Abstract
Infections with goose circovirus (GoCV) are associated with growth retardation and developmental problems in farmed geese. An indirect immunofluorescence assay for detecting virus-specific serum antibody was developed for diagnostic and epidemiological purposes. In the absence of a method for growing GoCV in cell culture, the assay was based on the reaction of antibodies with the GoCV capsid protein produced within baby hamster kidney cells using the eukaryotic Semliki forest virus expression vector. Using an optimized test that involved screening sera at 1:50 dilution and the use of a fluorescein isothiocyanate anti-duck immunoglobulin conjugate, GoCV-specific antibody was detected in 141 (88.6%) of 159 samples obtained from 27 of 28 breeder flocks aged from 1 to 6 years. Testing also showed the presence of GoCV-specific antibody in 85 (40.9%) of 208 serum samples from birds aged 30 weeks or less. Although maternally derived antibody was detected in birds when 1 and 4 days old, actively acquired antibody was first detected in birds aged 53 days. Following experimental inoculation of 21-day-old geese with tissue homogenate containing GoCV, virus-specific antibody was detected in serum samples collected at 27 and 34 days post inoculation. It is concluded that the SFV expression vector approach may prove useful for developing serological tests for other viruses, including other avian circoviruses, that do not grow in cell culture.
Les infections dues au circovirus de l'oie (GoCV) sont associées à un retard de croissance et à des problèmes de développement dans les élevages d'oie. Un test d'immunofluorescence indirect pour la détection d'anticorps sériques spécifiques du virus a été développé à des fins diagnostique et épidémiologique. En absence de méthode permettant la culture du virus sur cellules, le test est basé sur la réaction des anticorps avec la protéine de capside du GoCV produite sur cellules de reins d'hamster nouveau né utilisant le Semliki Forest Virus (SFV) comme vecteur d'expression de gène. L'emploi d'un test optimisé comprenant le screening des sérums à la dilution au 1:50 et l'utilisation d'un conjugué immunoglobuline anti-canard marqué à l'isothiocyanate de fluorescéine, a mis en évidence la présence d'anticorps spécifiques anti-GoCv dans 88,6% des échantillons (141/159) obtenus à partir de 27 troupeaux sur 28, âgés de 1 à 6 ans. Il a également été mis en évidence la présence d'anticorps spécifiques anti-GoCv dans 40,9% des échantillons de sérums (85/208) d'animaux âgés de 30 semaines ou moins. Bien que les anticorps maternels aient été détectés chez les animaux âgés d'un à 4 jours, les anticorps induits ont été détectés, en premier, chez des animaux âgés de 53 jours. Après une infection expérimentale d'oies âgées de 21 jours avec un broyat de tissu contenant le GoCV, des anticorps spécifiques du virus ont été détectés dans les échantillons de sérum prélevés 27 et 34 jours après l'inoculation. Il a été conclu que l'utilisation du vecteur d'expression SFV peut être utile au développement de tests sérologiques pour d'autres virus, incluant d'autres circovirus aviaires, qui ne se multiplient pas sur culture cellulaire.
Infektionen mit Gänsecircovirus (GoCV) sind bei auf Farmen gehaltenen Gänsen mit Wachstumsverzögerungen und Entwicklungsstörungen verbunden. Für diagnostische und epidemiologische Zwecke wurde ein indirekter Immunfluoreszenztest zum Nachweis virusspezifischer Serumantikörper entwickelt. Mangels einer Anzüchtungsmethode für GoCV in der Zellkultur basiert der Test auf einer Antikörperreaktion mit GoCV-Kapsidprotein, das in Babyhamsternierenzellen unter Verwendung des eukaryotischen Semliki Forest-Virus (SFV)-Expressionsvektors produziert wird. Nach Optimierung des Tests, was die Untersuchung der Seren in 1:50 Verdünung und die Verwendung eines Fluoreszein-Isothiozyanat-anti-Ente-Immunglobulin-Knojugats bedeutete, wurden GoCV-spezifische Antikörper in 141 (88,6%) von 159 Proben aus 27 von 28 zwischen 1 und 6 Jahre alten Elterntierherden nachgewiesen. Auch die Untersuchung von 208 Serumproben aus 30 Wochen alten und jüngeren Tieren ließen in 85 Proben (40,9%) das Vorhandensein von GoCV-spezifischen Antikörpern erkennen. Obwohl maternale Antikörper bei ein- bis viertägigen Gänseküken entdeckt wurden, konnten aktiv gebildete Antikörper erst in 53 Tage alten Tieren ermittelt werden. Nach experimenteller Inokulation von 21-tägigen Gänseküken mit GoCV-haltigem Gewebehomogenat wurden virusspezifische Antikörper in Serumproben vom 27. und 24. Tag nach der Inokulation festgestellt. Daraus wird geschlossen, dass die SFV-Expressionsvektormethode sich auch für die Entwicklung serologischer Tests für andere Viren, einschließlich anderer aviärer Circoviren, die sich nicht in Zellkulturen vermehren, als nützlich erweisen wird.
