieron en seis grupos. Uno de los grupos se mantuvo como grupo control sin vacunar. Tres de los grupos restantes se vacunaron al día de vida con una vacuna viva frente APV o una o dos vacunas vivas frente a NDV (VG/GA o HB1). Los dos grupos restantes recibieron la vacuna frente APV en combinación con una de las dos vacunas frente a NDV al día de vida. Se monitorizaron a distintos tiempos tras la vacunación la distribución de los virus en los tejidos y las repuestas de anticuerpos humorales. Se obtuvieron pocos aislamientos de NDV a partir de cualquiera de los pollos vacunados frente a NDV, probablemente debido a la presencia de anticuerpos maternales frente a NDV. APV persistió durante más tiempo [hasta 21 días post vacunación (d.p.v.)] en los dos grupos vacunados frente a ambos agentes que en los grupos vacunados una única vez (hasta 14 d.p.v). Tras 14 d.p.v. los títulos de anticuerpos frente APV en ambos grupos vacunados dos veces se mantuvieron más altos que en los vacunados únicamente frente a APV. Se detectaron títulos similares de anticuerpos inhibidores de la hemoaglutinación (HI) de NDV en los vacunados una o dos veces con la vacuna de NDV VG/GA. Sin embargo, en los pollos vacunados doblemente frente a APV y NDV, con la vacuna HB1, los títulos de HI fueron significativamente mayores a los 21 y 28 d.p.v. que en los vacunados una única vez con HB1. Las diferencias observadas reflejan que los títulos de anticuerpos HI frente a NDV dependen de la vacuna utilizada frente a esta enfermedad.
Introduction
Avian metapneumoviruses (APVs) cause turkey rhinotracheitis and respiratory disease in chickens, which is sometimes associated with swollen head syndrome in chickens (Cook, Citation2000; Cook & Cavanagh, Citation2002). Newcastle disease caused by avian paramyxovirus serotype 1 (Aldous & Alexander, Citation2001) occurs throughout the world in poultry and is frequently associated with high mortality and morbidity (Alexander & Jones, Citation2001; Alexander, Citation2003). Both diseases are controlled by use of live and inactivated vaccines (Alexander & Jones, Citation2001; Alexander, Citation2003).
The introduction of a new vaccine into a vaccination programme raises questions about its compatibility with other vaccines in the existing programme. We recently (Ganapathy et al., Citation2005) demonstrated that, when groups of 1-day-old specific pathogen free (SPF) White-Leghorn chicks were vaccinated with APV Nemovac® and NDV Avinew® compared with single vaccination, the following occur: (i) increased invasiveness and probable multiplication of Newcastle disease virus (NDV) in the presence of APV early after vaccination; (ii) temporary suppression of APV replication in the presence of NDV; (iii) longer persistence of APV (up to 24 days post vaccination (d.p.v.)) following dual vaccination; (iv) increased humoral and local immune responses to NDV vaccine in the presence of APV vaccine; and (v) decreased humoral immune response to APV in the presence of NDV; but (vi) similar levels of local APV-specific IgA in lachrymal fluids irrespective of single or dual vaccination.
This paper reports on an experiment similar to the above but with the following differences: the birds used were a commercial strain of broiler chicks with NDV maternal antibodies but none for APV; two additional groups were included, each being given NDV strain HB1 (Bio B1) vaccine, either alone or with Nemovac® APV vaccine; and sampling was extended up to 42 d.p.v.
Materials and Methods
Chicks
One-day-old Newcastle disease maternal-antibody-positive but APV maternal-antibody-free broiler chicks were randomly allocated into six groups in separate isolation rooms. Food and water were provided ad libitum.
Vaccines
Two strains of NDVs, namely NDV strain VG/GA (Avinew®) and NDV strain HB1 (Bio B1; Merial Italia spa, Milan, Italy), and avian pneumovirus virus subtype B (Nemovac®) live vaccines were obtained from Merial Animal Health Limited, where they were mass produced in embryonated chicken eggs. VG/GA and HB1 viruses were isolated from turkeys and chickens, respectively, and are designated as lentogenic pathotypes (Beard et al., Citation1993; Alexander, Citation1998; Seal et al., Citation2002). The vaccines were prepared for use as recommended by the manufacturer, where one vial of each NDV vaccine was thoroughly mixed with 100 ml sterile water (SW). APV vaccine was prepared in a similar manner. For dual vaccination, one vial of APV vaccine and one vial of either of the two strains of NDV vaccine were reconstituted in 100 ml SW. Vaccines were given at commercial dose rates, which are presented in .
