Abstract
The gene fragment coding for the complement protein C3 (chC3d) from Arbor Acres (AA) chickens was cloned and expressed as a fusion protein for its application in the vaccine study of chicken, and for in vitro experiments. The chC3d fragment strengthened B-cell responses when complexed with antigen. Three potential vaccine construct units were engineered to contain two, four and six copies of the chC3d-P29 coding gene linked to the F gene of Newcastle disease virus, an economically important pathogen of chicken that is classified as a list A contagious disease of poultry by the Office International des Epizooties. Recombinant chC3d protein and C3d-P29 proteins that contained the F gene of Newcastle disease virus (C3d-F-P29.n) were generated separately in Escherichia coli and chick embryo fibroblast cells with the help of expression vectors. All recombinant proteins were analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and western blotting. Analysis of the immunogenicity of different repeats of C3d-F-P29.n revealed that C3d-P29 had an enhancing effect on the immunogenicity of antigens, and that six or more repeats of C3d-P29 may be necessary for efficient enhancement of antigen-specific immune responses. To date, published research into the adjuvant activities of C3d has been limited to experiments in mice, rabbits and cattle. The adjuvant properties of C3d have not been assessed in poultry using homologous C3d in association with antigens relevant to the target species. The chicken C3d fusion proteins detailed in this study are the first reported and they provide a basis for immunization trials in chicken, studies of receptor binding and cell activation of chicken lymphocytes, and investigations of new types of vaccine, including genetic recombinant and DNA vaccines against various pathogens.
Introduction
Recent advances in immunology have led to the recognition that activated products of the complement system can have profound effects on adaptive immune responses. Complement 3d (C3d), generated by proteolytic cleavage of activated C3, is capable of covalently attaching to potential antigens. In addition, C3d specifically binds to complement receptor type 2 (CR2) expressed on the surface of B lymphocytes and dendritic cells. Following the report of the in vitro evidence for the ability of C3d to enhance the antibody response (Griffioen et al., Citation1991), the first in vivo study that supported a role for C3d as a molecular adjuvant was the demonstration that immunization of mice with hen egg-white lysozyme (HEL) genetically linked to two or three tandem copies of C3d resulted in a dramatically enhanced HEL-specific antibody response, compared with immunization with unmodified HEL (Dempsey et al., Citation1996). Since then, several other studies have convincingly demonstrated that conjugation of target antigens to multiple copies of C3d by genetic means can dramatically enhance the antibody responses (Green et al., Citation2001, Citation2003; Li et al., Citation2003; Watanabe et al., Citation2003; Wang et al., Citation2004). Conjugation of C3d to pneumococcal capsular polysaccharide by chemical means has also been reported to enhance the immunogenicity of the target polysaccharide antigen (Test et al., Citation2001).
Enhanced immunogenicity of C3d–antigen complexes has been attributed to synergistic B-cell activation associated with the co-ligation of the B-cell antigen-specific receptor and CR2. Support for this concept is based on in vitro studies in which cross-linking of the B-cell antigen-specific receptor using anti-IgM antibodies, together with the concomitant cross-linking of CR2 by either CR2-specific antibodies or polymerized C3d, leads to enhanced B-cell activation (Carter et al., Citation1988, Citation1989). A more recent report appears to indicate that the enhanced immunogenicity exhibited by C3d–antigen conjugates may be more complex, owing to the observation that C3d–antigen complexes have been shown to elicit elevated antibody responses to antigens in the absence of CD21/35 expression in mice (Haas et al., Citation2004).
To date, published research into the immunomodulatory properties of C3d has been limited to experiments in mice, rabbits and cattle (Firth et al., Citation2006). The adjuvant properties of C3d have not been assessed in poultry using homologous C3d in association with antigens relevant to the target species. Experimentally, C3d from one species may retain adjuvant activity in another species (Green et al., Citation2003; Firth et al., Citation2006). Homologous C3d can be expected to have higher activity. In addition, the use of heterologous C3d may induce antibody responses to C3d, which may reduce adjuvant effects following subsequent vaccinations or may lead to autoimmune disease due to induction of cross-reacting antibodies with C3d. To express the recombinant chicken C3d protein, it was necessary to generate cDNA coding for chC3d. Although nucleotide sequences were available for various small fragments of chicken C3, none were available for the C3d fragment. Fusion proteins of antigen with multiple fragment copies of chicken C3d (connected by appropriate linker sequences) were created for vaccine studies, since more than one copy of C3d is needed in a fusion protein for optimal adjuvant activity (Dempsey et al., Citation1996; Firth et al., Citation2006).
