Abstract
Although the effects of chicken anaemia virus (CAV) infection have frequently been investigated in young chickens, there have been few studies of the pathogenesis of CAV infection in older birds. The aim of the work reported here was to study viral loads in 6-week-old chickens and to compare these with those seen in younger birds. Specific pathogen free chickens were inoculated at 1 day or at 6 weeks of age with 104 median tissue culture infective doses of CAV by the intraocular route. Chicks infected when 1 day old were euthanized at day 14, 18 or 22 post inoculation (p.i.), and those infected when 6 weeks old at day 16, 18 or 20 p.i. Their body and thymus weights were determined and samples were collected from their spleen, liver and thymus. A quantitative polymerase chain reaction assay was developed and used to determine the number of viral genome copies in the tissue samples. In both age groups, viral genome concentrations increased in all organs up to day 18 p.i. and reached a peak in the spleen and liver at day 18 p.i. The peak viral concentrations in the thymus were detected at day 18 in the younger birds and at day 20 p.i. in older chickens. These studies have shown that exposure to CAV in older birds leads to similar levels of active viral replication to those seen in younger birds, and may result in subclinical infections in older birds with the potential to increase susceptibility to other infectious agents.
Bien que les effets de l'infection par le virus de l'anémie du poulet (CAV) ont souvent été investigués chez des jeunes poulets, il y a peu d'études de pathogénie de l'infection par le CAV chez des poulets plus âgés. Le but de ce travail a été d'étudier les charges virales chez des poulets âgés de six semaines et de les comparer à celles d'animaux plus jeunes. Des poulets, exempts de microorganismes pathogènes spécifiés, ont été inoculés aux âges d'un jour ou de six semaines avec 104 DICC50 de CAV administré par instillation oculaire. Les poussins qui ont été infectés à un jour, ont été euthanasiés à 14, 18 ou 22 jours après l'inoculation (p.i.) et ceux infectés à l'âge de six semaines l'ont été à 16, 18 or 20 jours p.i.. Les poids du corps et du thymus ont été déterminés et des échantillons de rate, de foie et de thymus ont été prélevés. Une réaction de polymérisation en chaîne quantitative a été développée et utilisée pour déterminer le nombre de copies de génome viral dans les échantillons de tissu. Dans les deux groupes d'âges différents les concentrations de génome viral a augmenté dans tous les organes jusqu'à 18 jours p.i. et a atteint un pic dans la rate et le foie le jour 18 p.i. Le pic des concentrations virales dans le thymus a été détecté à 18 jours p.i. chez les animaux les plus jeunes et à 20 jours p.i. chez les poulets les plus âgés. Ces études ont montré que l'exposition au CAV des oiseaux les plus âgés a induit des niveaux similaires de réplication virale à ceux observés chez les animaux les plus jeunes et peut entraîner des infections subcliniques chez les animaux les plus âgés avec la possibilité d'augmenter la sensibilité aux autres agents infectieux.
Obwohl die Auswirkungen einer Infektion mit dem Hühneranämievirus (CAV) auf junge Hühnerküken bereits öfter untersucht worden sind, gibt es nur wenige Studien zur Pathogenese der CAV-Infektion in älteren Hühnern. Ziel dieses Versuchs war es, die virale Belastung in sechs Wochen alten Hühnern zu ermitteln und mit der zu vergleichen, die in jüngeren Tieren beobachtet worden war. Spezifisch pathogen freie Hühner wurden am ersten Lebenstag oder im Alter von sechs Wochen intraokular mit 104 Gewebekultur infektiösen Dosen50 CAV inokuliert. Die am ersten Lebenstag infizierten Küken wurden 14, 18 oder 22 Tage post inoculationem (p.i.) und die mit sechs Wochen infizierten Tiere wurden 16, 18 oder 20 Tage p.i. euthanasiert. Ihre Körper- und Thymusgewichte wurden ermittelt und aus Milz, Leber und Thymus wurden Proben entnommen. Ein quantitativer Polymerasekettenreaktionstest wurde entwickelt und zur Bestimmung der Anzahl der viralen Genomkopien in den Gewebeproben eingesetzt. In beiden Altersgruppen stiegen die viralen Genomkonzentrationen in allen Organen bis zum 18. Tag p.i. an und erreichten in Milz und Leber an diesem Tag ihren Höchststand. Im Thymus wurden die größten Virusgehalte bei den jüngeren Tieren 18 Tage p.i. und bei den älteren Tieren am 20. Tag p.i. festgestellt. Dieser Versuch hat gezeigt, dass die Infektion älterer Hühner mit CAV zu ähnlich aktiven Virusreplikationsraten führt wie bei den jungen Hühnerküken. Dies kann bei den älteren Tieren zu subklinischen Infektionen mit der Gefahr einer erhöhten Empfänglichkeit für andere Infektionserreger führen.