Las infecciones por circovirus de ganso (GoCV) están asociadas a retraso en el crecimiento y problemas del desarrollo en gansos de granja. Se desarrolló un ensayo de inmunofluorescencia indirecta para detectar anticuerpos virus-específicos en suero con fines diagnósticos y epidemiológicos. A falta de un método para replicar GoCV en cultivo celular, el ensayo se basaba en la reacción de anticuerpos con proteínas de la cápside de GoCV producidas en células de riñón de crías de hamster mediante el uso de un vector de expresión eucariótico basado en el virus Semliki Forest. Mediante una prueba optimizada que suponía testar sueros a la dilución 1:50 y el uso de un anticuerpo anti-inmunoglobulina de pato conjugado con isotiocianato de fluoresceína, se detectaron anticuerpos específicos de GoCVen 141 (88.6%) de 159 muestras obtenidas de 27 de 28 lotes de reproductores de 1 a 6 años. El muestreo también mostró la presencia de anticuerpos específicos de GoCV en 85 (40.9%) de 208 muestras de sueros de aves de 30 o menos semanas de vida. Pese a que se detectaron anticuerpos maternales en aves de 1 a 4 días de vida, los primeros anticuerpos adquiridos de manera activa se detectaron en aves de 53 días de vida. Tras la inoculación experimental de gansos de 21 días de vida con homogeneizados que contenían GoCV, se detectaron anticuerpos en muestras de suero obtenidas a los 27 y 34 días post inoculación. Se concluye que la aproximación a través de la expresión en vector de SFV podría ser útil para el desarrollo de pruebas serológicas para otros virus, incluyendo otros circovirus aviares que no crecen bien en cultivo celular.
Introduction
Infection of geese with a circovirus-like virus was first reported in a large commercial flock in Germany that was experiencing increased levels of mortality and runting (Soike et al., Citation1999). Disease signs were restricted to growth retardation, and feathering and developmental disorders. Histopathological examination showed lymphocyte depletion and histiocytosis in the bursa of Fabricius, spleen and thymus, and electron microscopic examination of these tissues identified the presence of a small, icosahedral virus about 15 nm diameter. It was suggested that the immunosuppression induced by the circovirus-like virus could be a predisposing factor for secondary infections by other infectious agents (Soike et al., Citation1999). Subsequent nucleotide sequencing confirmed the presence within this flock of a novel circovirus, named goose circovirus (GoCV), which shared genomic similarity with previously characterized circoviruses (Todd et al., Citation2001).
Circoviruses are small (diameter 15 to 25 nm), non-enveloped icosahedral viruses that contain single-stranded DNA genomes (Todd, Citation2000). Chicken anaemia virus is classified as the only member of the genus Gyrovirus of the family Circoviridae (Pringle, Citation1999), while the genus Circovirus comprises two mammalian circoviruses, porcine circovirus types 1 and 2, and a number of avian circoviruses, including psittacine beak and feather disease virus, pigeon circovirus (Mankertz et al., Citation2000; Todd et al., Citation2001), GoCV (Todd et al., Citation2001), canary circovirus (Phenix et al., Citation2001b) and duck circovirus (Hattermann et al., Citation2003). Like other members of the genus, GoCV has an ambisense genomic organization, containing two major open reading frames. ORF V1, which is located on the virus-sense strand, encodes the replication-associated protein (Rep), while ORF C1, located on the complementary sense strand, encodes the capsid protein, which in GoCV comprises 250 amino acids.