The present research was undertaken to facilitate studies of C3d activity in chicken by generating C3d fusion proteins as potential vaccine constructs and by expressing the fragment of P29 gene that was derived from chicken complement component C3d (chC3d) as an in vivo self-protein for use as in vitro DNA vaccine adjuvant studies. Two, four and six copies of the C3d-P29 coding gene, amplified by PCR and modified by restriction digestion with endonucleases, were subcloned into the pUC19. Different repeats of C3d-P29 and the F gene of strain F48E9 of Newcastle disease virus (NDV) were separately linked into the eukaryotic expression vector pcDNA3.1(+). In order to study the immunogenicity of recombinant C3d-P29 repeats, the plasmids of different repeats of C3d-P29 fused with the F gene of NDV (pCDNA3.1-F-C3d-P29.n) were injected intramuscularly into 1-day-old specific-pathogen free (SPF) chickens to determine whether these C3d-P29 repeats could enhance immunogenicity. The challenge/protection experiments were carried out in the fifth week after immunization. This study aims to provide the basis for investigations of new types of vaccine, including genetic recombinant and DNA vaccines, for the future control of NDV and other poultry pathogens.
Materials and Methods
Cloning and sequencing of the chicken C3d gene fragment
Two weeks before slaughter, frozen liver tissue from Arbor Acres (AA) chickens (Beijing Arbor Acres Poultry Breeding Co. Ltd, Beijing, China) were injected with the oil emulsion of Escherichia coli-inactivated vaccine (E. coli O1:O2:O78=1:1:1), in doses of 0.5 ml once per week, to obtain more C3d products. RNA was extracted with Trizol reagent (Invitrogen, Burlington, Ontario, Canada) as per the manufacturer's instructions. Reverse transcription (RT) was carried out with Moloney murine leukaemia virus reverse transcriptase (Invitrogen) using C3dr primer (Takara, Tokyo, Japan), designed on the basis of the published sequences of C3 gene using Primer Premier software (version 5.0; PREMIER Biosoft International, Palo Alto, California, USA).
Polymerase chain reaction (PCR) with primers C3df and C3dr () amplified a fragment of the C3 gene including the C3d coding region, and sufficient nucleotides flanking the coding region to avoid overlap of degenerate primers with the C3d sequence. PCR was performed in 50 µl reaction volumes using Taq DNA polymerase (Invitrogen), or Platinum Pt-pfx DNA polymerase (Invitrogen) when blunt-ended amplicons were desired. Standard conditions consisted of initial denaturing at 94°C (5 min), followed by 30 cycles of denaturing at 94°C (30 sec), annealing at 58°C (30 sec), and extending at 72°C (1 min), and then a 10-min final extension at 72°C. The PCR product was cloned into the pUC19, pET-32a and pMD18-T plasmids (Takara) and the sequence was confirmed.
Table 1. Primers used in the present study
Virus propagation and cloning of Newcastle disease virus F gene
Three-time plaque-purified F48E9 NDV stocks (China Institute of Veterinary Drug Control, Beijing, China) prepared on primary chicken embryo fibroblasts were grown in 9-day-old to 10-day-old SPF chicken embryos (from Shandong SPF Chicken Research Center). RNA from 200 µl infected allantoic fluid was extracted with the ‘RNAeasy’ kit (QIAGEN, GmbH, Germany), according to the manual. Similarly, RNA from 200 µl clinical samples (10% w/v homogenate) was also extracted with TRIZOL reagent (Life Technologies Inc., USA). RT-PCR was carried out to amplify the full-length F gene of NDV with primers designed from the published sequences () in a 50 µl reaction volume in reaction conditions of 94°C for 1 min, and 35 cycles 94°C for 1 min, 54°C for 1 min, and extension of 72°C for 2 min. The PCR products were cloned into the pMD18-T plasmid (Takara) and confirmed by nucleotide sequencing. The NDV F gene was further amplified by PCR from recombinant plasmid pMD18-T-F with primers P3 and P4 () with NheI and HindIII restriction enzyme sites to facilitate cloning into pcDNA3.1 plasmid.
Construction of plasmids
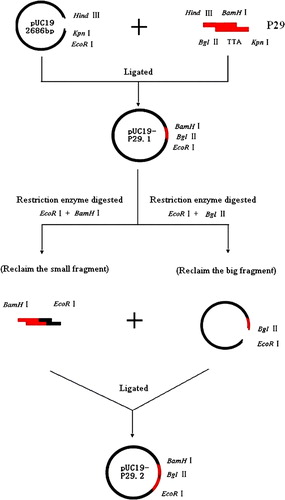
The gene encoding C3d-P29 gene amplified from plasmid pMD18-T-C3d was inserted into pUC19 vector with standard molecular biological techniques (). Briefly, the P29 fragments digested with HindIII and BamHI were inserted into pUC19 plasmid and downstream KpnI and BglII sites for construction of different C3d-P29 repeats. The process was repeated for construction of vectors with two, four and six copies of C3d-P29 (C3d-P29.2, C3d-P29.4 and C3d-P29.6, respectively). Finally, the C3d-P29.n was inserted into pCDNA3.1(+) plasmid by restriction digestion of EcoRI and HindIII. All recombinant plasmids were verified by PCR, restriction endonuclease digestion and DNA sequencing.
