Abstract
Low pathogenic avian influenza subtype H9N8 was diagnosed on a Korean native chicken farm in Gyeonggi province, South Korea, in late April 2004. Clinical signs included moderate respiratory distress, depression, mild diarrhoea, loss of appetite and a slightly elevated mortality (1.4% in 5 days). Pathologically, mucopurulent tracheitis and air sacculitis were prominently found with urate renal deposition. The isolated A/chicken/Kr/164/04 (H9N8) had an Ala-Ser-Gly-Arg (A/S/G/R) motif at the cleavage site of haemagglutinin, which has been commonly found in H9N2 isolated from Korean poultry. Phylogenetic analysis of the haemagglutinin and neuraminidase genes of the H9N8 avian influenza virus (AIV) isolate showed that reassortment had occurred. Its haemagglutinin gene was similar to that of Korean H9N2 AIVs, but its neuraminidase gene was closely related to that of A/WBF/Kr/KCA16/03 (H3N8) isolated from the faeces of wild birds in Korea. The pathogenicity of the isolate was tested on 6-week-old specific pathogen free chickens. The inoculated virus (H9N8) was recovered from most tested organs, including the trachea, lung, kidney, spleen, and caecal tonsil. This is the first report of an outbreak of low pathogenic avian influenza in chickens caused by AIV subtype H9N8.
Un cas d'influenza aviaire faiblement pathogène (LPAI) de sous type H9N8 a été diagnostiqué dans une ferme de poulets indigènes, dans la province de Gyeonggi, en Corée du Sud, en Avril 2004. Les symptômes consistaient en une détresse respiratoire modérée, une diarrhée peu importante, une perte d'appétit et une mortalité légèrement élevée (1,4% en 5 jours). Sur le plan pathologique, une trachéite mucopurulente et une aérosacculite ont été les lésions les plus souvent observées avec un dépôt d'urate au niveau des reins. La souche isolée A/chicken/Kr/164/04 (H9N8) présentait un motif Ala-Ser-Gly-Arg (A/S/G/R) au niveau du site de clivage de l'hémagglutinine (HA) qui a souvent été observé chez les souches H9N2 isolées de volailles en Corée. L'analyse phylogénétique des gènes de HA et de la neuraminidase (NA) de la souche de virus de l'influenza aviaire (AIV) H9N8 a montré qu'il s'était produit un réassortiment. Son gène HA était similaire à celui des AIVs coréens H9N2, mais son gène NA était très proche de celui de la souche A/WBF/Kr/KCA16/03 (H3N8) isolée de fèces d'oiseaux sauvage en Corée. La pathogénicité de l'isolat a été testée chez des poulets exempts de microorganismes pathogènes spécifiés âgés de 6 semaines. Le virus inoculé (H9N8) a été réisolé de la plupart des organes testés incluant la trachée, les poumons, les reins, la rate et les amygdales cæcales. C'est le premier rapport d'un cas de LPAI chez des poulets causé par un AIV de sous type H9N8.
Im späten April 2004 wurde auf einer koreanischen Farm mit einheimischen Hühnern in der Gyeonggi-Provinz ein schwach pathogenes aviäres Influenzavirus (LPAI) Subtyp H9N8 isoliert. Die klinischen Symptome umfassten geringe Atembeschwerden, Störung des Allgemeinbefindens, milde Diarrhoe, Appetitlosigkeit und eine geringfügig erhöhte Mortalität (1,4 % in 5 Tagen). Pathologisch-anatomisch wurden als herausragende Veränderungen eine mukopurulente Tracheitis und Aerosacculitis sowie renale Harnsäureablagerungen gefunden. Das Isolat A/chicken/Kr/164/04 (H9N8) besaß ein Ala-Ser-Gly-Arg (A/S/G/R)-Motiv an der Spaltstelle des Hämagglutinins (HA), das auch üblicherweise bei den aus Geflügel in Korea isolierten H9N2-Stämmen gefunden worden war. Die phylogenetische Analyse der HA- und Neuraminidase (NA)-Gene des aviären H9N8-Influenzavirus (AIV)-Isolats ließ erkennen, dass ein Reassortment statt gefunden hat. Sein HA-Gen war dem der koreanischen H9N2-AIVs ähnlich, während sein NA-Gen eng verwandt war mit dem des A/WBF/Kr/KCA16/03 (H3N8)-Stamm, der aus dem Fäzes von Wildvögeln in Korea isoliert worden war. Die Pathogenität des Isolats wurde an sechs Wochen alten spezifisch pathogen freien Hühnerküken überprüft. Das inokulierte Virus (H9N8) wurde aus fast allen untersuchten Organen einschließlich Trachea, Lunge, Niere, Milz und Zäkaltonsillen reisoliert. Dies ist die Erstbeschreibung eines durch den AIV-Subtyp H9N8 verursachten LPAI-Ausbruch in Hühnern.