To date, GoCV has not been grown in cell culture and specific antibodies for detection of viral antigen are not available. GoCV infections are therefore currently diagnosed by histological and electron microscopic examination as originally described by Soike et al. (Citation1999), or by polymerase chain reaction (PCR) and dot blot hybridization, which rely on the detection of virus DNA (Ball et al., Citation2004). In a previous study we have shown that GoCV infections were detected by PCR in 103 (48.1%) of 214 bursa of Fabricius (BF) samples from sick or dead birds that were submitted to the Central Veterinary Laboratory, Budapest, Hungary, for diagnostic investigation (Ball et al., Citation2004). That study showed that infections were detected in samples from 49 of 76 flocks and in samples from birds aged from 1 to 13 weeks (Ball et al., Citation2004). Chen et al. (Citation2003) have also described the detection of GoCV in 16 of 21 goose flocks in Taiwan.
Although PCR has proved useful for demonstrating that GoCV infections are common, a test for detecting virus-specific antibody in serum would be of considerable use as a more convenient method for determining the prevalence of infection and for providing epidemiologically useful information. However, the lack of a cell culture propagation system for GoCV has restricted the development of serological tests such as indirect immunofluorescence (IIF) and virus neutralization assays. In this paper we describe the development and application of an IIF assay for detecting GoCV-specific antibodies that utilizes the GoCV capsid protein antigen produced within baby hamster kidney (BHK) cells by the eukaryotic Semliki forest virus (SFV) expression vector.
Materials and Methods
Serum samples from commercial geese
Young geese. Sets of serum samples were obtained from 218 geese from 33 flocks in Hungary. The birds ranged in age from 1 to 210 days and the majority were being reared for meat production.
Older geese (>1 year old). Serum samples were obtained from 159 breeder geese, aged between 1 and 6 years, from 28 farms.
Experimental infection
A small-scale experimental infection of 21-day-old geese was undertaken to provide additional serum samples for test validation. Twenty-nine geese were orally inoculated with a suspension that was prepared from a BF tissue sample shown by PCR to be positive for GoCV DNA. The sample was homogenized as a 10% suspension in nutrient broth and clarified before inoculation. Testing by virus isolation in cell culture showed that this inoculum was free of goose parvovirus and reovirus. For control purposes, five geese remained uninoculated and were housed separately. Five inoculated birds were killed at days 7, 13, 20, 27 and 34 post inoculation (p.i.). Blood was collected immediately before the birds were killed and samples of BF were collected at necropsy for PCR testing. The five uninoculated geese were killed at 20 days p.i. and were similarly sampled.
Polymerase chain reaction
Extracts of DNA from BF tissue from the experimentally inoculated geese were tested for the presence of GoCV DNA using the PCR test method described previously by Ball et al. (Citation2004).
Construction of the SFV/GoCVcap defective virus DNA
The GoCV capsid gene region was amplified by PCR from the cloned GoCV genome (Todd et al., Citation2001) using the primers SFV GoCV F (5′-GCA TGG ATC CTA TTA TAG CAC CAT GCC GCT GTA TCG CGC GAG-3′) and SFV GoCV R (5′-GCA TGG ATC CTT ATG GTG AAA GCC CAG TCC-3′). These primers contained an engineered BamHI site (underlined) to enable insertion of the amplified GoCVcap PCR product into the SFV-1 vector. PCR was carried out using the Qiagen Hot Start PCR kit (Qiagen, West Sussex, UK) and the conditions used were an initial denaturation at 94°C for 15 min followed by 35 cycles of 94°C for 30 sec, 45°C for 30 sec and 72°C for 1 min, followed by a final extension at 72°C for 7 min. The GoCVcap PCR product was purified using the Qiaquick PCR cleanup kit (Qiagen, UK) and cut using BamHI. The SFV-1 vector was also cut using BamHI and treated with calf intestinal alkaline phosphatase according to the manufacturer's instructions (New England Biolabs, Hertfordshire, UK). The linearized SFV-1 vector and the GoCVcap PCR product were ligated together in a 1:3 molar ratio following size selection on a 2% agarose gel and purification using the Qiaquick gel extraction kit (Qiagen, UK). The ligation mixture was used to transform TOP 10 cells (Invitrogen, Paisley, UK), and these were inoculated onto LB-agar plates containing ampicillin. Miniplasmid preparations of individual SFV/GoCVcap clones were prepared and their nucleotide sequence determined to confirm both the presence and orientation of the GoCVcap insert in the SFV-1 vector.
