Abstract
Between 1997 and 1999 several cases of a new disease in Muscovy ducks were reported in Pennsylvania, USA. The cases were characterized by locomotor dysfunction, weakness, recumbency, 40 to 60% morbidity and 10 to 40% mortality. The most characteristic microscopic lesions were moderate to severe degenerative rhabodomyopathy. In order to characterize the aetiological agent, virus isolation was attempted from the spleen, liver, heart, skeletal muscle and intestine by inoculation of 14-day-old Muscovy duck embryos with tissue homogenates. Deaths occurred on the second egg passage and parvoviruses were isolated by serial passage of allantoic fluid from dead embryos and then in Muscovy duck embryo fibroblast (MDEF) cultures. Parvovirus particles were observed in allantoic fluids and supernatants of MDEF cultures by transmission electron microscopy. Two genomic fragments, comprising 1108 nucleotides of the right open reading frame that codes for the structural viral proteins 1, 2 and 3, were amplified by polymerase chain reaction from one of the isolates, Muscovy duck parvovirus (MDPV)/PSU-31010. Comparison of this fragment with available sequences of other MDPV and related goose parvovirus (GPV) isolates showed that it had only 84.5% sequence identity with other MDPV isolates and 84.6% identity with the GPV isolates. This region shares over 99% identity among previously sequenced MDPV isolates and 95% identity among the related GPV isolates. This suggests that MDPV/PSU-31010 is divergent from all other sequenced MDPV and GPV isolates, and may represent a new group of avian parvoviruses.
Entre 1997 et 1999 plusieurs cas d'une nouvelle maladie observée chez les canards de Barbarie ont été rapportés en Pennsylvanie, USA. Les cas étaient caractérisés par un disfonctionnement locomoteur, de la faiblesse, des sujets couchés, 40-60% de morbidité et 10-40% de mortalité. Les lésions microscopiques les plus caractéristiques ont été une rhabodomyopathie modérée à aiguë. Dans le but de caractériser l'agent étiologique, des essais d'isolement de virus ont été réalisés à partir de la rate, du foie, du cœur, des muscles du squelette et de l'intestin par inoculation à des embryons de canard de Barbarie âgés de 14 jours avec des broyats de tissu. Les mortalités sont apparues au 2ème passage sur œufs et des parvovirus on été isolés par passages en série du liquide allantoïdien d'embryons morts et puis sur des cultures de fibroblastes d'embryon de canard de Barbarie (MDEF). En microscopie électronique, des particules de parvovirus ont été observées à partir des liquides allantoïdiens et des surnageants des cultures de MDEF. Deux fragments génomiques, comprenant 1108 nucléotides au niveau du cadre ouvert de lecture droit qui code les protéines virales de structure (VP)1, 2 et 3, ont été amplifiés par PCR à partir d'une des souches isolées, de parvovirus du canard Barbarie (MDPV)/PSU-31010. La comparaison de ce fragment aux séquences disponibles d'autres MDPV et des souches), virus apparenté, de parvovirus de l'oie (GPV) a montré qu'il y avait seulement 84,5% d'identité avec les autres souches de MDPV et 84,6% d'identité avec les souches de GPV. Cette région présente plus de 99% d'identité avec celle des MDVP séquencés antérieurement et 95% d'identité avec celle des GPV), virus apparenté. Ceci suggère que la souche MDPV/PSU-31010 est différente des autres souches de MDPV ET GPV séquencées et qu'elle peut représenter un nouveau groupe de parvovirus aviaires.