Se diagnosticó influenza aviar de baja patogenicidad (LPAI) del subtipo H9N8 en una granja de pollos nativos de Korea en la provincia de Gyeonggi, Korea del Sur, a finales de Abril del 2004. Los signos clínicos incluían insuficiencia respiratoria moderada, depresión, diarrea leve, pérdida de apetito e incremento leve de mortalidad (1.4% en 5 días). Microscópicamente, se observó principalmente traqueítis mucopurulenta y aerosaculitis asociados a depósito de uratos en los riñones. El aislamiento A/chicken/Kr/164/04 (H9N8) tenían un motivo Ala-Ser-Gly-Arg (A/S/G/R) en el punto de corte de la hemaglutinina (HA), el cual se había encontrado frecuentemente en los aislamientos H9N2 de las aves de Korea. El análisis filogenético de los genes de la HA y de la neuraminidasa (NA) del aislamiento del virus de influenza aviar H9N8 mostró que había ocurrido un reordenamiento genético. Su gen de HA era similar al de los AIVs H9N2 de Korea, pero su gen NA estaba relacionado estrechamente con el virus A/WBF/Kr/KCA16/03 (H3N8) aislado de heces de aves salvajes de Korea. Se testó la patogenicidad del aislamiento en pollos libres de patógenos específicos de 6 semanas de vida. El virus inoculado (H9N8) se recuperó de la mayoría de los órganos testados incluyendo tráquea, pulmón, riñón, bazo y tonsila cecal. Esta es la primera descripción de un brote de LPAI en pollos causado por un AIV del subtipo H9N8.
Introduction
Avian influenza (AI) is an infectious disease caused by viruses of the influenza A genus of the Orthomyxoviridae family. The avian influenza virus (AIV) is distributed throughout the world in many domestic birds, including chickens, turkeys, quails, geese and ducks, and in wild waterfowl, gulls and shorebirds (Alexander, Citation2000). Poultry are not the natural hosts of this virus, but infections with AIVs produce a variety of syndromes ranging from asymptomatic, to respiratory disease with low mortality, to highly pathogenic forms with high mortality (Hinshaw et al., Citation1980; Swayne & Halvorson, Citation2003). The viruses are classified into subtypes based on the antigenic differences between their two surface glycoproteins, haemagglutinin (HA) and neuraminidase (NA) (Webster et al., Citation1992). Sixteen HA subtypes (H1 to H16) and nine NA subtypes (N1 to N9) have been identified among influenza A viruses (Kawaoka et al., Citation1990; Rohm et al., Citation1996; Fouchier et al., Citation2005), and viruses of all subtypes and of the majority of possible combinations have been isolated from avian species (Alexander, Citation2000).
Epidemiological data suggest that H9 AIVs have been isolated worldwide from birds, pigs and humans since its first isolation from turkey in Wisconsin in 1966 (Banks et al., Citation2000; Xu et al., Citation2004; Li et al., Citation2003, Citation2005). H9 viruses were isolated regularly from ducks in South China and Hong Kong before the 1990s, and infections of the H9 subtype AIV in chickens have been reported in many Asian countries since (Guo et al., Citation2000; Choi et al., Citation2005). In South Korea, a low pathogenic avian influenza (LPAI) outbreak due to H9N2 was also reported in 1996 (Mo et al., Citation1997; Lee et al., Citation2000). Subsequently, the virus was commonly isolated from layers and broiler breeders national-wide (Seo & Kim, Citation2004; Choi et al., Citation2005).