Production of replication defective SFV/GoCVcap particles
Five micrograms of SFV/GoCVcap construct, the SFV/helper vector and the SFV/LacZ control vector were individually linearized using SpeI. The linearized fragments were size selected on a 2% agarose gel and purified using the Qiaquick gel extraction kit. RNA transcripts were generated from the linearized SFV/GoCVcap, SFV/LacZ and SFV/helper constructs using a T7 transcription kit according to the manufacturer's instructions (Ambion, Cambridgeshire, UK). BHK cells were treated with a trypsin versene solution and washed once using Dulbecco's phosphate-buffered saline (PBS) (Invitrogen). Following resuspension in PBS at 1×107 cells/ml, 800 µl cells were transferred to a 4 mm diameter electroporation cuvette. The SFV/GoCVcap and SFV/LacZ transcripts were each co-electroporated with SFV/helper transcript using two pulses with a capacitance of 25 µF and a potential difference of 0.85 kV using a gene pulser (Bio-Rad, Hertfordshire, UK). Following incubation at 37°C for 48 h, the cell supernatants containing replication defective recombinant virus particles were harvested by rapid freezing in liquid nitrogen.
Production of coverslip BHK cell cultures containing GoCV capsid antigen
BHK cells were diluted 1:4 in BHK growth medium and inoculated onto coverslips in Iwaki six-well tissue culture plates (Bibby-Sterilin, Staffordshire, UK) and incubated for 24 h at 37°C. For biosafety reasons the recombinant SFV particles were produced in a non-infectious form, in which the SFV glycoprotein required for cell penetration was present in an uncleaved, inactive state. Generation of infectious recombinant virus (activation) required proteolytic cleavage of the glycoprotein. To do this, 20 µl aliquots of SFV/GoCVcap and SFV/LacZ virus particles were activated by adding 0.4 µl CaCl2 (100 mM) and 1 µl chymotrypsin (10 mg/ml). Following incubation on ice for 30 min, 9 µl aprotonin protease inhibitor (Sigma-Aldrich, Dorset, UK) was added to stop the cleavage reaction. The activated virus particles were made up to a volume of 3 ml using serum-free medium. The coverslips carrying BHK monolayers were washed three times using pre-warmed sterile PBS, and 500 µl activated virus particles were added to each well and allowed to adsorb for 1 h at 37°C. Following adsorption, 4 ml BHK growth medium was added and the coverslips were incubated for 24 hr at 37°C. The coverslips were harvested, fixed in acetone for 10 min and stored at −20°C over silica gel.
Indirect immunofluorescence assay
During the development and optimization stage, BHK cell coverslips containing the SFV-expressed GoCV capsid protein were incubated for 1 h at 37°C with serum samples (100 µl) diluted between 1:5 and 1:400 in PBS. Following washing in PBS for 10 min, dilutions (1:50 to 1:400) of fluorescein isothyocyanate (FITC)-conjugated rabbit anti-duck IgG (100 µl; Autogen Bioclear, Wiltshire, UK), which cross-reacts with goose IgG, was added and the coverslips incubated for a further 1 h at 37°C. Following washing in PBS for 10 min the coverslips were mounted in Citifluor (Citifluor Ltd., London, UK) and examined by ultraviolet microscopy for fluorescence. BHK coverslips containing the SFV-expressed β-galactosidase gene were similarly stained and used as a GoCV negative control throughout the experiment.
