Abstract
Previous studies in our laboratory using a combination of polymerase chain reaction and restriction fragment length polymorphism have identified at least five different genotypes of infectious laryngotracheitis virus (ILTV). However, the virulence of these classes of ILTV was not investigated. In this study, five groups (16 birds each) of 3-week-old specific pathogen free chickens were inoculated via the intratracheal route with 103 median embryo infected dose of five different strains of ILTV. Three further groups of chickens were inoculated similarly with the vaccine strains SA2 and A20 or with sterile phosphate-buffered saline (PBS) for comparison. Four days post-inoculation, clinical signs were monitored for scoring, and eight chickens from each group were subsequently euthanized, weighed and subjected to pathological and histopathological examinations. The remaining birds were monitored for clinical signs and mortality until 21 days post-inoculation.
All groups inoculated with ILTV strains showed moderate to severe clinical signs 4 days after inoculation. The strain Q1-96 caused only minimal breathing symptoms with a median score that was not significantly different to that of the group inoculated with PBS, but was significantly different to those of the groups inoculated with other ILTV strains. The strain Q1-96 caused severe photophobia and conjunctivitis with a median score that was significantly higher than those of all other groups except for the group inoculated with the strain N3-04. All ILTV strains caused a significant reduction in weight gain when compared with the group inoculated with PBS. The strain Q1-96 caused an average weigh loss of 14% that was significantly higher than those of other ILTV strains. The strains S2-04 and Q1-96 induced only minor microscopic tracheal lesions while all the other ILTV strains, including the vaccine strains A20 and SA2, induced moderate to severe microscopic tracheal lesions. Median scores for microscopic tracheal lesions were well correlated with the number of viral genomes detected in trachea.
The results revealed that there is considerable variation among ILTV strains in their tropism for trachea or conjunctiva. In addition it was revealed that ILTV strains with high affinity for conjunctiva can severely affect weigh gain. The ILTV numbers and microscopic lesions in trachea were not found to be reliable indicators of virulence since they are not necessarily correlated with mortality rate in ILT.
Des études antérieures conduites dans notre laboratoire utilisant une réaction de polymérisation en chaîne et de polymorphisme de taille des fragments de restriction (PCR-RFLP) avaient identifié au moins 5 génotypes différents de virus de la laryngotrachéite infectieuse aviaire (ILTV). Cependant, la virulence de ces classes d'ILTV n'avait pas été investiguée.
Dans cette étude, 5 groupes de 16 poulets SPF âgés de 3 semaines ont été inoculés par voie trachéale avec 103 EID50 de 5 souches différentes d' ILTV. Trois autres groupes de poulets ont été inoculés de façon similaire avec les souches vaccinales SA2 et A20 ou avec une solution de tampon phosphate (PBS) stérile pour comparaison. Quatre jours après l'inoculation, les symptômes ont été relevés et leur intensité enregistrée, huit poulets de chaque groupe ont ensuite été euthanasiés, pesés et soumis à des examens pathologiques et histopathologiques. Les sujets restants ont fait l'objet d'un suivi clinique et la mortalité a été enregistrée jusqu'à 21 jours après l'inoculation.
Tous les groupes inoculés avec les souches d'ILTV ont présenté des symptômes modérés à graves 4 jours après l'inoculation. La souche Q1-96 a entraîné seulement des symptômes respiratoires minimums avec un score moyen qui n'était pas significativement différent de celui du groupe inoculé avec le PBS, mais était significativement différent de ceux des groupes inoculés avec les autres souches d'ILTV. La souche Q1-96 a causé une photophobie sévère et une conjonctivite avec un score médian qui a été significativement supérieur à celui des autres groupes à l'exception du groupe inoculé avec la souche N3-04. Toutes les souches d'ILTV ont entraîné une diminution significative du gain de poids comparé au groupe inoculé avec le PBS. La souche Q1-96 a entraîné une perte de poids moyenne de 14% qui a été significativement supérieure à celle des autres souches d'ILTV. Les souches S2-04 et Q1-96 ont induit seulement quelques lésions trachéales microscopiques mineures alors que toutes les autres souches d'ILTV incluant les souches vaccinales A20 et SA2 ont induit des lésions trachéales microscopiques modérées à sévères. Les scores moyens des lésions trachéales microscopiques sont bien corrélés au nombre de génome viral détecté dans la trachée.