Figure 1. Structure of recombinant chicken C3d constructs. Restriction enzyme sites HindIII and KpnI of the P29 fragment were used for interlinking with the pUC19 plasmid and C3d-P29. The BamHI and BglII sites were used for the construction of different C3d-P29 repeats. Briefly, the C3d-P29 gene coding region was amplified from plasmid pMD18-T-C3d by PCR and cloned into the cloning vector pUC19 to construct the pUC19-P29.1 plasmid. The pUC19-P29.1 plasmid, which had EcoRI, BamHI, and BglII restriction enzyme sites, was digested with restriction enzyme EcoRI and BamHI or EcoRI and BglII, respectively. The small fragment that was digested with EcoRI and BamHI, and the large fragment that was digested with EcoRI and BglII, were ligated to construct the pUC19-P29.2 plasmid. The figure only shows the process of how to construct pUC19-P29.2; the other C3d-P29 repeats, such as C3d-P29.4 and C3d-P29.6, were constructed similarly to pUC19-P29.2.
Prokaryotic expression of C3d and western blotting
E. coli DH5α cells transformed with pET-32a-C3d plasmids were grown in Luria-Bertani broth supplemented with 100 mg/ml ampicillin. Bacteria were grown at 30°C until mid-log phase (OD600 ∼ 0.4) and induced with 0.2% arabinose and 1 mM Isopropyl-β-D-thiogalactopyrananoside, and samples were collected at 2 h, 3 h, 5 h, 7 h and overnight for 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western immunoblot analysis. Resolved proteins were transferred onto a nitrocellulose membrane (Bio-Rad) and incubated with a 1:3000 dilution of synthetic 21-mer peptide (1272SEVDDAFLSDEDITSRSLFPE1292) of chicken C3 complement (Beijing Scilight Biotechnology Ltd) described previously (Ma et al., Citation2006). After extensive washing, bound 21-mer peptide antibodies were detected using a 1:5000 dilution of horseradish peroxidase-conjugated rabbit anti-chicken antiserum and enhanced chemiluminescence (Beijing Biosynthesis Biotechnology Ltd, Beijing, China).
Eukaryotic expression and western blot analysis of recombinant proteins
Primary chick embryo fibroblasts (5×105 cells) were transfected with 2 µg DNA using Lipofectamine reagent (Life Technologies, Grand Island, New York, USA) and were detected by indirect immunofluorescence assay with Fluorescein isothiocynate-conjugated goat anti-rabbit IgG (Beijing Boisynthesis Biotechnology Ltd). The transfected cells and 1.5% of diluted supernatant in SDS sample buffer (Bio-Rad, Hercules, California, USA) were loaded onto 10% polyacrylamide/SDS gel. The resolved proteins were transferred onto a nitrocellulose membrane (Bio-Rad) and incubated with a 1:3000 dilution of polyclonal chicken NDV antisera in phosphate-buffered saline (PBS) containing 0.05% Tween-20 and 5% foetal calf serum. After extensive washing, bound chicken antibodies were detected with a 1:5000 dilution of horseradish peroxidase-conjugated rabbit anti-chicken antiserum and enhanced chemiluminescence.
Immunization of SPF chickens with plasmid DNA and challenge
Vaccine trials and challenge protection experiments (all experiments were repeated three times) were performed on SPF chickens (from Shandong Specific-Pathogen-Free Chicken Research Center, Jinan, China). For this, 252 chickens were divided into six groups (14 per group) in a randomized block design. Plasmid preparations of pCDNA3.1, pCDNA3.1-F, pCDNA3.1-F-C3d-P29.2, pCDNA3.1-F-C3d-P29.4, and pCDNA3.1-F-C3d-P29.6 were purified by polyethylene glycol precipitation (Sambrook et al., Citation1989), and after quantitation (Ultrospec3000; Pharmacia Biotech Inc.) were resuspended in PBS (pH 7.2) at a final concentration of 0.5 µg/µl. Desired concentrations of the plasmid stocks were administered by intramuscular injection in a 200 µl volume at the leg muscle. Inactivated oil-emulsion vaccine of F48E9 virus was also injected as a control group. In all of the immunization experiments, sera were collected regularly (from the first week to the fifth week after immunization) post vaccination for detection of antibody. Chickens were challenged with 100 median embryo lethal dose F48E9 viruses in 0.1 ml intranasally at the fifth week after immunization, and observed daily for disease signs and death for 2 weeks post challenge.