In contrast to the extensive investigations undertaken on HA subtypes, NA subtypes have been limited to N1 and N2 from avian and human isolates. N8 subtype AIVs have been isolated from mammals, including equines and humans, more frequently than from avian species. Moreover, N8 had been isolated from wild waterfowl in some countries but not from chickens (Bragstad et al., Citation2005).
During the past 10 years the National Veterinary Research and Quarantine Service (NVRQS) in South Korea, through routine diagnostic casework, has diagnosed AI on two occasions in commercial poultry (1996, H9N2; 2003, H5N1) (Mo et al., 1997; Lee et al., 2000;Wee et al., Citation2006). In the migratory wild birds, many different H subtypes, including H3N8, H4N6, H9N2 and H10N8, have also been isolated since 2003 (Kim et al., Citation2004;Wee et al., Citation2006). In 2004, an AI outbreak caused by H9N8 was first detected in chickens at Korean Native chicken farms. However, although the infection of chickens with various AIVs has been described in many countries, to our knowledge this is the first report of H9N8 infection in this host. We therefore describe the molecular and pathological properties of this Korean H9N8 AI isolate.
Materials and Methods
Animals
On 22 May 2004, Gyeonggi Regional Livestock and Veterinary Service submitted eight dead Korean native chickens (74 days old) from a poultry farm that exhibited moderate mortality and clinical respiratory signs to the Avian Disease Division, NVRQS, Anyang, South Korea.
In the farm located in northern South Korea (Gyeonggi province), a disease characterized by depression, respiratory signs, diarrhoea, a decrease in feed and water consumption, and a moderately increased mortality (1.4% in 5 days) occurred on the farm. At the time of the outbreak, the farm contained 40,000 meat birds (Korean native chickens) and four poultry houses. A final diagnosis of LPAI was made based on laboratory tests, which included pathological and virological test results. The Gyeonggi Department of livestock placed an immediate quarantine at the site at the time of the AIV diagnosis. Movement of all animals and equipment was prohibited until no AIV was isolated from the faeces or tissues (i.e., caecal tonsils, tracheas and kidneys).
Pathology
The surfaces of birds were wetted with disinfectant and necropsies were routinely performed. Tissues samples were taken (trachea, lung, heart, liver, spleen, kidney, proventriculus, pancreas, intestine, caecal tonsil, and brain) and fixed in 10% neutral buffered formalin, were processed, and were embedded in paraffin blocks. Sections were made at 5 µm and stained with haematoxylin and eosin.
Virology
For virological examination, 10% homogenates of caecal tonsils, faeces, and kidneys were inoculated into the allantoic cavity of 9-day-old specific pathogen free embryonated eggs. Allantoic fluids from the eggs were then harvested after 4 days inoculation and tested using the agar gel precipitation test for type A influenza virus. The isolate was typed as influenza A H9N8 virus by haemagglutination and neuraminase inhibition testing with a panel of antisera (provided by OIE Reference Laboratory, Veterinary Agency, Surrey, UK).
Bacteriology
For bacterial examination, lungs and air sacs were aseptically collected using sterilized cotton-tipped swabs. Swabs collected were streaked on both 5% sheep blood agar (Komed Co., Ltd., Korea) and MacConkey's agar, and inoculated into 10 ml trypticase soy broth as growth media. The solid media plates and broths were then incubated at 37°C for 24 to 48 h under aerobic and anaerobic conditions.
Sequencing and phylogenetic analyses of the influenza HA and NA genes
Viral genes were sequenced and analysed as described previously (Choi et al., Citation2005). Briefly, viral RNA was extracted from the allantoic fluid of embryonated eggs using RNeasy Mini Kits (Qiagen, Chatsworth, California, USA). Reverse transcription and polymerase chain reaction (PCR) amplification were carried out under standard conditions using influenza-specific primers (Hoffmann et al., Citation2001). PCR products were purified using QIAquick PCR purification kits (Qiagen). Nucleotide sequences were analysed by directly sequencing PCR products on an automated 3700 DNA sequencer.