Les résultats ont révélé qu'il existait une variation considérable parmi les souches d'ILTV dans leur tropisme pour la trachée ou la conjonctive. De plus, il a été révélé que les souches d'ILTV possédant une forte affinité pour la conjonctive peuvent gravement affecter le gain de poids. Les nombres de ILTV et les lésions microscopiques au niveau de la trachée ne se sont pas révélé être des indicateurs fiables de la virulence puisqu'ils ne sont pas nécessairement corrélés avec le taux de mortalité dans la ILT.
In den verhergehenden Studien in unserem Labor waren mittels einer Kombination aus Polymerasekettenreaktion und Restriktionsfragmentlängenpolymorphismus (PCR-RFLP) mindestens fünf verschiedene Genotypen des Virus der infektiösen Laryngotracheitis (ILTV) identifiziert worden. Die Virulenz dieser ILTV-Genotypen war jedoch nicht untersucht worden.
In dieser Studie wurden fünf Gruppen von jeweils 16 dreiwöchigen SPF-Hühnerküken intratracheal mit je 103 EID50 von fünf verschiedenen ILTV-Stämmen inokuliert. Zum Vergleich wurden drei weiteren Hühnergruppen auf ähnliche Weise die Vakzinestämme SA2 und A20 oder steriler Phosphatpuffer (PBS) verabreicht. Vier Tage nach der Inokulation wurden die klinischen Symptome erhoben und bewertet. Anschließend wurden aus jeder Gruppe 8 Küken euthanasiert, gewogen und einer pathologisch-anatomischen und pathohistologischen Untersuchung unterzogen. Bei den verbliebenen Tieren wurden bis zum 21. Tag nach der Inokulation auftretende klinische Symptome und die Mortalität protokolliert.
Alle mit den ILTV-Stämmen inokulierten Gruppen zeigten am 4. Tag nach der Inokulation mittel- bis hochgradige klinische Symptome. Der Stamm Q1-96 verursachte nur minimale Atemwegssymptome mit einem gemittelten Bewertungsgrad, der sich nicht signifikant von dem der mit PBS inokulierten Gruppe unterschied, der aber signifikante Unterschiede zu denen der Gruppen aufwies, die mit den anderen ILTV-Stämmen infiziert worden waren. Der Stamm Q1-96 induzierte jedoch eine hochgradige Photophobie und Konjunktivitis deren mittlerer Schweregrad signifikant höher war als der der anderen Gruppen außer dem der mit dem Stamm N3-04 inokulierten Gruppe. Alle ILTV-Stämme führten im Vergleich zu der mit PBS inokulierten Gruppe zu einer signifikanten Reduktion der Gewichtszunahmen. Der Stamm Q1-96 verursachte einen mittleren Gewichtsverlust von 14 %, der signifikant über denen der anderen ILTV-Stämme lag. Die Stämme S2-04 und Q1-96 induzierten nur geringgradige mikroskopische Trachealläsionen, während alle anderen ILTV-Stämme einschließlich der Vakzinestämme A20 und SA2 mittel- bis hochgradige mikroskopisch sichtbare Läsionen in der Trachea hervorriefen. Der mittlere Schweregrad dieser Läsionen korrelierte gut mit der Anzahl der in der Trachea nachgewiesenen Virusgenome.
Diese Ergebnisse zeigen, dass bei den ILTV-Stämmen erhebliche Unterschiede hinsichtlich ihres Tropismus für Trachea oder Konjunktiva bestehen. Außerdem wurde offensichtlich, dass ILTV-Stämme mit Affinität für die Konjunktiva erhebliche Auswirkungen auf die Gewichtszunahmen haben können. Es wurde festgestellt, dass die Anzahl der ILTV-Partikel sowie die mikroskopischen Läsionen keine zuverlässigen Virulenzindikatoren sind, da sie nicht unbedingt mit der Mortalitätsrate bei einer ILT korrelieren.