Table 1. Design of an experiment to study the effects of vaccination with APV and NDV strains alone or simultaneously
Experimental design
The allocation of groups is shown in . In the control group, each chick received 50 µl SW via the ocular route and another 50 µl orally. Chickens in other groups were inoculated with vaccine viruses at the same volume by the same routes of application. Chickens in the single vaccination groups were inoculated with APV vaccine or NDV vaccines VG/GA or HB1. For the dual-vaccination groups, APV vaccine was mixed with either of the two NDV vaccines before administration. Doses received by each bird after confirmation by titration are presented in . The chicks were monitored daily for clinical signs.
Oropharyngeal and cloacal swabs
Dry swabs from the oropharynx and cloaca were collected at 2, 5, 10, 16, 24 and 37 d.p.v. for detection of NDV and APV by reverse-transcriptase polymerase-chain reaction (RT-PCR) (Cavanagh et al., Citation1999). At the same time, a separate set of oropharyngeal swabs, previously moistened in TOC medium (Eagle's serum-free minimum essential medium with glutamine, streptomycin (50 µg/ml) and penicillin (50 IU/ml)), was taken for attempted re-isolation of the vaccine viruses. Wet swabs were previously found to be superior to dry swabs for virus isolation (K. Ganapathy & R. C. Jones, unpublished observations). At each occasion, swabs were randomly collected from five birds and were pooled for RT-PCR.
Tissues
At 3, 7, 14, 21, 28, 35 and 42 d.p.v., five chicks per group were humanely killed and pieces of the turbinate, trachea, lung, proventriculus, caecal tonsil and liver were collected aseptically for attempted isolation of vaccine viruses. The same samples were also examined by RT-PCR for the presence of APV. Tissues were examined individually for virus isolation, but for RT-PCR, like tissues from the same group, were pooled together at each sampling interval.
Sera
At 7, 14, 21, 28, 35 and 42 d.p.v., blood was obtained from eight chicks per group for detection of antibodies against the vaccines.
Detection of vaccine viruses
Oropharyngeal (OP) swabs and selected homogenized tissues from the chicks were examined to attempt vaccine virus isolation and RT-PCR, as described elsewhere (Cook et al., Citation1976; Alexander, Citation1998; Cavanagh et al., Citation1999; Aldous & Alexander, Citation2001; Ganapathy et al., Citation2005). Cloacal swabs were only examined by RT-PCR (Cavanagh et al., Citation1999; Aldous & Alexander, Citation2001; Ganapathy et al., Citation2005).
Detection of vaccinal antibodies
Haemagglutination inhibition (HI) assays and enzyme-linked immunosorbent assays (ELISAs) were used to detect antibodies against NDV and APV vaccines, respectively; the procedures used were the same as described elsewhere (Ganapathy et al., Citation2005).
Statistics
The mean antibody titres were compared using Student's t test.
Results
Clinical signs and necropsy lesions
No clinical signs or postmortem lesions were observed in any of the groups at any time.
Detection of vaccine viruses (isolation/RT-PCR)
No NDV or APV was detected in the control chicks.
OP and cloacal swabs
APV was detected in OP swabs by RT-PCR on days 10, 5 and 16 in the single APV, APV + VG/GA and APV + HB1 groups, respectively (). Cloacal swabs were positive for APV on day 5 in both the single APV and dual APV + HB1 vaccinates. Successful virus isolation from OP swabs was made on day 5 in both dual-vaccination groups and on day 10 from the single APV vaccinates. Virus isolation was not attempted from cloacal swabs.
Table 2. Detection of APV vaccine in swabs and tissues by RT-PCR and passage in tracheal organ cultures (no virus detected at 2, 24, 28, 35 and 42 d.p.v.)
NDV was isolated from the OP swabs from all NDV-vaccinated groups on days 2, 5 and 10, except on day 10 in the APV + HB1 dual vaccinates (). OP and cloacal swabs were negative when examined by RT-PCR (data not shown). Virus isolation from cloacal swabs was not attempted.