For comparison, we included in the phylogenetic analysis sequences from AIVs established in southern China and middle east Asia since the mid-1990s (Dk/HK/Y280/97-like and Ck/Pakistan/4/99-like H9N2 viruses); five Korean H9N2 viruses (Ck/Kr/MS96/96-like); sequences from H3N8 viruses isolated from equine and avian species in Eurasian or North America; and AIVs recently isolated from poultry farms and wild birds in Korea. The nucleotide regions used in the phylogenetic analyses were 35 to 1680 for HA and 1 to 1463 for NA. Gene sequences determined during this study have been deposited at GenBank (accession number DQ885991 to DQ885998). The assembly of sequencing contigs was performed using Vector NTI; sequence data were aligned and analysed using Clustal X (version 1.83); and phylogenetic relationships were established using the neighbour-joining algorithm. The robustnesses of groupings were assessed by bootstrap resampling of 1000 replicate trees.
Pathogenicity of the virus in chickens
To investigate the pathogenicity of the virus in chickens, twelve 6-week-old specific pathogen free chickens were intravascularly inoculated with 0.1 ml of 106.5 median embryo infective dose (EID50) of the virus. Four of these chickens were randomly sacrificed at 4 days post inoculation (p.i.), and visceral organ, including the tracheas, lungs, caecal tonsils, kidneys, spleens and brains, oropharyngeal and cloacal swab specimens were collected. Virus titration was performed by egg inoculation. All tissues were sampled with sterilized separate scissors to prevent cross-contamination. The remaining birds were monitored for survival over a 10-day period. On day 14 p.i., chickens were bled and euthanized, and sera were tested for the presence of antibodies using the agar gel precipitin test. All birds were housed in isolation cabinets that ventilated under negative pressure with filtered air.
Results
Pathological findings
At necropsy, prominent lesions were observed in the respiratory tract, especially in sinuses and tracheas. Intraorbital sinuses were swollen, and mucopurulent nasal discharge was evident. Tracheal mucosae were oedematous, congested with/without haemorrhages with fibrinopurulent exudates. In some cases, fibrinous air sacculitis or pale and swollen kidneys with urate deposition were observed.
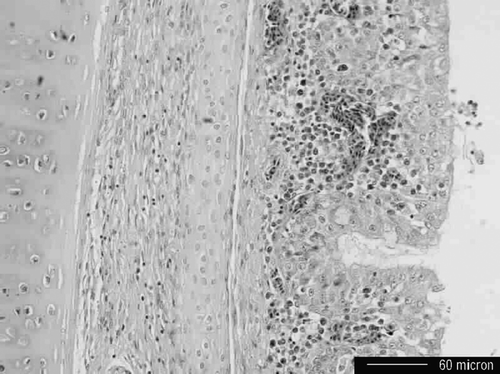
Histopathologically, severe congestion and occasional oedema of pulmonary parenchyma were evident. Tracheal epithelium and submucosa were congested with loss of cilia, and in some cases focal serofibrinous exudates containing epithelial cells debris and rare heterophils lined the epithelium (). In addition, lymphocytic interstitial nephritis was occasionally found with minerals in tubules.
Bacteriology
Escherichia coli was identified in aerobic cultures from the air sacs and lungs of the birds examined.
Pathogenicity testing
The virus induced moderate depression with a reluctance to move in chickens at 4 to 5 days p.i. However, mortality or any other typical AI clinical signs were not observed after 10 days of observation. All of the chickens had sero-converted on day 10 after inoculation ().
Table 1. Pathogenicity and replication of H9N8 influenza virus in chickensa
To investigate the distribution of virus in infected chickens, we randomly killed four chickens on day 4 p.i. to titrate virus in organs, which included the tracheas, lungs, caecal tonsils, kidneys and spleens. The inoculated virus was recovered from the majority of organs tested when inoculated intravascularly, but viral titres differed. The highest titres were found in caecal tonsils followed in decreasing order by the tracheas, lungs, kidneys, and spleens. These finding suggest that this virus replicates preferentially in the intestinal and respiratory tracts.