Zwischen 1997 und 1999 wurde aus Pennsylvania von mehreren Fällen einer neuen Erkrankung bei Moschusenten berichtet. Das Krankheitsbild war durch Bewegungsstörungen, Schwäche und Festliegen bei einer Morbidität von 40-60 % und einer Mörtalität von 10-40 % gekennzeichnet. Histopathologisch war eine gering- bis hochgradige degenerative Rhabdomyopathie die charakteristische Veränderung. Zur Ermittlung des ätiologischen Agens wurde mittels Inokulation von Gewebehomogenaten in 14-tägige embryonierte Moschusenteneier eine Virusisolierung aus Milz, Leber, Herz, Skelettmuskel und Darm versucht. Nach zwei Eipassagen begannen die Embryonen zu sterben und Parvoviren wurden durch weitere Passagierung der Allantoisflüssigkeit von toten Embryonen im Ei und dann in Moschusentenembryofibroblasten (MEEF)-Kulturen isoliert. Elektronenmikrokopisch konnten in den Allantoisflüssigkeiten und den MEEF-Kulturüberständen Parvoviruspartikel nachgewiesen werden. Aus einem der Isolate, dem Flugentenparvovirus (MEPV)/PSU-31010 wurden mittels Polymerasekettenreaktion zwei Genomfragmente bestehend aus 1108 Nukleotiden des rechten offenen Leserahmens, der für die Virusstrukturproteine (VP) 1, 2 und 3 kodiert, amplifiziert. Der Vergleich dieses Fragments mit verfügbaren Sequenzen anderer MEPV und verwandten Gänseparvovirus (GPV)-Isolaten ließ erkennen, dass es eine nur 84,5%-ige Sequenzübereinstimmung mit anderen MEPV-Isolaten und eine 84,6%-ige Übereinstimmung mit den GPV-Isolaten hatte. Bei den bislang sequenzierten MEPV-Isolaten weist diese Region eine 99%-ige Identität und unter den verwandten GPV-Isolaten eine 95%-ige Identität auf. Dies legt nahe, dass MEPV/PSU-31010 sich von allen anderen sequenzierten MEPV- und GPV-Isolaten unterscheidet und wahrscheinlich eine neue Gruppe aviärer Parvoviren repräsentiert.
Entre 1997 y 1999 se describieron varios casos de una nueva enfermedad en patos Criollos en Pennsylvania, USA. Los casos se caracterizaron por la presencia de disfunción locomotora, debilidad, letargo, morbilidad del 40-60% y mortalidad del 10-40%. Las lesiones microscópicas más características fueron rabdomiopatía degenerativa moderada a grave. Con el objetivo de caracterizar el agente causal se realizó aislamiento vírico mediante inoculación de embriones de pato Criollo de 14 días de vida con homogeneizados de bazo, hígado, corazón, músculo esquelético e intestino. Se produjeron mortalidades en el segundo pase en huevo y se aislaron parvovirus mediante pase seriado del líquido alantoideo de los embriones muertos en cultivos de fibroblastos de embriones de pato Criollo (MDEF). Las partículas de parvovirus se observaron mediante microscopía electrónica en los líquidos alantoideos y en los sobrenadantes de los cultivos de MDEF. Se amplificaron mediante reacción en cadena de la polimerasa dos fragmentos del genoma, que comprendían 1108 nucleótidos del fragmento de lectura abierta derecho que codifica para las proteínas estructurales (VP) 1, 2 y 3, de uno de los aislamientos, parvovirus de pato Criollo MDPV9/PSU-31010. La comparación de este fragmento con las secuencias disponibles de otros MDPV y aislamientos relacionados de parvovirus de gansos (GPV) mostró que sólo tenía una identidad de la secuencia del 84.5% con otros aislamientos de MDPV y del 84.6% con aislamientos de GPV. Esta región comparte aproximadamente un 99% de identidad entre aislamientos de MDPV secuenciados previamente y un 95% de identidad entre aislamientos de GPV relacionados. Esto sugiere que MDPV/PSU-31010 diverge del resto de aislamientos de MDPV y GPV secuenciados y podría representar un nuevo grupo de parvovirus aviares.