Estudios anteriores realizados en nuestro laboratorio utilizando una combinación de la reacción en cadena de la polimerasa y polimorfismo de la longitud de de los fragmentos de restricción (PCR-RFLP) identificaron al menos 5 genotipos distintos del virus de laringotraqueítis infecciosa (ILTV). Sin embargo, no se estudió la virulencia de estos tipos de ILTV. En este trabajo, 5 grupos de pollos SPF de 3 semanas de vida (16 en cada grupo) se inocularon vía intratraqueal con 103 EID50 de ILTV de 5 cepas distintas. Otros tres grupos de pollos se inocularon de manera parecida con las cepas vacunales SA2 y A20 o con solución salina fosfatada tamponada estéril para comparar. Cuatro días post inoculación, se valoraron los signos clínicos mediante puntuación, y a continuación ocho pollos de cada grupo se sacrificaron, se pesaron, y se les realizaron exámenes patológicos e histopatológicos. En las aves restantes se valoraron los signos clínicos y la mortalidad hasta 21 días post inoculación. Todos los grupos inoculados con cepas de ILTV mostraron signos clínicos moderados a graves a los 4 días post inoculación. La cepa Q1-96 sólo causó síntomas respiratorios leves con una valoración media que no se diferenció significativamente de la de los grupos inoculados con PBS, pero sí de la de los grupos inoculados con otras cepas de ILTV. La cepa Q1-96 causó fotofobia grave y conjuntivitis con una valoración media significativamente mayor que la del resto de grupos a excepción del grupo inoculado con la cepa N3-04. Todas las cepas de ILTV causaron una disminución significativa en la ganancia de peso en comparación al grupo inoculado con PBS. La cepa Q1-96 produjo una pérdida de peso media del 14% que fue significativamente mayor que la del resto de cepas de ILTV. Las cepas S2-04 y Q1-96 sólo indujeron lesiones microscópicas leves en la tráquea mientras que el resto de cepas de ILTV, incluyendo las cepas vacunales A20 y SA2, produjeron lesiones moderadas a graves en tráquea. Se observó una buena correlación entre las valoraciones medias de las lesiones traqueales y el número de copias de genoma vírico detectado en la tráquea. Los resultados revelaron que hay una variación considerable del tropismo hacia tráquea o conjuntiva entre las cepas de ILTV. Además se mostró que las cepas de ILTV con elevada afinidad por la conjuntiva pueden afectar gravemente la ganancia de peso. El número de ILTV y las lesiones microscópicas traqueales no fueron indicadores fiables de la virulencia dado que no se correlacionaron necesariamente con la tasa de mortalidad.
Introduction
Infectious laryngotracheitis (ILT) is a significant respiratory disease of chickens in many countries and can cause reduced egg production and predisposition to other respiratory pathogens (Guy & Bagust, Citation2003). Outbreaks of mild to moderate forms of ILT are not uncommon in commercial layer flocks worldwide, while sporadic outbreaks of ILT in broiler flocks have also been recognized in recent years as an emerging problem in several countries including Australia (Critchley, Citation2004; Wells, Citation2004). Two live attenuated vaccines, SA2 and A20 (Fort Dodge, Australia, Pty. Ltd.), are used to immunize chickens against ILT in Australia. While the A20 vaccine is considered relatively safe and can be used in very young chickens, the SA2 vaccine is less attenuated and is only recommended for use in adult birds or as a booster vaccine after A20 administration.
Although antigenically homogenous, infectious laryngotracheitis virus (ILTV) strains may vary in their virulence in chickens, chicken embryos and cell culture. Differentiation of ILTV strains of varying virulence, particularly wild-type and modified live vaccine viruses is an important practical problem. In a recent study, polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) of a number of ILTV genes/genomic regions was utilized to examine a number of historical and contemporary Australian ILTV field and vaccine strains (Kirkpatrick et al., Citation2006). The ILTV strains were classified into five different classes, with most strains distinguishable from the vaccine strains. The main purpose of the present study was to determine and compare the tissue tropism and virulence of the ILTV strains belonging to different PCR-RFLP classes by assessment of clinical signs, mortality, pathological lesions and effect on weight gain.