Genetic and phylogenetic analyses of HA and NA
To determine the genetic relatedness of selected genes of chicken H9N8 virus to those of other influenza A viruses isolated from avian and other hosts over time, the extended nucleotide sequences of the HA(35 to 1680) and NA(1 to 1463) genes were determined and compared with the gene sequences of influenza A viruses in Genbank.
Haemagglutinin
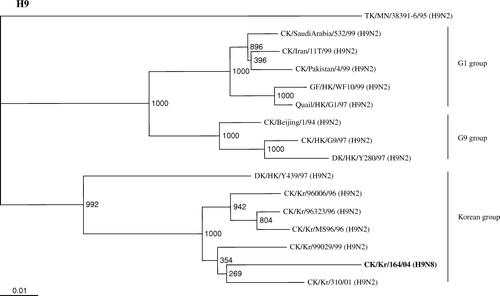
AIVs of subtype H9, especially H9N2, have become panzootic in Eurasia, including Korea, during the past decades, and have been divided into three lineages based on phylogenetic analysis: the G1 group, represented by A/quail/Hong Kong/G1/97(H9N2); the G9 group, represented by A/chicken/Hong Kong/G9/97(H9N2); and the Korean group, represented by A/duck/Hong Kong/Y439/97(H9N2). Phylogenetic analysis of the HA gene showed that the chicken/Kr/164/04 isolate belonged to the Korean group (), whereas the HA gene of the isolate closely resembled that of chicken/Kr/310/01 (H9N2), with 94.5% nucleotide homology, indicating that the HA gene originated from chicken H9 influenza virus in Korea. The deduced amino acid sequence of H9N8 at the cleavage site of HA protein differed from those of known highly pathogenic viruses. The isolate had an Ala-Ser-Gly-Arg (A/S/G/R) motif at this cleavage site, which has been commonly found in H9N2 isolated from Korean poultry (Lee et al., Citation2000; Choi et al., Citation2005).
Figure 2. Phylogenetic relationship between full-length HA nucleotide sequences of H9 viruses in the influenza sequence database and the A/Ck/Kr/164/04(H9N8) virus (shown in bold) constructed by the Clustal X program with 1000 bootstrap replicates. Numbers at branching points are the bootstrap value. Provisional designations of genotypes are indicated on the right. CK, chicken; DK, duck; GF, Guinea Fowl; HK; Hong Kong; Kr, Korea; H9, subanalysis of the influenza A virus isolate H9 subtypeH9 influenza viruses isolated. Bold type signifies the virus.
Neuraminidase
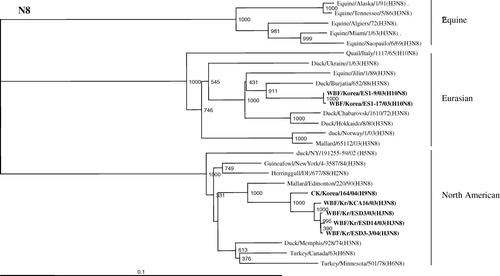
The NA nucleotide sequence of the chicken/Kr/164/04 isolate shared 99.2% nucleotide identity with N8 from A/WBF/Kr/KCA16/03(H3N8) isolated from wild birds in Korea. Previous phylogenetic analysis of the AIV N8 neuraminidases of different animals revealed that the N8 genes have evolved into at least three major lineages; that is, the equine, Eurasian, and North American lineages (Saito et al., Citation1993, Citation1994; Bragstad et al., Citation2005). The N8 gene of Korean H9N8 clustered phylogenetically with viruses isolated in North American, as did the four H3N8s isolated from wild birds in Korea between 2003 and 2004, with a 92.4 to 99.8% nucleotide homology (). These findings indicate that the NA genes originated directly from wild birds. In contrast, the NA genes of two H10N8s, isolated from wild birds in Korea in 2003, belong to the Eurasian lineage, and nucleotide identities between Korean H9N8 and H10N8 were less than 78%. The homologies of viruses of the Korean H3N8 subtype and H10N8 were less than 80%, suggesting that they evolved independently.