Table 3. Isolation of NDV vaccine from swabs and tissues by passage in embryonated chicken eggs
Tissue samples
summarizes APV detections by RT-PCR and virus isolation using TOC. In the APV-inoculated group, the virus was detected by RT-PCR on day 14 in the turbinate samples and by isolation from one tracheal sample on day 7. In the dual APV + VG/GA vaccinates, APV was also detected by RT-PCR in the turbinate samples on day 14 and in the tracheal samples at 3 and 21 d.p.v. However, the virus was only isolated from the trachea of one bird on day 7.
In the APV + HB1 vaccinates, APV was detected by RT-PCR at 21 d.p.v. in both turbinate and trachea samples. It was isolated from turbinates of two birds on day 3 and one bird on day 14, and from the trachea of two birds on day 3 and 7 and one other bird on day 14. No APV was detected after 21 d.p.v. in any of the groups.
The results of attempted NDV re-isolations are summarized in . From a total of 840 tissue samples processed, only 21 were positive, two on day 3 and the rest on day 7. For single NDV VG/GA vaccinates, virus was isolated only from turbinate and tracheal samples on days 3 and 7. In the single NDV HB1 group, there were no isolations other than from tracheal and lung samples at 7 d.p.v. For dual APV + VG/GA vaccinates, one tracheal and one caecal tonsil sample were positive, both on day 7. Unlike other groups, the APV + HB1 group yielded the highest proportion of isolations (10 out of 21). Most were obtained from the respiratory tissues and the rest from proventriculus and caecal tonsil samples. All were isolated on 7 d.p.v.
Serum NDV and APV antibodies
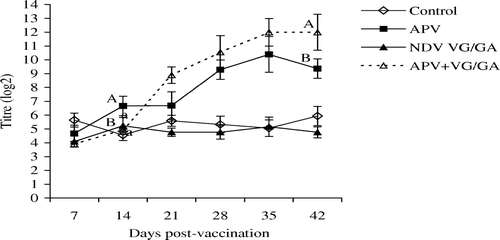
In all groups, levels of derived NDV HI antibodies were high at 7 d.p.v. but thereafter the titres had declined rapidly by 21 days in unvaccinated or single APV-vaccinated chickens ().
Figure 1. NDV HI antibodies in the unvaccinated control, APV, VG/GA or dual-vaccinate APV + VG/GA groups of chickens. Bar = standard deviation.
Interaction between live APV and NDV strain VG/GA vaccines
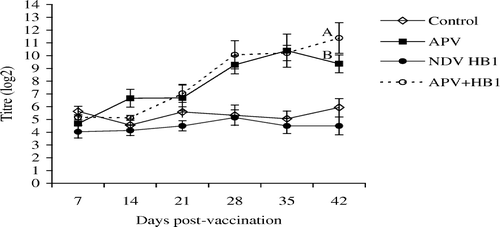
No significant differences were seen when levels of HI antibodies in the single NDV VG/GA group were compared with those found in the dual vaccinates of APV + VG/GA (). For APV ELISA antibody titres (), no significant differences were found between single and dual vaccinates except on days 14 and 42. On day 14, the single vaccinates had significantly higher titres, while the reverse was observed on day 42.
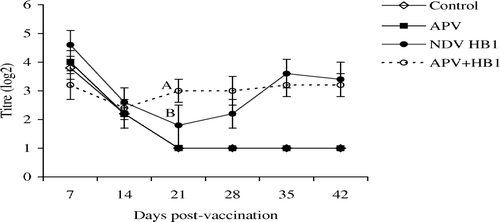
Interaction between live APV and NDV HB1 vaccines
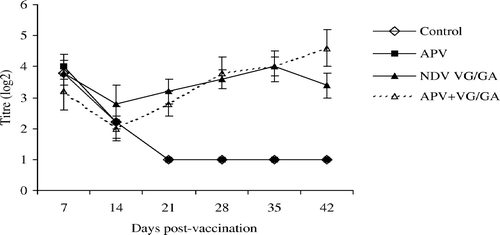
shows that at 21 d.p.v. only were HI antibodies in the dual vaccinates significantly higher than in the single NDV vaccinates. For APV, levels of specific antibodies were similar in both APV-vaccinated groups, except at 42 d.p.v., when antibody titres in the dual vaccinates were significantly higher than in the singly APV-vaccinated chickens.