Enzyme-linked immunosorbent assay for detection of anti-NDV antibodies
An endpoint enzyme-linked immunosorbent assay (ELISA) was performed to assess the titres of NDV F gene-specific antibody responses using the recombinant NDV F protein as coating antigen. Briefly, the 96-well ELISA plates (Costar, Cambridge, UK) were coated overnight at 4°C with 100 µl recombinant chicken NDV antigen at a concentration of 5 µg/ml in 100 mM carbonate buffer (pH 9.6). Plates were washed three times with PBS containing 0.05% Tween-20 and blocked for 1 h with PBS containing 1% bovine serum albumin. After washing, serum samples diluted in blocking buffer were applied to the wells (100 µl/well). Subsequently, NDV-specific antibodies were detected by horseradish peroxidase-conjugated rabbit anti-chicken IgG. The reaction was detected with 3,3′,5,5-tetramethylbenzidine substrate and measured at 490 nm with a Titertek Multiskan Plus, MK II microplate reader (Labsystems, Eragny/Oise, France).
Statistical analysis
The protective effect of vaccination was evaluated using a Student's t test comparing differences between the empty vector control group and vaccine groups. Experimental results were analysed by variance analysis or t test with SPSS software. If P < 0.05, the results were considered statistically significant.
Results
Cloning and sequencing of the chicken C3d gene fragment
A single, approximately 990 base pair (bp) PCR product of the C3d gene was amplified from RNA extracted from AA chicken liver tissue. The fragment was cloned into the pMD18-T plasmid and sequenced. The nucleotide sequence for C3d (accession number EF632299) was 99.5% identical to published Leghorn chicken (accession number DQ291160) C3d sequences. The predicted protein sequences for AA chicken C3d were 98.8% identical to published Leghorn chicken (accession number AAB92374) sequences, as determined by BLASTp analysis.
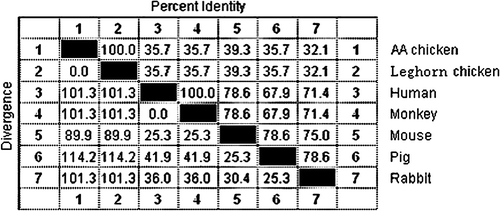
Sequence comparison of the CR2-binding sites on C3d (KVLMKFSKDGTHWAERNAHTYNIEGTSYA) is shown in . The sequence showed a lower nucleotide similarity and greater genetic distance than that obtained from different animals. However, the nucleotide similarity between Leghorn chicken C3d and AA chicken C3d was 100%.
Virus propagation and cloning of Newcastle disease virus F gene
Using the sets of primers F1–F2, an estimated 1700 bp fragment of the F gene of NDV F48E9 strain was amplified, and recombinant plasmids containing the cloned insert were validated by sequence analysis. The NDV F gene was also amplified from recombinant plasmid pMD18-T-F with primers P3 and P4 and was used for the pCDNA3.1-F construct.
Construction of plasmids
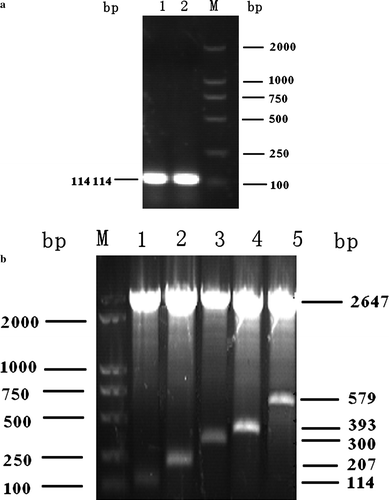
The gene encoding C3d-P29 amplified from plasmid pMD18-T-C3d by PCR was cloned into the cloning plasmid vector pUC19 (a). Different C3d-P29 repeats were constructed as described and were analysed by restriction endonuclease double digestion of NheI and HindIII. The digestion products included inserted P29 gene fragments 114 bp, 207 bp, 300 bp, 393 bp and 579 bp, respectively (b).