Introduction
In the mid-1960s, a highly fatal disease, characterized by anorexia, prostration and death within 2 to 5 days, was described in goslings (Derzsy, Citation1967; Woolcock et al., Citation2000). This disease, named Derzsy's disease, was caused by goose parvovirus (GPV) (Kisary & Derzsy, Citation1974). During the late 1980s a new disease caused by a related parvovirus appeared in Muscovy ducks (Cairina moschata) in several parts of the world (Fournier & Gaudry, Citation1992; Jestin et al., Citation1991; Lu et al., Citation1993). This disease was characterized by mortalities of between 10 and 80%, nervous, locomotor and enteric signs, abnormal feather development and stunting (Barnes, Citation1997). Infection in older ducks caused degenerative skeletal muscle myopathy and growth retardation (Glavits et al., Citation2005). The virus isolated from Muscovy ducks is closely related to the GPV based on Southern hybridization assays (Zadori et al., Citation1994) and shares 81.9% sequence identity with GPV (Zadori et al., Citation1995). The genome of Muscovy duck parvovirus (MDPV) consists of single-stranded DNA of 5132 nucleotides, and strands of both polarities are encapsidated (Zadori et al., Citation1995). The genome contains two major open reading frames (ORFs). The left ORF (ORF1) encodes the pleiotropic regulatory proteins, and the right ORF (ORF2) encodes the capsid viral proteins VP1, VP2 and VP3. The latter two proteins are produced by alternate splicing and are completely located within the carboxyl terminus of VP1 (Zadori et al., Citation1995). Waterfowl parvoviruses can be categorized into GPV-related or MDPV-related groups based on their nucleotide sequences. Tsai et al. (Citation2004) analysed the sequences of VP1 of five GPV and three MDPV isolates. The two groups of viruses differed by between 20.9 and 24.4% in nucleotide sequence, confirming the results reported by Zadori et al. (Citation1995). The differences in nucleotide sequences within the MDPV group were only between 0.1 and 1.9%. Woolcock et al. (Citation2000) isolated a MDPV from Muscovy ducklings in California and found the sequence of the VP1 gene of this isolate to be identical to the reference MDPV strain 89384, which was isolated in France (Jestin et al., Citation1991). The aim of the studies described here were to isolate and characterize the viruses associated with an outbreak of disease in Muscovy ducklings in Pennsylvania, USA.
Material and Methods
Case history
The first of a series of submissions of Muscovy ducklings from disease outbreaks with high morbidity and mortality was presented to the Animal Diagnostic Laboratory at Pennsylvania State University (ADL-PSU) on 30 October 1997. Similar cases were presented at ADL-PSU from additional farms through until early 1999. The sources included semi-integrated poultry companies and small independent farm enterprises in central and southeastern Pennsylvania. Most of the ducks were raised for sale into the northeast live bird marketing system. Flock sizes, housing and management styles were highly variable. Feed sources and formulations differed. The strain of Muscovy duck in the initial cases was a hybrid developed for meat production in Europe. Both males and females were represented among the cases, and some of the farms had multiple ages of ducks.
Virus isolation in Muscovy duck embryos
At ADL-PSU, tissues from dead Muscovy ducks were collected. These tissues, including the spleen, liver, heart, skeletal muscle and intestines, were used for virus isolation. Tissue homogenates were prepared as a 1:10 dilution in viral transport medium, consisting of 500 ml minimum essential medium (MEM), 7.5 ml of 1 M Hepes buffer, 10 ml of 10 mg gentamicin/ml, 2.5 ml of 10,000 µg kanamycin/ml, 5 ml antibiotic/antimycotic mixture (10,000 units penicillin-G sodium/ml, 10,000 µg streptomycin sulfate/ml; 25 µg amphotericin B/ml in 0.85% saline) and 5 ml heat-inactivated horse serum, and centrifuged at 250 x g for 10 min at 4°C. Supernatants were filtered through 0.45 µm filters and stored at 4°C for use within 48 h or stored at –80°C for use after 48 h. Fertile Muscovy duck eggs were obtained from breeder flocks in Pennsylvania that were free of MDPV antibodies, did not have a history of parvovirus disease and had not produced progeny suspected of parvovirus disease. The allantoic cavities of three to five 14-day-old embryonated Muscovy duck eggs/specimen were inoculated with 0.2 ml/egg of each of the filtered tissue homogenates. Allantoic fluids were harvested from inoculated eggs 6 to 7 days post inoculation and from embryos that died more than 24 h after inoculation. Serial egg passages were performed in attempts to isolate MDPV.