Figure 3. Phylogenetic relationship between available full-length NA nucleotide sequences of N8 viruses in the influenza sequence database and the A/chicken/Kr/164/03 (H9N8) virus constructed by the Clustal X program with 1000 bootstrap replicates. Numbers at branching points are the bootstrap value. Provisional designations of genotypes are indicated on the right. Bold type signifies the viruses tested in this study.
Discussion
Pathogenicity testing, by intravenous inoculation into SPF chickens, showed that this Korean H9N8 isolate did not cause mortality under laboratory conditions. Moreover, sequence analysis revealed that the virus did not possess multiple basic amino acids at the HA1/HA2 cleavage site. A LPAI virus A/S/G/R motif has previously been described for Korean H9N2 viruses (Lee et al., Citation2000; Choi et al., Citation2005). In addition, the signs of disease that were observed in Korean native chickens on a gross and histopathological level most resembled those reportedly caused by LPAI viruses (Swayne & Halvorson, Citation2003).
The H9 nucleotide sequence of the Korean H9N8 isolate shared 94.5% homology with the H9 sequence from A/chicken/Kr/310/01, and its N8 nucleotide sequence shared 99.2% homology with the N8 sequence from A/WBF/Kr/KCA16/03. Based on these analyses, it is suggested that the new H9N8 might be a recent re-assortment of a H9N2 subtype virus isolated from migratory wild birds which showed a closer similarity with the Korean lineage and a Korean H3N8 subtype virus of the North American lineage. However, the data on the N8 gene are very minimal and may actually indicate poor separation of the N8 gene between the continents until now. Further characterization of the genes coding for the internal protein of the Korean H9N8 isolate may reveal the actual relationship between this isolate and other AIVs isolated from domestic and wild birds in Korea.
AIVs (involving at least three subtypes, H5, H7, and H9) have emerged as an important pathogen in the poultry industry, and are a major global health concern. In 1996, the first AI outbreak case was reported in Korea, and type A H9N2 AIV was isolated from several broiler breeder flocks (Mo et al., Citation1997; Lee et al., Citation2000). At that time, five H9N2 viruses were isolated from five broiler breeder farms throughout the country. Most of the affected birds showed typical clinical signs of influenza (i.e. a drop in egg production and up to 40% mortality), but neither the virus nor the different Korean H9N2 isolates produced any detectable signs of disease after experimental infection (Mo et al., Citation1997; Choi et al., Citation2005); this finding concurs with the present study. However, in the field, disease or death in poultry farms from which the LPAI virus, including the H9 subtype, were isolated may not be caused by the virus alone and may well have been the result of co-infection with other pathogens (Kishida et al., Citation2004).
Our studies showed that replication patterns differ among H9 subtype AIVs. North American H9 subtype isolates from turkeys (Ty/WI/66) (H9N2) and shore birds (Sb/DE/9/96) (H9N2) showed a limited ability to replicate mainly in intestinal tracts (Guo et al., Citation2000). In contrast, the Korean H9N8 isolate replicated mainly in intestinal and respiratory tracts, including the lungs, which suggests that the HA gene of the chicken/Kr/164/04 (H9N8) isolate, the most important virulent protein of AIVs (Suarez & Schultz-Cherry, Citation2000), may have originated directly from the chicken, and further adapted to this host. Thus, influenza viruses isolated from different avian species differ in terms of host range restriction (Webster et al., Citation1978; Hinshaw et al., Citation1980).