Expression and western immunoblot detection of recombinant proteins
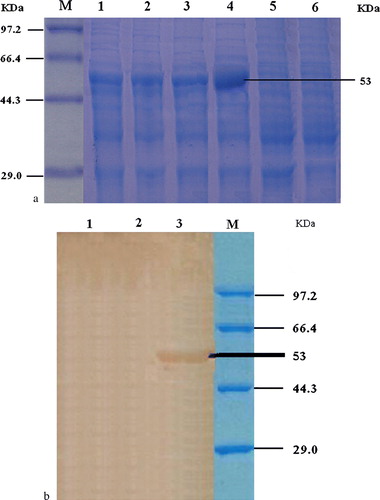
Induction of E. coli transformed with pET-32a-C3d plasmids in Luria-Bertani revealed that the highest expression products were observed after overnight induction (a). The expression of the protein was further confirmed by western immunoblot analysis, which showed the presence of an approximately 53 kDa protein (b). These proteins were not present in the uninduced controls.
Figure 4. 4a: SDS-PAGE result of the recombinant bacteria expressing C3d. Lane M, low-molecular-weight standard protein marker; lane 1, products of pET-32a-C3d (with IPTG induction for 3h); lane 2, products of pET-32a-C3d (with IPTG induction for 5h); lane 3, products of pET-32a-C3d (with IPTG induction for 7h); lane 4, products of pET-32a-C3d (with IPTG induction overnight); lane 5, products of pET-32a-C3d (without IPTG); and lane 6, DH5a control. 4b: Western blot analysis of recombinant C3d protein. Lane 1, pET-32a(+) only; lane 2, not induced by IPTG; lane 3, result of bacteria with pET-32a(+)-C3d; and lane M, protein molecular weight marker. Western immunoblot analysis was also done to reveal the presence of expressed proteins at approximately 53 kDa as expected. These reactive proteins were not present in the uninduced controls.
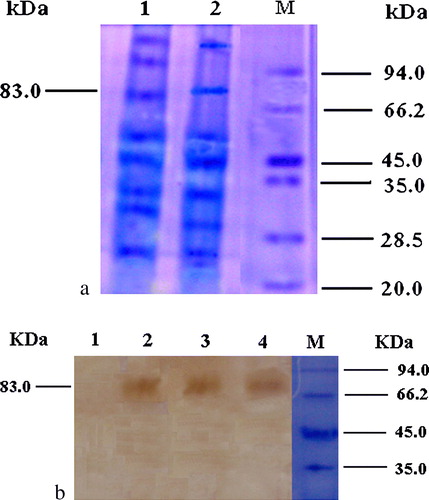
The primary chicken embryo fibroblasts (5×105cells/transfection) was transfected with 2 µg DNA using Lipofectamine reagent (Life Technologies) according to the manufacture's guidelines and were detected by indirect immunofluorescence assay, which proved that pcDNA3.1-F-C3d-P29.N was expressed as F protein in chick embryo fibroblast cells. Western blot analysis of the proteins separated by SDS-PAGE (a) revealed an expected band of 83 kDa.
Figure 5. 5a: Expression of vaccine constructs in vitro. Chick embryo fibroblast cultures were transfected with 2 µg each plasmid. Transfected cells were collected and 1.5% of total volume subjected to 10% PAGE. Lane 1, pCDNA3.1-F-C3d-P29.2; lane 2, pCDNA3.1-F-C3d-P29.6; and lane M, protein molecular weight markers. 5b: Western blot analysis of recombinant C3d protein. Lane 1, negative control; lane 2, pCDNA3.1-F-C3d-P29.2; lane 3, pCDNA3.1-F-C3d-P29.4; lane 4, pCDNA3.1-F-C3d-P29.6; and lane M, protein molecular weight marker. The presence of the approximately 83 kDa expressed proteins is indicated.
Immunization of SPF chickens with plasmid DNA and challenge
Five groups of SPF chickens (n=14) were immunized with a 200 µl volume of plasmids pCDNA3.1, pCDNA3.1-F, pCDNA3.1-F-C3d-P29.2, pCDNA3.1-F-C3d-P29.4, and pCDNA3.1-F-C3d-P29.6, respectively, into the leg muscles. Inactivated vaccine of F48E9 virus was also injected similarly as a control group. The experiments were repeated three times. Serological analysis showed that SPF chickens vaccinated with DNA expressing fusions of the F gene to P29 (two, four or six repeats) elicited a higher level of primary humoral response compared with chickens vaccinated with DNA expressing only the pCDNA3.1 vector and pCDNA3.1-F group (P < 0.05), although these were not as effective as inactivated oil-emulsion vaccine. The increase in antibody response elicited by six copies of P29 was higher than the four copy constructs (P < 0.05), which in turn was higher than two copy constructs (P < 0.05). However, the results demonstrated that the antibody levels in chickens immunized primarily with six copies of the P29 group were not only significantly higher than those with the two copies of P29 group (P<0.01), but were also higher than the level of response in the groups primed with pCDNA3.1-F (P<0.01) and the groups primed with plasmid pCDNA3.1 alone (P<0.01), as well as those with four copies of P29 (P<0.05). The results of immunizations also showed the inactivated oil-emulsion vaccine induced the highest antibody titres () and a 100% protection ratio and a 100% protection ratio (). The pCDNA3.1-F-C3d-P29.6 construct has the highest antibody titres () and an 88.1% protection ratio () in the pCDNA3.1-F-C3d-P29.n groups. The results of protection experiments are presented in . All chickens that received PBS solution (PBS + pCDNA3.1 vector) showed serious clinical signs and died 2 to 8 days post challenge. Differences in the enhancement of the antibody levels raised by the fusions of pCDNA3.1-P29 to variable repeats of P29 appeared to be determined by the different repeats of P29, in which six or more copies of P29 might be necessary to achieve the most modest antibody responses.