Pese a que los efectos de la infección con el virus de la anemia infecciosa (CAV) han sido estudiados frecuentemente en pollos jóvenes, existen pocos estudios de la patogénesis de la infección en pollos mayores. El objetivo del trabajo que se describe aquí fue estudiar las cargas víricas en pollos de seis semanas de vida y compararlas con las observadas en pollos más jóvenes. Se inocularon pollos libres de patógenos específicos a un día o seis semanas de vida con 104 dosis infectivas medias en cultivo de tejido de CAV vía intraocular. Los pollos infectados al día de vida se sacrificaron a los 14, 18 o 22 días post inoculación (p.i.) y aquellos infectados a las seis semanas de vida a los 16, 18 o 20 días p.i.. Se determinaron los pesos corporales y del timo y se obtuvieron muestras de bazo, hígado y timo. Se desarrolló una reacción en cadena de la polimerasa cuantitativa y se usó para determinar el número de copias de genoma vírico en las muestras de tejido. En los dos grupos de edad, las concentraciones de genoma vírico aumentaron en todos los órganos hasta los 18 días p.i. y alcanzaron un máximo en bazo e hígado a los 18 días p.i. Los picos de concentración vírica en timo se detectaron a los 18 días p.i. en los pollos más jóvenes y al día 20 p.i. en pollos mayores. Estos estudios muestran que la exposición a CAV en pollos más viejos comporta niveles de replicación vírica activa similares a los observados en pollos más jóvenes, y podría resultar en infecciones subclínicas en pollos más viejos con el posible incremento de la susceptibilidad a otros agentes infecciosos.
Introduction
Chicken anaemia virus (CAV) is the causative agent of a disease in young chickens characterized by aplastic anaemia and generalized lymphoid atrophy. The genome of CAV consists of a small, single-stranded DNA that has three functional open reading frames encoding the viral proteins VP1, VP2 and VP3 (Schat, Citation2003).
Resistance to CAV-induced disease has been regarded as developing rapidly during the first week of life, becoming complete by the time birds reach 2 to 3 weeks of age (Adair, Citation2000; Schat, Citation2003). This resistance appears to be dependent on virus pathogenicity, dose, route of exposure and coinfection with other immunosuppressive agents (Rosenberger & Cloud, Citation1989; Imai et al., Citation1999). However, despite the age resistance to disease, birds remain susceptible to infection. The subclinical outcomes of CAV infection in broilers have been studied by some workers (McNulty et al., Citation1991; Goodwin et al., Citation1993; Jorgensen et al., Citation1995; Sommer & Cardona, Citation2003), but few studies have examined CAV infection in older birds. McConnell et al. (Citation1993) reported a decrease in lymphocyte and macrophage function in 3-week-old chickens inoculated orally with CAV, and Dren et al. (Citation1996) studied viraemia and virus shedding in 6-week-old birds infected with CAV by the intramuscular route and compared them with their neutralizing antibody levels. It has been shown that viral DNA may persist in reproductive and splenic tissue in mature commercial and specific pathogen free chickens, in spite of the presence of antibodies against CAV (Cardona et al., Citation2000a,Citationb; Miller et al., Citation2003; Brentano et al., Citation2005). However, the concentration of the virus in different organs has not been quantified in older birds, and there has been no definitive evidence that it is replicating. The aim of the study reported here was to examine the viral titres in the thymus, spleen and liver in 6-week-old chickens infected with CAV by the intraocular route. Viral titres were also determined in the same organs in birds infected at 1 day of age.