Virus propagation in Muscovy duck embryo fibroblast cultures
Two sources of Muscovy duck embryos were used to prepare Muscovy duck embryo fibroblast (MDEF) cultures from 14-day-old embryos using routine techniques for primary avian embryo fibroblast cultures (Schat & Purchase, Citation1998) and maintained in MEM supplemented with 10% foetal bovine serum at 37°C in 5% CO2. Initially, fertile eggs were used from the same sources that were used for embryo inoculations. For the preparation of viral DNA, specific pathogen free Muscovy duck eggs were imported from France through Grimaud Farms. MDPV/PSU-31010 was passaged seven times in Muscovy duck embryos, followed by six passages in MDEF cultures. Virus was harvested between 5 and 7 days post inoculation when 70 to 90% of the cells showed cytopathic effects, characterized by complete destruction of the monolayer of cells. For DNA extraction, MDPV/PSU-31010 was passaged once at Cornell University in tertiary specific pathogen free MDEF cultures seeded at 1.25 x 105 cells/ml. These cultures were incubated at 36°C in MEM supplemented with 0.25% calf serum. When cultures showed cytopathic effects, virus was collected by freeze/thawing three times, followed by centrifugation at 250 x g, and the supernatant fluids were harvested and stored at –80°C until use.
Transmission electron microscopy
Samples of chorioallantoic fluid and supernatant from the MDEF cultures were centrifuged at 55,000 x g for 1 h. Pellets were resuspended in 200 µl distilled water and then filtered through a 0.2 µm filter. The filtered suspension was mixed with 200 µl of 4% sodium phosphotungstic acid, pH 7.0, and this was sprayed onto 400-mesh carbon-Formvar-coated copper grids. Prepared grids were examined and photographed using a Phillips EM-4000 electron microscope.
Viral DNA extraction
Samples were treated with digestion buffer (100 mM NaCl, 10 mM Tris–HCl (pH 8.0), 25 mM ethylenediamine tetraacetic acid, 0.5% sodium dodecyl sulphate) containing 100 µg proteinase K/ml (Sigma, St Louis, Missouri, USA) and DNA was extracted with phenol:chloroform:isoamyl alcohol (50:49:1) and precipitated with ethanol (Sambrook et al., Citation1989).
Polymerase chain reaction assays
Six sets of primers were used. The sequence of primer pairs 1 and 3 to 6, the expected size of the amplicons, the genome location based on the FM strain (GenBank accession number U22967) (Zadori et al., Citation1995), and references are presented in . The sequences for primer pair 1 were provided by Dr J. Pederson (Ames, Iowa, USA) and correspond to nucleotides 2615 to 2634 and 3189 to 3208 of the MDPV FM strain. Primer pair 2 was derived from Chang et al. (Citation2000) and was based on the consensus sequence for VP3 of the FM strain of MDPV and GPV (GenBank accession number U25749) (Zadori et al., Citation1995). The forward primer of primer pair 1 and the reverse primer for primer pair 2 span nucleotides 2615 to 3723 of U22967. The forward primer for primer set 2 was located within the 3′ end of the amplicon expected using primer set 1. PCR assays were conducted using the touchdown protocol described by Xing & Schat (Citation2000). The touch-down steps consisted of two cycles of 70°C for 120 sec, 69°C for 115 sec, 68°C for 110 sec, 66°C for 100 sec, 65°C for 95 sec, 63°C for 85 sec, 61°C for 75 sec and 60°C for 70 sec, and these were followed by 30 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 120 sec, with a final incubation at 70°C for 7 min. All fragments were cloned and sequenced using the Topo TA cloning kit with the PCR@4-TOPO vector (Invitrogen Corp, Carlsbad, California, USA) according to the manufacturer's instructions. Four independently selected plasmids were sequenced for amplicons generated with primer sets 1 and 2. Sequencing was performed by the DNA sequencing facility at Cornell University using Big Dye terminators from Applied Biosystems, Inc. (Foster City, California, USA) and primers provided with the Topo TA cloning kit. Sequencing reactions were run on ABI 3700 DNA sequencers. The combined sequence was compared with other parvovirus sequences in GenBank using the Clustal V method incorporated in the MegAlign™ program within the Lasergene 6.1 package (DNA Star, Inc., Madison, Wisconsin, USA).