Discussion
In the current experiments in broiler chicks with ND maternal antibodies, differences were seen in serological responses depending on the Newcastle disease vaccine used. After dual vaccination with APV and NDV VG/GA vaccines there was no enhancement of NDV HI antibodies, in contrast to our previous study in SPF chickens (Ganapathy et al., Citation2005). This discrepancy is likely to have been due to the effect of NDV maternal antibodies causing reduced replication and invasiveness of NDV vaccine. However, in the SPF chicks, the HI titres after vaccination were of a higher order than those in the broilers reported here. Whether this difference related to the maternal antibodies in the broiler chicks and/or to fundamental differences between the breeds used is not known.
In contrast to the results with NDV VG/GA vaccine, dual vaccination of NDV maternal-antibody-positive broilers with APV and the HB1 vaccines resulted in HI titres on day 21 (significantly) and day 28 that were higher than those of the single vaccinates. These results mirrored those in the SPF birds, where APV vaccination appeared to enhance those of the NDV vaccine (Ganapathy et al., Citation2005), although in that work VG/GA vaccine was used. It seems possible that the nature of the serological response to NDV in NDV maternal-antibody-positive broilers may depend on the NDV vaccine used. Both NDV vaccines used were of lentogenic pathotype, with intracerebral pathogenicity indices lower than 0.4 (Alexander, Citation1998; P. Villegas, University of Georgia, Athens, Georgia, USA, personal communication). However, the HB1 was isolated from chickens and the VG/GA from turkeys, and it is not known whether this may have influenced the serological responses.
After 21 d.p.v., both dual-vaccinated groups showed higher APV antibody titres than the single APV vaccinates, except at 35 d.p.v. in the APV + HB1-vaccinated group. This result is in contrast to our previous work with SPF chickens (Ganapathy et al., Citation2005) where decreased humoral immune responses to APV were found. This discrepancy is probably a reflection of the effects of NDV maternal antibodies, allowing comparable loads of APV to be present in both single and dual vaccinates. It is probable that, with replication of NDV vaccine viruses being restricted due to the presence of Newcastle disease maternal antibodies, there was reduced competition for attachment and replication of the APV vaccine virus. Interestingly, in both dual-vaccination groups, significantly higher levels of APV antibodies were detected at 42 d.p.v., suggesting possible later replication of persisting APV vaccine virus.
In the present study, a relatively small number of NDV isolations was obtained from birds vaccinated with NDV vaccines, which further suggests that NDV maternal antibodies neutralized much of the virus (Russell & Ezeifeka, Citation1995). In SPF chickens, in contrast, increased isolation and invasiveness of NDV were reported in dual vaccinates compared with the single NDV vaccinates (Ganapathy et al., Citation2005). However, differences were seen between isolation rates for the two NDV vaccines in the dual-vaccinated groups. Isolation of HB1 vaccine virus was more frequent than VG/GA in all tissues except the lungs. This difference probably reflects the different tropisms of the vaccine viruses.
Detection of APV vaccine virus in single and dual vaccinates was broadly similar, except that tracheas of the dual vaccinates at 21 d.p.v. were positive by RT-PCR but not by virus isolation. The reason for this late APV detection is not known. It appears that in the early stage after vaccination, NDV may have proliferated at a rate that suppressed replication of APV, but insufficiently to completely eliminate it. Subsequently, with decreasing amounts of NDV, presumably the persisting APV multiplied until it was ultimately eliminated by the host immune responses. It has been shown that IBV can suppress replication of APV when they are given simultaneously (Jones et al., Citation1998; Cook et al., Citation2001). In contrast to our previous experiment in SPF birds (Ganapathy et al., Citation2005), detection of APV was similar in single and dual APV vaccinates but the duration of APV persistence differed slightly, from up to 21 d.p.v. in broilers compared with 24 d.p.v. in SPF chickens.
For regular detection of APV, oropharyngeal or tracheal swabs or respiratory tissues are preferred (Cook & Cavanagh, Citation2002) and intestinal samples are not normally examined. However, Hess et al. (Citation2004a) reported virus isolation and RT-PCR detection of virulent APV in cloacal swabs taken from APV-challenged chickens. The viral genome was detected from 7 to 28 days post challenge in birds challenged either intravenously or oculonasally, but those birds challenged by the intravenous route were also positive as measured by virus isolation. In another study, Hess et al. (Citation2004b) administered APV vaccine virus by the in ovo method and found, by RT-PCR, APV in the cloacal swabs. In the present study, using RT-PCR, APV vaccine virus was detected in cloacal swabs of single APV and dual APV + HB1 vaccinates at 5 d.p.v. These findings show that both virulent and avirulent APV viruses can sometimes be excreted via the cloaca. However, no report exists confirming the replication of APV in the gut, and the mechanism to explain the early detections reported here are unknown.