Materials and methods
Viral strain and birds
The Australian strain of CAV, CAU269/7 (Brown et al., Citation2000), originating from an Australian commercial breeder flock, was used for this study. CAU269/7 was provided by D. O'Rourke and T. J. Bagust (CSIRO Animal Health Laboratory, Parkville, Victoria, Australia). Specific pathogen free birds were obtained from Charles River Laboratories (Woodend, Victoria, Australia).
Experimental design
One-day-old chicks were randomly allocated into two groups of 18 birds, which were housed in separate isolators. Birds in the treatment group were inoculated intraocularly with 104 median tissue culture infective doses of CAV on day 1. The intraocular route was chosen because it enables rapid and reliable delivery of a defined dose to the gastrointestinal tract, while the dose used was one that previous work in our laboratory had shown to be capable of inducing subclinical disease in 1-day-old and 21-day-old chicks when delivered subcutaneously (Tivendale et al., unpublished data). Five, six and seven birds from each group were euthanized by exposure to halothane vapour at days 14, 18 and 22 post inoculation (p.i.), respectively. The weight of each bird was recorded, thymic lobes were dissected from each side of the neck and the weight of the thymus measured for each bird. Samples of the spleen, liver and thymus were collected from all birds and stored at −70°C, as previous studies have shown them to be major target organs for CAV.
Six-week-old chickens were randomly allocated into two groups of 22 birds and housed in separate isolators. Birds in the treatment group were inoculated intraocularly with 104 median tissue culture infective doses of CAV. On days 16, 18 and 20 p.i., seven, seven and eight birds, respectively, from each group were euthanized. The body and thymus weights for each bird were measured and samples from the spleen, liver and thymus were collected and stored at −70°C.
Sample preparation and DNA extraction
A small piece of each tissue was weighed and then homogenized by passage through a three-way tap in a 0.5 ml volume of phosphate-buffered saline. DNA from a 50 µl volume of each homogenized tissue sample was extracted using a Qiaex® II matrix (Qiagen) as described previously (Sykes et al., Citation2001).
Preparation of a standard curve
CAV ORF 2 (encoding VP2) was amplified from the Australian isolate, CAU269/7 (GenBank accession number AF227982), by polymerase chain reaction (PCR) using primers VP2-F (5′-CGGTCCGGATCCATGCACGGAAACGGCGGACAAC-3′) and VP2-R (5′-GGTTTGGAATTCTCACACTATACGTACCGGGGC-3′). A DNA band of the expected size for the PCR product (677 base pairs) was excised from a 1% agarose gel and ligated into the pGEX-4T-1 vector (Amersham Pharmacia Biotech, Little Chalfont, Bucks, UK), and this was used to transform Escherichia coli.
The resultant plasmid pGEX-4T1-VP2 was purified from transformants using a Plasmid Midi Kit (Qiagen, Hilden, Germany) and linearized by digestion with EcoRI. The concentration of the DNA was calculated by measuring the absorbance at 260 nm (A 260) and calculating the concentration of the plasmid using the following formula: number of molecules of DNA/ml = A 260 x 4.56 x 1016/N, where N is the number of base pairs in the molecule of DNA. Serial 10-fold dilutions of the purified plasmid solution containing between 51 and 510 000 copies of the plasmid were used in duplicate to produce a standard curve for quantitative PCR (qPCR).
Quantitative PCR assay
Primers QF1 (5′-GAATGTGCCGGACTTGAGGA-3′) and QR1 (5′-GGGTCGCAGGATCGCTT-3′), each at a final concentration of 0.3 µM, were used to amplify a 65-base-pair fragment of CAV ORF 2. After optimization of the qPCR conditions, 15 µl Platinum SYBR Green qPCR supermix UDG (Invitrogen, Carlsbad, California, USA) and 5 µl extracted DNA (diluted 1 in 100) or dilutions of the plasmid standard were assayed in duplicate.
Amplification conditions used an initial cycle of 2 min at 50°C and 2 min at 95°C, and then 35 cycles of 30 sec at 95°C and 30 sec at 60°C.