Figure 6. ELISA antibodies of chickens after immunization. Blood sera were collected post vaccination for detection of antibody in 5 weeks. The increase in antibody response elicited by six copies of P29 was higher than four copies of P29 (P < 0.05), which is also higher than two copies of P29 (P < 0.05). However, the results demonstrated that the antibody levels from chicken immunized primarily with the six copies of P29 group was significantly higher than that from the two copies of P29 group and the F gene alone vaccinated chicken group (P < 0.01) respectively. The challenge/protection experiments were carried out 5 weeks after immunity (results presented in Table 2).
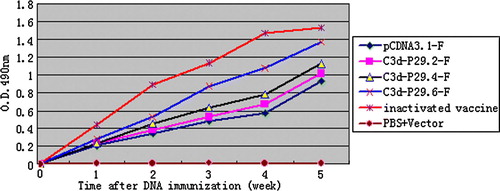
Table 2. Results of protection from immunized chickens after challenge
Discussion
NDV is an Office International des Epizooties list A pathogen that causes a serious respiratory and neurological disease in all species of birds and is an economically important infectious agent, causing substantial losses to the poultry industry. Newcastle disease varies in the degree of severity, ranging from an unapparent infection to severe disease causing 100% mortality. In China, the first Newcastle disease outbreak was recorded in 1928, and the standard strain F48E9 was isolated in 1948 for the first time (Liu et al., 2007). After the introduction of intensive vaccination programme against Newcastle disease in both large-scale poultry operations and village poultry farming, Newcastle disease has become endemic in many parts. Although DNA vaccines are generally immunogenic in several experimental models, they have not been as effective as live attenuated vaccines (Robinson, Citation1997; Donnelly et al., Citation1998; Robinson & Pertmer, Citation2000). In order to improve the efficacy of DNA vaccination, previous studies have shown that the complement protein C3d, when conjugated to an immunogen, can elicit high titre antibodies (Ross et al., Citation2000, Citation2001; Green et al., Citation2001, Citation2003; Mitchell et al., Citation2003). BALB/c mice vaccinated with DNA expressing sgp120 coupled to multiple copies of murine or human C3d elicit specific antibody titres 1 to 2 logs higher than mice vaccinated with sgp120-DNA (Ross et al., Citation2000; Green et al., Citation2003). Recently, Suradhat et al. (Citation2001) reported that two copies of murine C3d fused to bovine rotavirus VP7 or to bovine herpesvirus type 1 glycoprotein D did not enhance antibody titres to either antigen in C57BL/6 mice. Viewing these results collectively, it becomes apparent that the use of C3d as a molecular adjuvant may be efficacious in some and detrimental in other systems. While using C3d as a vaccine adjuvant is a very attractive strategy because of the small size of the molecule, its lack of immunogenicity and the relative simplicity of creating such fusion constructs, this cannot be viewed as a universal strategy for any antigen, but instead it requires careful case-to-case evaluation to determine whether this powerful small molecule will be useful or ineffective (Elke et al., Citation2006).
Although much progress has been made in the characterization of structure/function relationship of individual C components, unfortunately research to date has not advanced beyond laboratory models. Besides the research report by Firth et al. (Citation2006) with bovine C3d, no other studies using homologous C3d with antigens relevant to the target species have been reported, especially in chickens. The present research was undertaken to facilitate investigations of recombinant chicken C3d as an adjuvant in chicken. Our laboratory is involved into the research to examine the homology of such genes in different breeds or strains in chickens. We hope that this work will provide the basis for the construction of C3d as a molecular adjuvant in the near future.