Materials and Methods
Viruses
The origin of the ILTV strains used in this study has been previously described (Kirkpatrick et al., Citation2006). The commercial ILTV vaccine strains SA2 and A20 (Fort Dodge) and several recently isolated ILTV strains were used in this study (). The vaccine strain SA2 was derived from an Australian field isolate by attenuation through sequential passages in chicken embryo (Purcell & Surman, Citation1974), while strain A20 originated from SA2 through further passages in chicken embryonic cell culture to lessen residual virulence (T. J. Bagust, personal communication, 2005). All field strains were isolated from the upper respiratory tract of infected birds during outbreaks of ILT in Australia. Field strains V1-99 and Q1-96 were isolated by the Australian Government Diagnostic Laboratories (Department of Primary Industries, Victoria/Queensland). The field strain CSW was isolated from the upper respiratory tract of infected birds (Fahey et al., Citation1983). The field strains N3-04 and S2-04 were isolated within our laboratory during the course of a previous study. The field strain S2-04 was isolated from an aged (over 70 weeks of age) commercial layer flock in South Australia and associated with minimal mortality, moderate signs of conjunctivitis and/or tracheal rales, and lesions of mucoid to diphtheritic laryngotracheitis and stomatitis. This flock had been moulted and was in a second period of lay. Vaccination history of this flock included an A20 and a SA2 vaccination before the first lay period but none before the second lay period. The field strain N3-04 was from a 53-day-old non-vaccinated commercial broiler flock in New South Wales (Australia) with increasing daily mortality (up to 1%). This isolate was associated with signs of conjunctivitis, coughing, and lesions of mucoid, haemorrhagic or diphtheritic tracheitis. At the time of depopulation, the average body weight of this flock was approximately 300 g less than standard presumably due to reduced feed consumption.
Table 1. Details of the ILTV strains used in this study and comparisons of RFLP patterns generated by restriction digestion of PCR products of selected genes
Strains and isolates were chosen based on their different PCR-RFLP patterns of several ILTV genes, including gE, gG, TK, ICP4 and ORFB-TK as described previously (Kirkpatrick et al., Citation2006).
Titration of virus strains
All virus strains were propagated on the chorioallantoic membrane of 10-day-old chicken embryos using standard techniques (Tripathy & Hanson, Citation1989). Virus was purified by picking single plaques from infected chorioallantoic membranes. The virus strains were also titrated on the chorioallantoic membranes of embryonated chicken eggs. Viral stocks at 104 median embryo infected dose/ml were prepared for each virus strain and stored at − 70°C.
Experimental design
One hundred and twenty-eight 3-week-old specific pathogen free hybrid White Leghorn chickens (Charles River Laboratories Pty, Ltd., Woodend, Victoria, Australia) were randomly separated into eight groups of 16, housed in separate isolators and provided irradiated feed and water ad libitum. Birds within each group were weighed and inoculated within isolators with 103 median embryo infected dose of the relevant ILTV strains in a 300 µl volume per bird by the intra-tracheal route. Dilutions were made in sterile phosphate-buffered saline (PBS). The negative control birds received 300 µl sterile PBS only. Four days post-inoculation, eight randomly selected birds from each group were examined for clinical signs, weighed, euthanized by halothane and then subjected to pathological, histopathological and virological examinations. The remaining eight birds within each group were kept within isolators, monitored for clinical signs and mortality pattern, and euthanized at 21 days post-inoculation.
Scoring of clinical signs
Four days post inoculation, clinical signs were recorded with a digital video camera (recording 30 sec for each bird) and subsequently scored. Clinical signs were scored on the basis of the bird's breathing, the condition of their conjunctiva and their general demeanour. Breathing was scored on a scale of 0 (normal breathing), 1 (mild dyspnoea), 2 (mild dyspnoea and open mouth breathing), 3 (gasping) and 4 (gasping with an extended neck). The condition of the conjunctiva was scored on a scale of 0 (normal), 1 (swollen and/or partial closure of the eyes) and 2 (complete closure of the eyes). The demeanour was scored on a scale of 0 (normal), 1 (depressed) and 2 (severely depressed). An overall clinical sign score was then calculated for each bird by summing together the scores for each clinical sign.