Table 1. Primer sets used in PCR assays
Accession number
The sequence was submitted to the GenBank nucleic acid database and has been assigned accession number DQ413026.
Results
Clinical signs and lesions
In general, locomotor dysfunction, weakness, recumbency and increased mortality of Muscovy ducklings started at 9 to 10 days of age and peaked at 2 to 2.5 weeks. During the acute phase, morbidity was between 40 and 60%, and mortality was between 10 and 40%. Sequelae in recovered flocks were poor growth, decreased feed conversion efficiency, lingering locomotor deficits and an increased incidence of ascites. Total flock mortality at the end of the grow-out period typically ranged from 20 to 50%. Gross lesions were subtle, inconsistent or absent. Decreased skeletal muscle mass and pallor of the skeletal muscles of the leg were observed in some birds. Myocardial pallor, hydropericardium, cardiomegaly, splenomegaly, ascites, and fibrin accumulation on the epicardium and capsular surfaces of the spleen and liver were sometimes seen. Microscopically, the most characteristic lesion was moderate to severe degenerative rhabodomyopathy. Mild to moderate myocarditis, fibrinous epicarditis, periportal hepatitis and peripheral neuritis were seen in some sections. Nuclear changes suggestive of intranuclear viral inclusion bodies were seen within occasional myocardial tissues.
Epidemiology
By mid-1999, approximately 20 premises in Pennsylvania were affected with MDPV disease. Epidemiological evidence strongly suggested that the virus was introduced into Pennsylvania via hatching eggs and 1-day-old ducklings from the same breeder company. In some instances, there was potential for subsequent spread between farms by visitors. The disease was seen in successive flocks on some of the farms despite cleaning and disinfection.
Initial laboratory tests
Initial assays for ionophores, vitamin E and selenium failed to identify an aetiology. Routine bacteriology, mycology, serology and conventional virus isolation did not yield significant or consistent findings. As additional case histories revealed a common breeder source for all cases, suspicion shifted to vertically transmitted diseases and parvovirus was first considered as a possible aetiology (Dunn et al., Citation1999). Convalescent blood samples obtained from diseased flocks were submitted to the National CitationVeterinary Services Laboratory (Ames, Iowa, USA) and were found to contain antibodies against GPV by indirect immunofluorescence assays.
Virus isolation and propagation
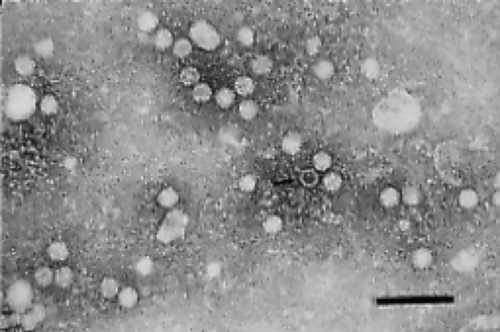
Embryo deaths occurred on the second egg passage and parvovirus particles were detected in the allantoic fluids by transmission electron microscopy (). Five additional passages were performed in Muscovy duck embryos and consistently induced embryo mortality. MDEF cultures were inoculated with preparations prepared from embryos to propagate virus isolates. Cytopathic effects were not observed until passages 4 to 5, and major destruction of monolayers occurred after passage 6. MDPV required initial isolation in Muscovy duck embryos before it could be adapted to MDEF cultures.