NDV classified under the genus Avulavirus in the family Paramyxoviridae (Mayo, Citation2002), encodes for six major structural and non-structural proteins (Millar et al., Citation1988). The fusion protein (F) present in the viral envelope is synthesized as the F0 precursor, which is activated after cleavage into F1 and F2 fragments, to initiate infection (Nagai et al., Citation1976; Umino et al., Citation1990). DNA vaccines containing F genes have been most extensively researched in chickens and have been shown to confer certain protective immunity. Therefore in this study we selected the NDV F gene as a model antigen and different repeats of P29 peptide, an active peptide corresponding to the CR2-binding site on C3d, as a molecular adjuvant to explore enhancing effects on the immune responses. As expected, SPF chickens vaccinated with DNA expressing fusions of the F gene to P29 (two, four or six repeats) elicited a higher level of primary humoral responses compared with chickens vaccinated with DNA expressing only the pCDNA3.1 vector, although their effects as vaccines still were not as effective as inactivated oil-emulsion vaccine. The increase in antibody response elicited by six copies of P29 was higher than four copies of P29 (P < 0.05), which is also higher than two copies of P29 (P < 0.05). This result is coincident with the results of protection experiments shown in . Interestingly, the results showed that the enhancing effect of various repeats of P29 in fusions of the F gene to P29 on primary response was different, in which six copies or more of P29 might be necessary to achieve the most modest antibody response ( and ). It was indicated that the most modest antibody response appeared to be determined by the different repeats of P29. These results further explained the conflict between reports by Ross et al. (Citation2001) and by Suradhat et al. (Citation2001). Despite demonstrating a positive effect on the immunogenic response, more work needs to be undertaken to understand the role played by the C3d-P29 as a molecular adjuvant in the immune response. This is the first report to demonstrate that immunization with DNA expressing antigen–P29 fusions results in enhancing antibody responses. These findings argue that six or more repeats of C3d-P29 may be necessary for efficient enhancement of antigen-specific immune responses, and thus C3d-P29—a short, active peptide derived from the CR2-binding site on complement C3d—may be another promising molecular adjuvant and may assist the future design of vaccines for health-threatening pathogens.
Acknowledgements
The present work was supported by Shandong Natural Science Foundation (grant number Y2007D47). While this manuscript was in preparation, the sequence of AA chicken C3d was deposited in GenBank (accession number EF632299) by our colleagues X.X. Chu, L.X. Weng and Z.Z. Niu. The authors would like to thank them for their significant previous research work, and sincerely thank Dr Ma Xiao-li from Anhui University of Architecture for her kind help in editing the manuscript.
References
- Carter , R.H. , Spycher , M.O. , Ng , Y.C. , Hoffman , R. and Fearon , D.T. 1988 . Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes . Journal of Immunology , 141 : 457 – 463 .
- Carter , R.H. and Fearon , D.T. 1989 . Polymeric C3dg primes human B lymphocytes for proliferation induced by anti-IgM . Journal of Immunology , 143 : 1755 – 1760 .
- Dempsey , P.W. , Allison , M.E. , Akkaraju , S. , Goodnow , C.C. and Fearon , D.T. 1996 . C3d of complement as a molecular adjuvant: bridging innate and acquired immunity . Science , 271 : 348 – 350 .
- Donnelly , J.J. , Ulmer , J.B. and Liu , M.A. 1998 . DNA vaccines . Developments in biological standardization , 95 : 43 – 53 .
- Elke , S.B. , Wolfgang , W.L. and George , C.T. 2006 . Complement 3d: from molecular adjuvant to target of immune escape mechanisms . Clinical Immunology , 121 : 177—185
- Firth , M.A. , Moore , D.P. , Pei , Y.L. , Shewen , P.E. , Lo , R.Y.C. , Yoo , D.W. and Hodgins , D.C. 2006 . Cloning of a gene fragment encoding bovine complement component C3d with expression and characterization of derived fusion proteins . Veterinary Immunology and Immunopathology , 114 : 61 – 71 .
- Green , T.D. , Newton , B.R. , Rota , P.A. , Xu , Y. , Robinson , H.L. and Ross , T.M. 2001 . C3d enhancement of neutralizing antibodies to measles hemagglutinin . Vaccine , 20 : 242 – 248 .
- Green , T.D. , Montefiori , D.C. and Ross , T.M. 2003 . Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d . Journal of Virology , 77 : 2046 – 2055 .
- Griffioen , A.W. , Rijkers , G.T. , Janssens-Korpela , P. and Zegers , B.J. 1991 . Pneumococcal polysaccharides complexed with C3d bind to human B lymphocytes via complement receptor type 2 . Infection and Immunity , 59 : 1839 – 1845 .