Histopathology
Transverse segments of upper and lower trachea were fixed in Bouin's solution overnight, embedded in paraffin and stained with haematoxylin and eosin for histopathological examinations. Microscopic lesions were scored according to a previously described method (Guy et al., Citation1990).
Real-time PCR
A transverse section of proximal trachea was collected during the postmortem examination and transferred to a microcentrifuge tube containing 500 µl of 4 M guanidinium isothiocyanate, 15 mM PIPES (pH 7.6) and 5 µl β-mercaptoethanol. Viral DNA from these sections was extracted using Qiaex II suspension (Qiagen) for detection and quantification of ILTV genome by a quantitative PCR. The quantitative PCR was performed using the Mx4000 thermocycler (Stratagene Pty Ltd) and Platinum SYBR Green qPCR SuperMix (Invitrogen). The forward (TTGCTGTCGTATTTCGCGTG) and reverse (GTAAATCGTTAGTGCGGCAT) primers used amplified a 115-base-pair region from the ILTV UL15 gene. The template was 2 µl extracted DNA (or sterile water as control) in each set of reaction, or 5 µl volumes of 10-fold dilutions of 4.0×108 copy number of the ILTV UL15 sequence in pGEM-T were included to form a standard curve. The amplification conditions were 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min at 60°C. This assay provided a threshold of detection of 2.98 ILTV genome equivalents per section of trachea.
Statistical analysis
The Mann–Whitney U test was used to compare the median figures for clinical signs, histological tracheal scores and viral copy numbers. The Student t test was used for comparison of the mean percentage weight changes. The Fisher's exact test was used to compare the incidence of mortalities. P < 0.05 was considered significant.
Results
Percentage weight gain
The mean percentage weight gain at 4 days post-inoculation of each of the groups is presented in . All groups except for the Q1-96 group had positive percentage weight gains and all, except for the N3-04 group, had a significantly different (P≤0.002) average weight gain to that of the negative control group. The mean percentage weight gain of the birds inoculated with the strain Q1-96 was negative and significantly different (P≤0.001) from those of all other groups.
Table 2. Percentage weight gain of 3-week-old chickens at 4 days post-inoculation with different strains of ILTV
Clinical signs
Median and range of scores for clinical sign for each group are presented in . The group inoculated with strain Q1-96 showed only very minimal clinical signs related to breathing with a median score that was significantly different (P≤0.005) from those of groups inoculated with other ILTV strains, but not from that of the negative control group. Most birds of the Q1-96 group showed clinical signs of conjunctivitis and/or closure of the eyes. The median of conjunctiva-related clinical signs of the Q1-96 group was 1.0, which was significantly higher (P≤0.046) than all other groups, except for that of group N3-04. The group inoculated with Q1-96 also showed moderate to severe signs of depression and had a significantly different (P=0.022) median clinical sign score for demeanour to that of the negative control group. The median scores for demeanour of the groups SA2, A20, CSW, V1-99, N3-04 and S2-04 were not significantly different from that of the negative control group. The sum of scores for breathing, condition of conjunctiva and demeanour of all the groups inoculated with an ILTV strain were not significantly different from each other but were all significantly different (P≤0.001) from that of the negative control group.
Table 3. Scores for the median (range) clinical signs of 3-week-old chickens at 4 days post-inoculation with different strains of ILTV
Microscopic tracheal lesions
The histological lesion scores for the upper and lower trachea for each group are summarized in . All ILTV strains induced moderate to severe microscopic lesions in the upper trachea, with medians of scores that were significantly different (P≤0.005) from that of the negative control group. Except for A20, all ILTV strains induced no or only minor microscopic lesions in the lower trachea and had medians of scores that were not significantly different from that of the negative control group. The median score of lesions in the lower tracheal for the A20-inoculated group was significantly different (P=0.005) from that of the negative control group. The sum of median scores for upper and lower tracheal microscopic lesions of the groups SA2, A20, CSW, V1-99 and N3-04, but not groups SA-04 or Q1-96, were significantly different (P≤0.032) from that of the negative control group.