DNA sequence
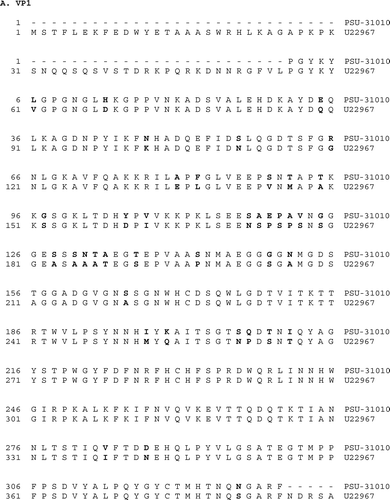
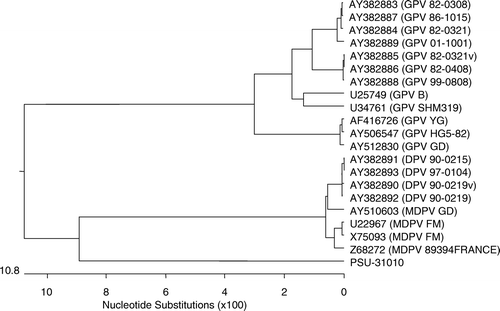
Primer sets 1 and 2 produced the expected amplicons of 593 and 539 base pairs, respectively, while all other primer sets failed to amplify the expected fragments. Sequencing of four independently selected plasmids confirmed that these amplicons were derived from MDPV sequences, and the combined sequence of 1109 bases spanned nucleotides 2615 to 3723 of ORF2, including parts of all capsid proteins (). The putative unusual start codon ACG for VP2 is located at nucleotides 2885 to 2887 and the start codon for VP3 is found at nucleotides 3044 to 3046. Comparison with one GPV and three MDPV sequences detected many differences between MDPV/PSU-30010 and both the MDPV and the GPV sequences (). Similar sequences in the GenBank database that had greater than 80% identity with MDPV/PSU-30010 were used to generate a phylogenic tree using MegAlign™ 6.1 (DNASTAR Inc.). The sequence of MDPV/PSU-31010 differed considerably from those of characterized isolates (). The differences in the nucleotide sequence () also resulted in differences in the predicted amino acid sequences for VP1 and VP3 (). The predicted amino acid sequence for VP2 was not examined because of the unusual start codon.
Figure 2. Sequence comparison for the 1109 nucleotide fragment of MDPV/PSU-31010 (GenBank accession number DQ413026) with three MDPV isolates (GenBank accession numbers U22967, X75093, and Z68272) and one goose parvovirus (Genbank accession number U25749).
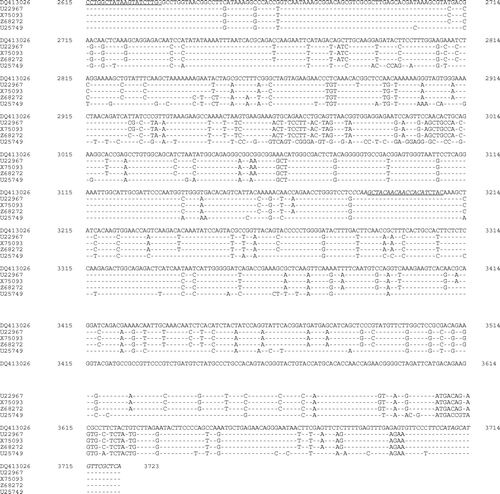
Figure 3. Phylogenetic tree based on the sequence of the 1109 nucleotide of MDPV isolate MDPV/PSU-31010 and available sequences in GenBank for MDPV and GPV. The GenBank accession numbers are shown with the parvovirus type and strain/isolate name in parentheses.
Discussion
Prior to the outbreak described here, MDPV disease had not been recognized in the Americas, although around the same time MDPV was isolated in California (Dunn et al., Citation1999; Woolcock et al., Citation2000). The clinical signs, gross and microscopic lesions, and clinical course of the cases in Pennsylvania were comparable with those previously described (Dalibard et al., Citation1993).