Results
Development of the indirect immunofluorescence assay
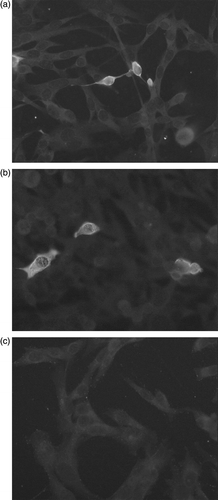
Ten serum samples were selected initially from farms that were known to be infected with GoCV on the basis of earlier PCR testing. Preliminary testing was undertaken using a range of serum dilutions between 1: 5 and 1: 400, in conjunction with selected dilutions (1:50 to 1:400) of the FITC-conjugated rabbit anti-duck immunoglobulin secondary antibody. With some of the serum samples, intracytoplasmic staining was observed in approximately 5% of the cells that had been infected with the recombinant SFV-GoCVCap replication-defective particles. This staining was not observed in cells that had been infected with the recombinant SFV-LacZ replication-defective particles (). A screening dilution of 1:50 was chosen on the basis that lower dilutions of sera, including 1:5, 1:10 and 1:25, produced higher levels of non-specific staining and that higher dilutions, including 1:100, 1:200 and 1:400, generally produced weaker staining. A decision was taken to test serum samples from geese aged from 1 to 21 days old at a dilution of 1:5 as well as at 1:50 in order to attempt to detect low levels of maternally derived antibody.
Figure 1. Detection of GoCV-specific antibody using indirect immunofluorescence. BHK cells grown on coverslips and infected with replication-defective particles of the recombinant SFV/GoCVCap (1a and 1b) or with replication-defective particles of the recombinant SFV/LacZ vector (1c) were incubated with 1:50 dilutions of goose sera. Reactive goose antibodies were detected using an FITC anti-duck immunoglobulin conjugate. Sera containing GoCV-specific antibodies produced cytoplasmic fluorescent staining in cells with the recombinant GoCV antigen but not in cells with the recombinant LacZ antigen.
Testing serum and BF samples from experimentally infected geese
At 7 days p.i. the BF of one out of five birds was positive for GoCV DNA by PCR testing, but none were positive at 13 days p.i. (). At 20, 27 and 34 days p.i. the proportions of birds testing positive by PCR were 40%, 60% and 100% respectively. Using the IIF assay, based on the GoCV capsid protein produced by the recombinant SFV vector, antibody to GoCV was first detectable at 27 days p.i., when one out of five birds tested positive. The level of seropositivity was increased at 34 days p.i., when five out of nine birds were positive for GoCV antibody.
Table 1. Detection of GoCV-specific antibody by IIF and GoCV DNA by PCR in samples from experimentally infected geese
Testing of serum samples from geese aged over 1 year
A total of 141 of 159 (88.7%) samples from 27 of 28 (96.4%) flocks were positive for GoCV-specific antibody. Five samples from birds from one breeder flock aged over 5 years were negative, while the other flocks tested had seroprevalences of 60% or greater. It was noted that the intensities of immunofluorescent staining obtained with sera from these older geese were generally greater than those obtained with samples from birds less than 1 year old.
Testing of serum samples from young geese
Taken overall, GoCV-specific antibody was detected in 85 (40.9%) of 208 serum samples derived from 34 sample sets that were collected from geese aged between 1 and 210 days old (). Antibody to GoCV was detected in samples from 1-day-old and 4-day-old birds and in samples from birds aged 53 days or over. Birds sampled between these timepoints were negative. The level of seroconversion broadly increased with age thereafter, reaching 100% in geese aged 182 days and 210 days. The flock from which sample set number 34 was obtained, which contained 210-day-old birds and from which 15 of 15 samples tested positive for GoCV antibody, was considered to have a high health status.