In Korea, the Korean HPAI (H5N1) epidemic between mid-December 2003 and late March 2004 was eventually controlled by combined depopulation and eradication by the NVRQS, the provincial livestock veterinary service, and the local poultry industry, aided by intense targeted diagnostic surveillance and producer reporting of the prominent clinical signs (i.e. an eerie flock quietness preceding an abrupt spike in mortality) (Kwon et al., Citation2005; Lee et al., Citation2005). However, the fact that H9N8 influenza viruses are not highly pathogenic for poultry makes an endemic more likely, as was demonstrated by the Korean H9N2 viruses that have been distributed nationwide since 1996. Therefore, continued active surveillance in both symptomatic and asymptomatic flocks is needed for early detection of H9N8 infected flocks, along with proper quarantine procedures in H9N8 AIV-positive flocks. This will improve understanding of the effect and distribution of this AIV subtype in the poultry industry in Korea.
References
- Alexander , D.J. 2000 . A review of avian influenza in different bird species . Veterinary Microbiology , 74 : 3 – 13 .
- Banks , J. , Speidel , E.C. , Harris , P.A. and Alexander , D.J. 2000 . Phylogenetic analysis of influenza A viruses of H9 hemagglutinin subtype . Avian Pathology , 29 : 353 – 360 .
- Bragstad , K. , Jorgensen , P.H. , Handberg , K.J. , Mellergarrd , S , Corbet , S. and Fomsgaard , A. 2005 . New avian infleunza A virus subtype combination H5N7 identified in Danish mallard ducks . Virus Research , 109 : 181 – 190 .
- Choi , Y.K. , Seo , S.H. , Kin , J.A. , Webby , R.J. and Webster , R.G. 2005 . Avian influenza viruses in Korean live poultry markets and their pathogenic potential . Virology , 332 : 529 – 537 .
- Fouchier , R.A. , Munster , V. , Wallensten , A. , Besterbroer , T.M. , Herfst , S. , Smith , D. , Rimmelzwann , G.F. , Olsen , B. and Osterhaus , A.D. 2005 . Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls . Journal of Virology , 79 : 2814 – 1822 .
- Guo , Y.J. , Krauss , S. , Senne , D.A. , Mo , I.P. , Lo , K.S. , Xiong , X.P. , Norwood , M. , Shortridge , K.F. , Webster , R.G. and Guan , Y. 2000 . Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia . Virology , 267 : 279 – 288 .
- Hinshaw , V.S. , Webster , R.G. and Turner , B. 1980 . The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl . Canadian Journal of Microbiology , 26 : 622 – 629 .
- Hoffmann , E. , Stech , J. , Guan , Y. , Webster , R.G. and Perez , D.R. 2001 . Universal primer set for the full-length amplification of all influenza A viruses . Archives of Virology , 146 : 2275 – 2289 .
- Kawaoka , Y. , Yamnikowa , S. , Chambers , T.M. , Ivov , D.K. and Webster , R.G. 1990 . Molecular characterization of a new hemagglutinin subtype H14 of influenza A virus . Virology , 179 : 757 – 767 .
- Kim , J.H ., Sung , H.W ., Lee , Y.L ., Choi , J.G ., Lee , E.K ., Kwon , Y.K ., Joh , S.J ., Kim , M.C ., Jang , H ., Wee , S.H ., Park , C.K ., & Park , J.M ., ( 2004 ). Virological and serological surveillance of domestic ducks and wild birds during outbreaks of highly pathogenic avian influenza in Korea . In Proceedings of the 13th Federation of Asian Veterinary Associations (pp. 172 ), Seoul, South Korea .
- Kishida , N. , Sakoda , Y. , Eto , M. , Sunaga , Y. and Kida , H. 2004 . Co-infection of Staphylcoccus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza A virus infection in chickens . Archives of Virology , 149 : 2095 – 2104 .
- Kwon , Y.K. , Joh , S.J. , Kim , M.C. , Sung , H.W. , Lee , Y.J. , Choi , J.G. , Lee , E.K. and Kim , J.H. 2005 . Highly pathogenic avian influenza (H5N1) in the commercial domestic ducks of South Korea . Avian Pathology , 34 : 367 – 370 .