The MDPV/PSU-31010 isolate is of interest because the nucleotide sequence, and the predicted amino acid sequences for VP1 and VP3 are clearly different from other MDPV and GPV sequences. The approximately 1.1 kb region within ORF2 shares over 99% sequence similarity among MDPV isolates, and the GPV isolates share more than 95% similarity. However, the available sequence of the MDPV/PSU-31010 isolate has an average sequence identity with other MDPV and GPV isolates of 85%. Most of these isolates are from Europe or Asia. Another US isolate, the MDPV isolate from California (Woolcock et al., Citation2000), had 100% sequence identity with the reference strain 89384 (Z68272) (Jestin et al., Citation1991), whereas the MDPV/PSU-31010 isolate shared only about 85% identity with Z68272. These results clearly show that the MDPV/PSU-31010 isolate is markedly different from any of the MDPV or GPV isolates described in the literature. The differences are most probably also present in other parts of the genome because the other primer combinations failed to amplify the expected fragments. The differences are of considerable interest because these are located in the coding region of the structural viral proteins. The start codons for VP1, VP2 and VP3 are located at nucleotides 2450 to 2452, 2885 to 2887, and 3044 to 3046, respectively, in the MDPV FM genome. The VP2 and VP3 start codons for MDPV are located at the same locations as for the FM isolate. The predicted MDPV/PSU-31010 VP2 start codon has the same unusual sequence, ACG, as that reported for the reference strain 89394 from France (Zadori et al., Citation1995).
The reasons for this divergence are not clear. The number of passages (seven in Muscovy duck embryos and seven in MDEF cultures) is unlikely to account for such variation. An MDPV vaccine strain (90-0219V), which had undergone more than 100 passages in embryonated eggs or embryo fibroblast cultures, exhibited only a 0.1% nucleotide difference from its parental field strain, whereas a GPV vaccine strain (82-0321V), which had undergone a total of 70 passages in embryonated eggs or embryo fibroblast cultures, exhibited only a 3.3% nucleotide difference from its parental field strain (Tsai et al., Citation2004). It is possible that MDPV/PSU-31010 actually represents a new parvovirus and additional studies will be needed to completely resolve this question, including the cloning and sequencing of the complete MDPV/PSU-31010 genome.
Acknowledgments
The authors thank Dr A. Castro and Dr D. Gaudry for their contributions to this study, and Kathy Hillard (ADL-PSU), Tom Doman (ADL-PSU) and Priscilla O'Connell (Cornell University) for excellent technical assistance. The studies at Cornell University were supported in part by a grant from Grimaud Farms, Stockton, California, USA.
References
- Barnes , H.J. ( 1997 ) . Muscovy duck parvovirus . In B.W. Calnek , H.J. Barnes , C.W. Beard , L.R. McDougald , & Y.M. Saif ( Eds ) Diseases of Poultry 10th edn (pp. 1032 – 1033 ). Ames , IA : Iowa State University Press .
- Chang , P.-C. , Shien , J.-H. , Wang , M.-S. and Shieh , H.K. 2000 . Phylogenetic analysis of parvoviruses isolated in Taiwan from ducks and geese . Avian Pathology , 29 : 45 – 49 .
- Chu , C.Y. , Pan , M.J. and Cheng , J.T. 2001 . Genetic variation of the nucleocapsid genes of waterfowl parvovirus . Journal of Veterinary Medical Science , 63 : 1165 – 1170 .
- Dalibard , V. , Plassiart , G. , Cherel , Y. , Hurtrel , M. and Wyers , M. 1993 . Characterization of histological lesions of spontaneous parvovirus of the Muscovy duck (Carina moschata). Differential histopathological diagnosis with reovirus . Recueil de Médecine Vétérinaire , 169 : 763 – 772 .