Table 2. Detection of GoCV-specific antibody in sera from young geese
Two sample sets, numbers 22 and 31, were obtained from the same flock at two different timepoints, at 49 and 66 days of age. None of the six samples were positive for GoCV-specific antibody when the birds were aged 49 days, whereas 15 of 20 were positive when the birds were aged 66 days. PCR testing of DNA extracted from the same serum samples indicated that four of six samples from 49-day-old birds were positive for GoCV DNA, whereas only two of 20 samples from 66-day-old birds were positive by PCR. The two PCR-positive serum samples from geese aged 66 days also contained GoCV-specific antibody.
Discussion
Serological tests for detecting virus-specific antibodies are important tools for the diagnosis of infection and in epidemiological studies. This is particularly true in the case of infections with novel or recently identified viruses including circoviruses of pigeons, geese and ducks, for which knowledge of the prevalence of infections, the age at which infection occurs and the range of susceptible host species can be important in formulating disease control strategies. The application of commonly used serological tests, such as the virus neutralization test, IIF and enzyme-linked immunosorbent assay, usually depends on the ability to grow the virus in cell culture. Although both porcine circovirus types can be grown in continuous pig kidney cell lines, to date there have been no reports describing the propagation of avian circoviruses, such as beak and feather disease virus, pigeon circovirus and GoCV, in cell culture, and this has restricted development of serological tests.
In the present investigation, the SFV expression vector has been employed to produce GoCV capsid protein within BHK cells. This eukaryotic expression system has been used successfully in our laboratory to express genes from other avian viruses, including infectious bursal disease virus (Phenix et al., Citation2001a), as well as mammalian (unpublished results) and fish viruses (Phenix et al., Citation2000). Although the SFV vector can be used by transfecting cells with recombinant RNA that has been transcribed in vitro, we have adopted the alternative approach involving the use of a helper vector to generate replication-defective SFV particles that contain the recombinant RNA and that can be used to “infect” cells (Liljestrom & Garoff, Citation1991).
We have found that the direct transfection procedure using electroporation results in an unacceptable level of non-specific immunofluorescence, whereas the use of replication-defective particles to deliver the recombinant RNA does not. To facilitate the IIF assay, the proportion of cells containing the recombinant antigen can be controlled by dilution of the replication-defective particles used for infection. However, the SFV expression vector is not suitable for producing large amounts of virus protein, such as the amounts that would be required for an indirect antibody-detecting enzyme-linked immunosorbent assay.
In this investigation, the IIF test based on the SFV-expressed GoCV capsid gene was validated using serum samples from a small-scale experimental infection of 21-day-old geese with a tissue homogenate known to contain GoCV particles. Evidence of virus replication was obtained using PCR, which showed that the proportion of birds in which virus DNA was detected in BF tissue increased from 20 days p.i. (two of five birds) to 34 days p.i. (nine of nine birds). Antibody was first detected by IIF at 27 days p.i., when one out of five birds was positive. In this preliminary experiment, tissue and serum samples were only available from uninoculated geese at 20 days p.i., which prevented us from being certain that the uninoculated birds had remained free of infection. However, the detection of an antibody response after virus replication makes it likely that the SFV GoCV-based IIF test is detecting GoCV-specific antibody. In the experimental infection, no clinical signs, such as growth retardation and or feathering disorders, were observed, and histological examination of the BF failed to detect botryoid inclusions, which are characteristic of circovirus infections. Although it is possible that clinical disease and histopathology may be difficult to reproduce experimentally with GoCV, as is the case with porcine circovirus type 2 experimental infections, it is also possible that the duration of the experiment was insufficient to allow development of lesions and clinical signs.
Serological testing of younger geese indicated that actively acquired antibody to the virus was first detected in geese aged 53 days and that the proportion of seropositive birds increased with age, reaching 75% for birds aged 63, 66 and 70 days and 100% for birds aged 182 and 210 days. Earlier work from our laboratory suggested that a substantial proportion of geese become infected in the first few weeks of life (Ball et al., Citation2004). The late apparent onset of seroconversion may reflect the possibility that the IIF assay developed for GoCV antibody lacks sensitivity. Alternatively, it is possible that circovirus infections may be slow to elicit antibody responses in comparison with other viral pathogens due to the lymphocytic depletion caused by circovirus infections.