Optimal dilution of extracted DNA from tissue samples
As tissue samples may contain substances with an inhibitory effect on PCR (Tichopad et al., Citation2004), it was attempted to find the optimal dilution of extracted DNA from tissue samples that does not have any inhibitory effect. Serial 10-fold dilutions from 1 to 10–4 extracted DNA from each tissue from two randomly chosen birds in the infected group were prepared in duplicate and subjected to qPCR. Analysis of the amplification plots showed that the differences between the threshold cycle (Ct) values for some samples did not correlate with the dilution factor. The minimum reliable dilution was 1:100, and this dilution of the extracted DNA was used for all samples in the study.
Statistical analysis
Mean body weights, thymus to body weight ratios and CAV genome concentrations in infected and uninfected birds in both 1-day-old and 6-week-old groups were compared using Student's t tests. Variances in CAV genome concentrations at different time points in each trial were compared using F tests. P values less than 0.05 were considered significant.
Results
Body weights and thymus to body weight ratios
The mean thymus to body weight ratios of the infected birds were significantly lower than uninfected birds at day 18 p.i. in the chicks infected when 1 day old. However, there were no significant differences at 14 or 22 days p.i. ().
Table 1. Mean body weights and thymus to body weight ratios at different time points after inoculation of 1-day-old chicks
In birds inoculated when 6 weeks old there was a significant decrease in the mean body weight of infected birds in comparison with uninfected birds at day 20 p.i., but there were no significant differences between the groups in mean thymus to body weight ratios at different time points ().
Table 2. Mean body weights and thymus to body weight ratios at different time points after inoculation of 6-week-old chickens
Quantitative PCR
The standard curve generated from amplication plots of 10-fold dilutions of pGEX-4T1-vp2 demonstrated a strong linear correlation between Ct values and the number of copies of the plasmid. The square of the coefficient of regression (R 2) and efficiency of amplification were within the ranges of 0.993 to 0.999 and 95.3% to 101%, respectively. The reproducibility of the standard curve in nine different qPCR runs is shown in .
Figure 1. Reproducibility of the pGEX-4T1-VP2 standard curve. The mean and standard deviation of the cycle threshold at each dilution of the plasmid for nine qPCR runs are shown.
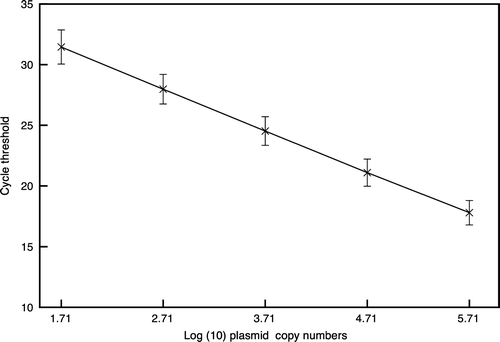
CAV genome (>50 copies/g) was detected in the liver, spleen and thymus samples from all the infected birds, but not from any of the uninfected birds.
In the chicks infected when 1 day old, the highest concentrations of CAV genome were detected in the thymus, followed by the spleen, and then the liver. Peak CAV genome concentrations were detected at day 18 p.i. in all three tissues. There were significant differences in the mean CAV genome concentrations in the thymus at each of the time points (). The variance in CAV genome concentration at day 18 in the thymus was significantly lower and at day 14 in the spleen the variance was significantly higher than at the other time points.
Table 3. Mean CAV genome concentration as detected by qPCR in different tissues at day 14, 18 and 22 after inoculation of 1-day-old chicks
In the chickens infected when 6 weeks old, the highest concentrations of CAV genome was detected in the thymus, followed by the spleen, and then the liver. Although the highest mean concentration was seen at day 20 in the thymus, this was not significantly different from that seen at the other time points. There was a significant increase in the viral genome concentration in the liver between days 16 and 18, but not between days 18 and 20 (). The variance in the CAV genome concentration in the liver was significantly lower at day 16 than at other time points.