- Derzsy , D. 1967 . A viral disease of goslings. I. Epidemiological, clinical, pathological and aethiological studies . Acta Veterinaria Academiae Scientiarum Hungaricae , 17 : 443 – 448 .
- Dunn , P.A. , Nesselrodt , A.J. , Miller , W.L. , Castro , A.E. & Ziegler , A.F. ( 1999 ) . Case report: Muscovy duck parvoviral disease in Pennsylvania ducklings . In Proceedings of the 71st Northeastern Conference on Avian Diseases (p. 34 ), Blacksburg , VA , USA .
- Fournier , D. & Gaudry , D. ( 1992 ) . Recent discoveries on waterfowl pathology: a new parvovirus of Muscovy ducks in France—Field vaccination trials . In M.S. McNulty & J.B. McFerran (Eds), Proceedings of the Commission of the European Communities Meeting on Virus Diseases of Poultry—New and Evolving Pathogens (pp. 183 – 194 ). Brussels : European Commission .
- Glavits , R. , Zolnai , A. , Szabo , E. , Ivanics , E. , Zarka , P. , Mato , T. and Palya , V. 2005 . Comparative pathological studies on domestic geese (Anser anser domestica) and Muscovy ducks (Cairina moschata) experimentally infected with parvovirus strains of goose and Muscovy duck origin . Acta Veterinaria Hungarica , 53 : 73 – 89 .
- Jestin , V. , le Bras , M.-O. , Cherbonnel , M. , le Gall , G. and Bennejean , G. 1991 . Mise en evidence de parvovirus (virus de la maladie de Derzsy) tres pathogenes dans les elevages de canards de Barbarie . Recueil de Médecine Vétérinaire , 167 : 849 – 857 .
- Kisary , J. and Derzsy , D. 1974 . Viral disease of goslings. IV. Characterization of the causal agent in tissue culture system . Acta Veterinaria Academiae Scientiarum Hungaricae , 24 : 287 – 292 .
- Lu , Y.S. , Lin , D.F. , Lee , Y.L , Liao , Y.K. and Tsai , H.J. 1993 . Infectious bill atrophy syndrome caused by parvovirus in a co-outbreak with duck viral hepatitis in ducklings in Taiwan . Avian Diseases , 37 : 591 – 596 .
- Sambrook , J. , Fritsch , E.F. and Maniatis , T. 1989 . Molecular Cloning. A Laboratory Manual , 2nd edn , Cold Spring Harbor , NY : Cold Spring Harbor Laboratory Press .
- Schat , K.A. & Purchase , H.G. ( 1998 ) . Cell-culture methods . In D.E. Swayne , J.R. Glisson , M.W. Jackwood , J.E. Pearson , & W.M. Reed (Eds.) , A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn (pp. 223 – 234 ). Kennett Square , PA : American Association of Avian Pathologists .
- Tsai , H.J. , Tseng , C.H. , Chang , P.C. , Mei , K. and Wang , S.C. 2004 . Genetic variation of viral protein 1 genes of field strains of waterfowl parvoviruses and their attenuated derivatives . Avian Diseases , 48 : 512 – 521 .
- Woolcock , P.R. , Jestin , V. , Shivaprasad , H.L. , Zwingelstein , F. , Arnauld , C. , McFarland , M.D. , Pedersen , J.C. and Senne , D.A. 2000 . Evidence of Muscovy duck parvovirus in Muscovy ducklings in California . Veterinary Record , 146 : 68 – 72 .
- Xing , Z. and Schat , K.A. 2000 . Expression of cytokine genes in Marek's disease virus-infected chickens and chicken embryo fibroblasts . Immunology , 100 : 70 – 76 .
- Zadori , Z. , Erdei , J. , Nagy , J. and Kisary , J. 1994 . Characteristics of the genome of goose parvovirus . Avian Pathology , 23 : 359 – 364 .
- Zadori , Z. , Stefancsik , R. , Rauch , T. and Kisary , J. 1995 . Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2 . Virology , 212 : 562 – 573 .