- Haas , K.M. , Toapanta , F.R. , Oliver , J.A. , Poe , J.C. , Weis , J.H. , Karp , D.R. , Bower , J.F. , Ross , T.M. and Tedder , T.F. 2004 . Cutting edge: C3d functions as a molecular adjuvant in the absence of CD21/35 expression . Journal of Immunology , 172 : 5833 – 5837 .
- Li , D.J. , Wang , H.M. , Li , L. , Zhao , X.R. , Wang , M.Y. , Zhu , Y. , Meng , Y. and Yuan , M.M. 2003 . Gene fusion of molecular adjuvant C3d to hCG( enhances the anti-hCG( antibody response in DNA immunization . Journal of Reproductive Immunology , 60 : 129 – 141 .
- Liu , X.F. , Wan , H.Q. , Ni , X.X. , Wu , Y.T. and Liu , W.B. 2003 . Pathotypical and genotypical characterization of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985–2001 . Archives of Virology , 148 : 1387 – 1403 .
- Ma , X.Y. , Han , J.Q. , Gao , S.X. and Niu , Z.X. 2006 . Study on isolating complement C3d from the chicken serum by the method of immuno affinity chromatography . Journal of Northwest Sci-Tech University of Agriculture and Forestry , 34 : 55 – 58 .
- Mayo , M.A. 2002 . Virus taxonomy . Archives of Virology , 147 : 1071 – 1076 .
- Millar , N.S. , Chambers , P. and Emmerson , P.T. 1988 . Nucleotide sequence of the fusion and hemaglutinin-neurominidase glycoprotein genes of Newcastle disease virus strain Ulster: molecular basis for variations in pathogenicity between strains . Journal of General Virology , 69 : 613 – 620 .
- Mitchell , J.A. , Green , T.D. , Bright , R.A. and Ross , T.M. 2003 . Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins . Vaccine , 21 : 902 – 914 .
- Nagai , Y. , Kenk , H.I. and Rott , R. 1976 . Proteolytic cleavage of the viral glycoprotein and its significance for the virulence of Newcastle disease virus . Virology , 72 : 494 – 508 .
- Robinson , H.L. 1997 . DNA vaccines for immunodeficiency viruses . AIDS , 11 ( Suppl A ) : S109 – S119 .
- Robinson , H.L. and Pertmer , T.M. 2000 . DNA vaccines for viral infections: basic studies and applications . Advances In Virus Research , 55 : 1 – 74 .
- Ross , T.M. , Xu , Y. , Bright , R.A. and Robinson , H.L. 2000 . C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge . Nature Immunology , 1 : 127 – 131 .
- Ross , T.M. , Xu , Y. , Green , T.D. , Montefiori , D.C. and Robinson , H.L. 2001 . Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins . AIDS Research and Human Retroviruses , 17 : 829 – 835 .
- Sambrook , J. , Fritsch , E.F. & Maniatis , T. , 1989 . Molecular Cloning—A Laboratory Manual 2nd edn (pp. 24 – 28 ). Cold Spring Harbor Laboratory Press , Woodbury , , NY , 1989 , pp. 24 – 28 .
- Suradhat , S. , Braun , R.P. , Lewis , P.J. , Babiuk , L.A. , van Drunen Littel-van den Hurk , S. , Griebel , P.J. and Baca-Estrada , M.E. 2001 . Fusion of C3d molecule with bovine rotavirus VP7 or bovine herpesvirus type 1 glycoprotein D inhibits immune responses following DNA immunization . Veterinary Immunology and Immunopathology , 83 : 79 – 92 .
- Test , S.T. , Mitsuyoshi , J. , Connolly , C.C. and Lucas , A.H. 2001 . Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide . Infection and Immunity , 69 : 3031 – 3040 .
- Umino , Y. , Kohama , T. , Sato , T.A. , Sugiura , A. , Klenk , H.D. and Rott , R. 1990 . Monoclonal antibodies to three structural proteins of Newcastle disease virus: biological characterization with particular reference to the conformational change of envelope glycoprotein associated with proteolytic cleavage . Journal of General Virology , 71 : 1189 – 1197 .
- Wang , L. , Sunyer , J.O. and Bello , L.J. 2004 . Fusion to C3d enhances the immunogenicity of the E2 glycoprotein of type 2 bovine viral diarrhea viruses . Journal of Virology , 78 : 1616 – 1622 .
- Watanabe , I. , Ross , T.M. , Tamura , S. , Ichinohe , T. , Ito , S. , Takahashi , H. , Sawa , H. , Chiba , J. , Kurata , T. , Sata , T. and Hasegawa , H. 2003 . Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin . Vaccine , 21 : 4532 – 4538 .