Table 4. Scores for the median (range) microscopic tracheal lesions for the upper trachea, lower trachea and total trachea of 3-week-old chickens at 3 days post-inoculation with different strains of ILTV
Viral copy numbers
The results of quantitative PCR for extracted DNA from the tracheas of birds sacrificed 4 days post-inoculation are summarized in . ILTV DNA was detected in all the birds in groups CSW, SA2 and A20, in few of the birds in groups N3-04 and S2-04, in most of the birds in groups Q1-96 and V1-99, but in none of the birds in the negative control group. Among ILTV strains, A20 produced the highest median viral copy number, while N3-04 produced the lowest median viral copy number in trachea.
Table 5. Median and range of viral copy numbers (determined by quantitative PCR) in trachea of 3-week-old chickens at 4 days post-inoculation with different strains of ILTV
Mortality
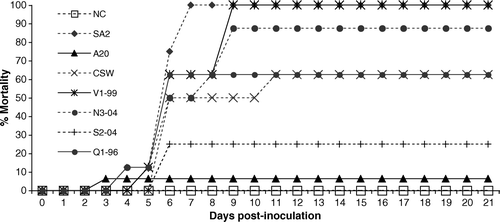
The pattern and rate of mortalities (including birds culled because of severe clinical signs) after inoculation with the different ILTV strains are shown in . Mortalities occurred in all groups inoculated with ILTV. Generally, the highest mortalities occurred between days 6 and 9 post-inoculation. Three groups, SA2, V1-99 and N3-04, produced greater than 80% cumulative mortality per group which was significantly different (P≤0.033) from the control group. Groups S2-04, Q1-96 and CSW produced 25, 50 and 62.5% cumulative mortality per group, respectively. The group inoculated with A20 was the only group to show mortality before the first time point of 4 days post-inoculation, after which no mortality was produced.
Discussion
Results from this study revealed that ILTV strains vary considerably in their capacity to induce mortality, clinical signs and lesions in different tissues. The ILTV field strain CSW and the vaccine strains SA2 and A20 were found to have a high affinity for the trachea but little affinity for conjunctival tissues. In contrast, the ILTV strain Q1-96 was found to have a particularly high affinity for conjunctival tissues but lower affinity for tracheal tissues compared with most other ILTV strains. Interestingly, the affinity of the ILTV strain Q1-96 for conjunctival tissue was found to be associated with a significant reduction in body weight. This is likely to be caused by the severe conjunctivitis and associated inability to locate the feed provided to the birds.
An interesting finding of this study was that the vaccine strains, in particular A20, had a high tendency to cause microscopic lesions in the trachea. Lesions induced by the A20 strain were noticeable even in the lower trachea. It is known that the use of the SA2 vaccine strain, particularly in young chickens, may be associated with clinical signs of respiratory disease (Purcell & Surman, Citation1974) but such an observation has not been reported with the A20 vaccine strain. Despite a high affinity for tracheal tissues, inoculation with the A20 ILTV strain, but not with the SA2 strain, was associated with only a minimal mortality in chickens, suggesting that mortality is not necessarily correlated with tracheal lesions in ILT. This finding is also supported by a previous study by Guy et al. ( Citation1990 ), where some field isolates such as 87-30900 scored relatively moderately (2.8) in terms of tracheal pathology while in contrast the vaccine strain 4 scored relatively high (4.5). Analysis of the results obtained in our study found a high correlation (Pearson's correlation = 0.812) between the scores for microscopic tracheal lesions and viral copy numbers in trachea of the birds. This result suggests that enhanced tracheal pathology is perhaps due to a high rate of viral replication in this tissue.
Unlike vaccine strains A20 and SA2, the field ILTV strain N3-04 did not show a high affinity for tracheal tissue as evidenced by the relatively low genomic copy number and moderate microscopic tracheal lesion score. In addition, only minimal conjunctiva-related clinical signs were seen in the birds infected with this strain. Nevertheless, this strain proved to be highly virulent causing severe mortality in infected birds. These results challenge the paradigm that ILTV is a local disease limited to the respiratory system (Hitchner et al., Citation1977; Bagust et al., Citation1986).