- Lee , C.W. , Song , C.S. , Lee , Y.J. , Mo , I.P. , Garcia , M. , Suarez , D.L. and Kim , S.J. 2000 . Sequence analysis of the hemagglutinin gene of H9N2 Korean avian influenza viruses and assessment of the pathogenic potential of isolate MS96 . Avian Diseases , 44 : 527 – 535 .
- Lee , C.W. , Suarez , D.L. , Tumpey , T.M. , Sung , H.W. , Kwon , Y.K. , Lee , Y.J. , Choi , J.G. , Joh , S.J. , Kim , M.C. , Lee , E.K. , Park , J.M. , Lu , X. , Katz , J.M. , Spackman , E. , Swayne , D.E. and Kim , J.H. 2005 . Characterization of highly pathogenic H5N1 avain influenza A viruses isolated from South Korea . Journal of Virology , 79 : 3692 – 3702 .
- Li , C. , Yu , K. , Tian , G , Yu , D. , Liu , L. , Jing , B. , Ping , J. and Chen , H. 2005 . Evolution of H9N2 influenza viruses from domestic poultry in mainland china . Virology , 340 : 70 – 83 .
- Li , K.S. , Xu , K.M. , Peiris , J.S.M. , Poon , L.L. , Yu , K.Z. , Yuen , K.Y. , Shortridge , K.F. , Webseter , R.G. and Guan , Y. 2003 . Characterization of H9 subtype influenza viruses from the ducks of southern china: a candidate for the next influenza pandemic in Human? . Journal of Virology , 77 : 6988 – 6994 .
- Mo , I.P. , Song , C.S. , Kim , K.S. & Rhee , J.C. ( 1997 ). An occurrence of non-highly pathogenic avian influenza in Korea . In Proceedings of the 4th International Symposium on Avian Influenza (pp. 379 – 383 ). Athens , GA , , USA .
- Rohm , C. , Zhou , N , Suss , J. , Mackenzie , J. and Webster , R.G. 1996 . Characterization of a novel influenza hemagglutinin, H15; criteria for determination of influenza A subtypes . Virology , 217 : 508 – 516 .
- Saito , T. , Kawaoka , Y. and Webster , R.G. 1993 . Phylogenetic analysis of the N8 neuraminidase gene of influenza A viruses . Virology , 193 : 868 – 876 .
- Saito , T. , Taylor , G. , Laver , W.G. , Kawaoka , Y. and Webster , R.G. 1994 . Antigenicity of the N8 influenza virus neuraminidase: existence of an epitope at the subunit interface of the neuraminidase . Journal of Virology , 68 : 1790 – 1796 .
- Seo , S.H. and Kim , H.S. 2004 . Epidemiology of influenza virus in Korean poultry . International Congress Series , 1263 : 758 – 761 .
- Suarez , D.L. and Schultz-Cherry , S. 2000 . Immunology of avian influenza virus: a review . Developmental and Comparative Immunology , 24 : 269 – 283 .
- Swayne , D.E. and Halvorson , D.A. 2003 . “ Influenza ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glison , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 135 – 160 . Ames , IA : Iowa State University Press .
- Webster , R.G.. , Yakhno , M. , Hinshaw , V.S. , Bean , W.J. and Murti , K.G. 1978 . Intestinal influenza: replication and characterization of influenza viruses in ducks . Virology , 84 : 268 – 278 .
- Webster , R.G. , Bean , W.J. , Gorman , O.T. , Chambers , T.M. and Kawaoka , Y. 1992 . Evolution and ecology of influenza A viruses . Microbiology , 56 : 152 – 179 .
- Wee , S.H. , Park , C.K. , Kim , C.H. , Yoon , H. , Kim , S.J. , Lee , E.S. , Lee , B.Y. , Kim , J.H. and Kim , C.S. 2006 . Outbreaks of highly pathogenic avian influenza (H5N1) in the Republic of Korea in 2003/04 . Veterinary Record , 158 : 341 – 344 .
- Xu , C. , Fan , W. , Wei , R. and Zhao , H. 2004 . Isolation and identification of swine influenza recombinant A/Swine/Shandong/1/2003(H9N2) virus . Microbes and Infection , 6 : 919 – 925 .