Table 4. Mean CAV genome concentration as detected by qPCR in different tissues at day 16, 18 and 20 after inoculation of 6-week-old chickens
Discussion
Our observation of a significant reduction of thymus to body weight ratio at day 18 p.i. in birds infected when 1 day old confirms findings from earlier studies on 1-day-old chicks (Goryo et al., Citation1989). In these studies the thymus to body weight ratio was lowest between 12 and 20 days p.i. Tan & Tannock (Citation2005) also reported a significant difference between uninfected and infected birds at days 15 to 20 and 25 p.i. in the thymus to body weight ratio. However, the mean body weight in our study was not in agreement with those from other studies, as there was no significance difference between infected and uninfected birds in 1-day-old chicks. This discrepancy could be explained by the fact that, in the other studies (Goryo et al., Citation1989; Tan & Tannock, Citation2005), birds were infected intramuscularly or with higher doses of virus than those used for our study.
A significant difference in weight was detected at day 20 p.i. in 6-week-old birds, supporting studies of the economic significance of subclinical CAV infection, which have found an effect on weight gain (McNulty et al., Citation1991; McIlroy et al., Citation1992).
In our study, a SYBR Green qPCR assay was developed and used to quantify CAV genome, and it was found to be sensitive, rapid and cost-effective.
In both age groups the highest viral genome concentrations were found in the thymus, in accordance with previous studies in 1-day-old chicks (van Santen et al., Citation2004; Tan & Tannock, Citation2005).
The increases in viral genome concentrations in all organs between days 14 and 18 p.i. in young birds and between days 16 and 18 p.i. in older birds suggest that virus was actively replicating in these organs.
The peak concentrations of CAV in the spleen and liver in both age groups were seen at day 18 p.i. The peak concentration in the thymus coincided with a significant reduction in the mean thymus to body weight ratio at day 18 p.i. in young chickens and in weight gain at day 20 p.i. in older chickens. Clinical disease was not observed in either of the groups of birds in this study, possibly because of the low dose of virus, but there was evidence that CAV took longer to achieve peak titres and to cause subclinical disease in the older birds. This may be because neutralizing antibody responses to CAV develop faster in 6-week-old to 9-week-old birds than in 1-week-old to 3-week-old chicks (Yuasa et al., Citation1983; Dren et al., Citation2000) and repopulation of the thymus with lymphocytes coincides with the commencement of antibody formation (Schat, Citation2003).
In conclusion, exposure of older birds to relatively low doses of CAV not only led to active viral replication in the spleen, liver and thymus at similar levels to those seen in young birds, but also caused subclinical disease resulting in a reduction in weight gain. Further study may be needed to fully elucidate the immunological and histopathological impact of subclinical infection in older birds and its economic significance.
References
- Adair , B.M. 2000 . Immunopathogenesis of chicken anemia virus infection . Developmental and Comparative Immunology , 24 : 247 – 255 .
- Brentano , L. , Lazzarin , S. , Bassi , S.S. , Klein , T.A. and Schat , K.A. 2005 . Detection of chicken anemia virus in the gonads and in the progeny of broiler breeder hens with high neutralizing antibody titers . Veterinary Microbiology , 105 : 65 – 72 .
- Brown , H.K. , Browning , G.F. , Scott , P.C. and Crabb , B.S. 2000 . Full-length infectious clone of a pathogenic Australian isolate of chicken anaemia virus . Australian Veterinary Journal , 78 : 637 – 640 .
- Cardona , C. , Lucio , B. , O'Connell , P. , Jagne , J. and Schat , K.A. 2000a . Humoral immune responses to chicken infectious anemia virus in three strains of chickens in a closed flock . Avian Diseases , 44 : 661 – 667 .
- Cardona , C.J. , Oswald , W.B. and Schat , K.A. 2000b . Distribution of chicken anaemia virus in the reproductive tissues of specific-pathogen-free chickens . Journal of General Virology , 81 : 2067 – 2075 .
- Dren , C. , Farkas , T. and Nemeth , I. 1996 . Serological survey on the prevalence of chicken anaemia virus infection in Hungarian chicken flocks . Veterinary Microbiology , 50 : 7 – 16 .
- Dren , C.N. , Kant , A. , Van Roozelaar , D.J. , Hartog , L. , Noteborn , M.H. and Koch , G. 2000 . Studies on the pathogenesis of chicken infectious anaemia virus infection in six-week-old SPF chickens . Acta Veterinaria Hungarica , 48 : 455 – 467 .