The high seroprevalence detected in adult breeder birds suggested that GoCV infections are probably endemic in farmed geese in Hungary and may be widespread in other countries where geese are farmed. The availability of a test capable of detecting GoCV-specific antibody provides a convenient way to determine whether other avian species are susceptible to infection with this virus. Preliminary testing of 30 sera from adult ducks using the same test and employing the same FITC anti-duck immunoglobulin conjugate provided no evidence of infection (unpublished results).
The widespread nature of GoCV infections makes it highly improbable that they will be controlled by eradication. Rather, control measures may take the form of reducing any adverse effects associated with infection. Benefits may be gained by ensuring that breeder birds have high levels of virus-specific antibody before lay, and the use of vaccines for administration to breeders may be considered. The development and evaluation of a GoCV vaccine, which can probably be produced as a recombinant protein, is likely to depend on the availability of a test for detecting GoCV-specific antibody such as that developed in this study. In conclusion, the SFV expression vector approach may prove useful for developing serological tests for other viruses, including other avian circoviruses that do not grow in cell culture.
Acknowledgments
This work was funded in part by the Biotechnology and Biological Sciences Research Council, UK.
References
- Ball , N.W. , Smyth , J.A. , Weston , J.H. , Borghmans , B.J. , Palya , V. , Glavitis , R. , Ivanics , E. , Dan , A. and Todd , D. 2004 . Diagnosis of goose circovirus infection in Hungarian geese samples using polymerase chain reaction and dot blot hybridization tests . Avian Pathology , 33 : 51 – 58 .
- Chen , C.-L. , Chang , P.-C. , Lee , M.-S. , Shien , J.-H. , Ou , S.-J. and Shieh , H.K. 2003 . Nucleotide sequences of goose circovirus isolated in Taiwan . Avian Pathology , 32 : 165 – 171 .
- Hattermann , K. , Schmitt , C. , Soike , D. and Mankertz , A. 2003 . Cloning and sequencing of duck circovirus (DuCV) . Archives of Virology , 148 : 2471 – 2480 .
- Liljestrom , P. and Garoff , H. 1991 . A new generation of animal cell expression vectors based on the semliki forest virus replicon . Biotechnology , 9 : 1356 – 1361 .
- Mankertz , A. , Hattermann , K. , Ehlers , B. and Soike , D. 2000 . Cloning and sequencing of columbid circovirus (CoCV), a new circovirus from pigeons . Archives of Virology , 145 : 1 – 11 .
- Phenix , K.V. , McKenna , B. , Fitzpatrick , R. , Vaughan , L. , Atkins , G.A. , Liljestrom , P. and Todd , D. 2000 . Cell culture evaluation of the semliki forest virus expression system as a novel approach for antigen delivery in fish . Marine Biotechnology , 2 : 27 – 37 .
- Phenix , K.V. , Wark , K. , Luke , C.J. , Skinner , M.A. , Smyth , J.A. , Mawhinney , K.A. and Todd , D. 2001a . Recombinant semliki forest virus vector exhibits potential for avian virus vaccine development . Vaccine , 19 : 3116 – 3123 .
- Phenix , K.V. , Weston , J.H. , Ypelaar , I. , Lavazza , A. , Todd , D. , Wilcox , G. and Raidal , S.R. 2001b . Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus Circovirus of the family Circoviridae . Journal of General Virology , 82 : 2805 – 2809 .
- Pringle , C.R. 1999 . Virus Taxonomy at the XIth International Congress of Virology, Sydney, Australia, 1999 . Archives of Virology , 144 : 2065 – 2070 .
- Soike , D. , Köhler , B. and Albrecht , K. 1999 . A circovirus-like infection in geese related to a runting syndrome . Avian Pathology , 28 : 199 – 202 .
- Todd , D. 2000 . Circoviruses: immunosuppressive threats to avian species: a review . Avian Pathology , 29 : 373 – 394 .
- Todd , D. , Weston , J.H. , Soike , D. and Smyth , J.A. 2001 . Genome sequence determinations and analyses of novel circoviruses from goose and pigeon . Virology , 286 : 354 – 362 .