Results presented in this study also emphasize the necessity to use the appropriate vaccine for vaccination of young chickens. The ILTV strain SA2 was found to cause clinical signs, mortality and reduced weight gain comparable with some of the field strains. This vaccine strain obviously still retains residual virulence and should only be used as a second vaccination (i.e. after vaccination with a less virulent strain such as A20) and its application should be restricted only to older birds.
It was found that the ILTV strains V1-99 and N3-04, which were previously shown to belong to the same RFLP class (Kirkpatrick et al., Citation2006), showed a similar level of virulence as determined by criteria used in this study (clinical signs, mortality, tracheal lesions and effects on weight gain). However, this finding was less expandable to some other ILTV strains, such as A20, SA2 and Q1-96, which have previously been shown to belong to the same RFLP class (class 1) and clearly exhibited different tissue tropisms, mortality rates and clinical signs. These results suggest that perhaps genes other than those used in the RFLP system may be involved in virulence of the ILTV strains and/or that RFLP does not fully examine the possible virulence-related region(s) of the genes assessed in our previous study (Kirkpatrick et al., Citation2006). Further studies examining differential expression of ILTV genes may be necessary to reveal the genetic mechanism of virulence/attenuation of an ILTV strain.
Acknowledgments
Funding for this project was provided by the Australian Egg Corporation Limited. The authors would like to thank Prof. Glen Browning and Dr Peter Scott for scientific input. The authors would also like to thank Denise O'Rourke, Peter Cowling, Brett Green and Tony Belfiore for technical support, the Queensland and Victorian Department of Primary Industries for the supply of some of the ILTV, strains and Dr Kim Critchley and Dr Ben Wells for submission of clinical cases that were suspected to involve ILT viruses.
References
- Bagust , T.J. , Calnek , B.W. and Fahey , K.J. 1986 . Gallid-1 herpesvirus infection in the chicken. 3. Reinvestigation of the pathogenesis of infectious laryngotracheitis in acute and early post-acute respiratory disease . Avian Diseases , 30 : 179 – 190 .
- Critchley , K. ( 2004 ). South Australian outbreaks of ILTV . In Proceedings of the Australian Veterinary Poultry Association Scientific Meeting . P. 7 Melbourne , Australia .
- Fahey , K.J. , Bagust , T.J. and York , J.J. 1983 . Laryngotracheitis herpesevirus infection in chicken: the role of humoral antibody in immunity to a graded challenge infection . Avian Pathology , 12 : 505 – 514 .
- Guy , J.S. and Bagust , T.J. 2003 . “ Laryngotracheitis ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. Swayne , D.E. 121 – 134 . Ames , IA : Iowa State Press .
- Guy , J.S. , Barnes , H.J. and Morgan , L.M. 1990 . Virulence of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates . Avian Diseases , 34 : 106 – 113 .
- Hitchner , S.B. , Fabricant , J. and Bagust , T.J. 1977 . A fluorescent-antibody study of the pathogenesis of infectious laryngotracheitis . Avian Diseases , 21 : 185 – 194 .
- Kirkpatrick , N.C. , Mahmoudian , A. , O'Rourke , D. and Noormohammadia , A.H. 2006 . Differentiation of infectious laryngotracheitis virus isolates by restriction fragment length polymorphic analysis of polymerase chain reaction products amplified from multiple genes . Avian Diseases , 50 : 28 – 34 .
- Purcell , D.A. and Surman , P.G. 1974 . Letter: aerosol administration of the SA-2 vaccine strain of infectious laryngotracheitis virus . Australian Veterinary Journal , 50 : 419 – 420 .
- Tripathy , D.N. and Hanson , L.E. 1989 . “ Laryngotracheitis ” . In A Laboratory Manual for Isolation and Identification of Avian Pathogens , 3rd edn , Edited by: Purchase , H.G. , Arp , L.H. , Domermuth , C.H. and Pearson , J.E. 85 – 88 . Kennett Square , PA : American Association of Avian Pathologists .
- Wells , B. ( 2004 ). New South Wales outbreaks of ILTV . In Proceedings of the Australian Veterinary Poultry Association Scientific Meeting , p. 52 Melbourne , Australia .