- Goodwin , M.A. , Smeltzer , M.A. , Brown , J. , Girshick , T. , McMurray , B.L. and McCarter , S. 1993 . Effect of so-called chicken anemia agent maternal antibody on chick serologic conversion to viruses in the field . Avian Diseases , 37 : 542 – 545 .
- Goryo , M. , Hayashi , S. , Yoshizawa , K. , Umemura , T. , Itakura , C. and Yamashiro , S. 1989 . Ultrastructure of the thymus in chicks inoculated with chicken anaemia agent (MSB1-TK5803 strain) . Avian Pathology , 18 : 605 – 617 .
- Imai , K. , Mase , M. , Tsukamoto , K. , Hihara , H. and Yuasa , N. 1999 . Persistent infection with chicken anaemia virus and some effects of highly virulent infectious bursal disease virus infection on its persistency . Research in Veterinary Science , 67 : 233 – 238 .
- Jorgensen , P.H. , Otte , L. , Nielsen , O.L. and Bisgaard , M. 1995 . Influence of subclinical virus infections and other factors on broiler flock performance . British Poultry Science , 36 : 455 – 463 .
- McConnell , C.D. , Adair , B.M. and McNulty , M.S. 1993 . Effects of chicken anemia virus on cell-mediated immune function in chickens exposed to the virus by a natural route . Avian Diseases , 37 : 366 – 374 .
- McIlroy , S.G. , McNulty , M.S. , Bruce , D.W. , Smyth , J.A. , Goodall , E.A. and Alcorn , M.J. 1992 . Economic effects of clinical chicken anemia agent infection on profitable broiler production . Avian Diseases , 36 : 566 – 574 .
- McNulty , M.S. , McIlroy , S.G. , Bruce , D.W. and Todd , D. 1991 . Economic effects of subclinical chicken anemia agent infection in broiler chickens . Avian Diseases , 35 : 263 – 268 .
- Miller , M.M. , Ealey , K.A. , Oswald , W.B. and Schat , K.A. 2003 . Detection of chicken anemia virus DNA in embryonal tissues and eggshell membranes . Avian Diseases , 47 : 662 – 671 .
- Rosenberger , J.K. and Cloud , S.S. 1989 . The effects of age, route of exposure, and coinfection with infectious bursal disease virus on the pathogenicity and transmissibility of chicken anemia agent (CAA) . Avian Diseases , 33 : 753 – 759 .
- Schat , K.A. 2003 . “ Chicken infectious anemia ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Fadly , A.M. , Glisson , J.R. , Mcdougald , L.R. and Swayne , D.E. 182 – 202 . Ames , IA : Iowa State Press .
- Sommer , F. and Cardona , C. 2003 . Chicken anemia virus in broilers: dynamics of the infection in two commercial broiler flocks . Avian Diseases , 47 : 1466 – 1473 .
- Sykes , J.E. , Allen , J.L. , Studdert , V.P. and Browning , G.F. 2001 . Detection of feline calcivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR . Veterinary Microbiology , 81 : 95 – 108 .
- Tan , J. and Tannock , G.A. 2005 . Role of viral load in the pathogenesis of chicken anemia virus . Journal of General Virology , 86 : 1327 – 1333 .
- Tichopad , A. , Didier , A. and Pfaffl , M.W. 2004 . Inhibition of real-time RT-PCR quantification due to tissue-specific contaminants . Molecular and Cellular Probes , 18 : 45 – 50 .
- van Santen , V.L. , Joiner , K.S. , Murray , C. , Petrenco , N. , Hoerr , F.J. and Toro , H. 2004 . Pathogenesis of chicken anemia virus: comparison of the oral and the intramuscular routes of infection . Avian Diseases , 48 : 494 – 504 .
- Yuasa , N. , Taniguchi , T. , Imada , T. and Hihara , H. 1983 . Distribution of chicken anemia agent (CAA) and detection of neutralizing antibody in chicks experimentally inoculated with CAA . National Institute of Animal Health Quarterly , 23 : 78 – 81 .