In conclusion, it appears that concurrent vaccination of NDV maternal-antibody-positive broilers chicks against NDV and APV with the vaccines used here is unlikely to have adverse effects on the efficacy of either vaccine, although there may be some differences in NDV HI antibody levels depending on the NDV vaccine used.
References
- Aldous , E.W. and Alexander , D.J. 2001 . Detection and differentiation of Newcastle disease virus (avian paramyxoviruses type 1) . Avian Pathology , 30 : 117 – 128 .
- Alexander , D.J. 1998 . “ Newcastle disease virus and other avian paramyxoviruses ” . In A Laboratory Manual for the Isolation and identification of Avian Pathogens , 4th edn , Edited by: Swayne , D.E. , Glisson , J.R. , Jackwood , M.W. , Pearson , J.E. and Reed , W.M. 156 – 163 . Pennsylvania , PA : American Association of Avian Pathologists Inc .
- Alexander , D.J. 2003 . “ Newcastle disease ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 64 – 87 . Ames , IA : Iowa State University Press .
- Alexander , D.J. and Jones , R.C. 2001 . “ Paramyxoviridae ” . In Poultry Diseases , 5th edn , Edited by: Jordan , F.T.W. , Pattison , M. , Alexander , D.J. and Faragher , T. 257 – 272 . London : W.B. Saunders, Harcourt Publishers Ltd .
- Beard , C. , Villegas , P. and Glisson , J. 1993 . Comparative efficacy of the B-1 and VG/GA vaccine strains against velogenic viscerotropic Newcastle disease virus in chickens . Avian Disease , 37 : 222 – 225 .
- Cavanagh , D. , Mawditt , K. , Britton , P. and Naylor , C.J. 1999 . Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions . Avian Pathology , 28 : 593 – 605 .
- Cook , J.K.A. 2000 . Avian pneumovirus infections of turkey and chickens . Veterinary Journal , 160 : 118 – 125 .
- Cook , J.K.A. and Cavanagh , D. 2002 . Detection and differentiation of avian pneumoviruses (metapneumoviruses) . Avian Pathology , 31 : 117 – 132 .
- Cook , J.K.A. , Darbyshire , J.H. and Huggins , M.B. 1976 . The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus . Archives of Virology , 50 : 109 – 118 .
- Cook , J.K.A. , Huggins , M.B. , Orbell , S.J. , Mawditt , K. and Cavanagh , D. 2001 . Infectious bronchitis virus vaccine interferes with the replication of avian pneumovirus vaccine in domestic fowl . Avian Pathology , 30 : 233 – 242 .
- Ganapathy , K. , Cargill , P. , Montiel , E. and Jones , R.C. 2005 . Interaction between live avian pneumovirus and Newcastle disease virus vaccines in specific pathogen free chickens . Avian Pathology , 34 : 297 – 302 .
- Hess , M. , Huggins , M.B. , Mudzamiri , R. and Heincz , U. 2004a . Avian metapneumovirus excretion in vaccinated and non-vaccinated specific pathogen free laying chickens . Avian Pathology , 33 : 35 – 40 .
- Hess , M. , Huggins , M.B. and Heincz , U. 2004b . Hatchability, serology and virus excretion following in ovo vaccination of chickens with an avian metapneumovirus vaccine . Avian Pathology , 33 : 576 – 580 .
- Jones , R.C. , Khehra , R.S. , Naylor , C.J. and Cavanagh , D. 1998 . “ Dual infection of tracheal organ culture and chicks with infectious bronchitis virus and avian pneumoviris ” . In Proceedings of the International Symposium on Infectious Bronchitis and Pneumovirus Infections in Poultry , Edited by: Kaleta , E.F. and Heffels-Redmann , Ursula . 97 – 106 . Rauischholzhausen, Germany .
- Russell , P.H. and Ezeifeka , G.O. 1995 . The Hitchner B1 strain of Newcastle disease virus induces high levels of IgA, IgG and IgM in newly hatched chicks . Vaccine , 13 : 61 – 66 .
- Seal , B. , Crawford , J. , Sellers , H. , Locke , D. and King , D. 2002 . Nucleotide sequence analysis of the Newcastle disease virus nucleocapsid protein gene and phylogenetic relationships among the Paramyxoviridae . Virus Research , 83 : 119 – 129 .