Abstract
The prevalence of Borrelia, Mycobacteria and avian influenza virus (AIV) infections, together with the distribution of different AIV subtypes, was studied in migratory waterfowl and terrestrial birds trapped in three localities in Slovakia during 2006. Samples obtained from waterfowl captured in the Senianske Ponds area of Eastern Slovakia showed the highest diversity of AIV isolates. A total of 13 different subtypes were detected in 19 samples from this location (H1N2, H2N2, H3N2, H6N6, H7N6, H9N2, H9N5, H9N6, H10N5, H10N6, H12N6, H13N6, and H16N6). H3N5 virus was detected in 50% of passerines testing positive for AIV in the Parizske Wetlands, with H7N2, H9N2, H9N5, H12N1, and H13N2 infections also recorded at this locality. H9N5 virus predominated in passerines captured at Trnava Ponds, with isolates H1N6, H6N5, H7N2, H7N6, H10N3, and H10N6 also detected at this location. There were five cases where different AIV infections were detected in oropharyngeal and cloacal samples originating from the same bird (H13N6 and H1N2; H10N5 and H12N6; H9N5 and H6N5; H10N6 and H7N6; and H9N2 and H3N5 in the oropharynx and cloaca, respectively). Between 21% and 52% of captured birds tested positive for Borrelia burgdorferi sensu lato, with the proportion infected depending on bird species and locality. Samples were characterized by polymerase chain reaction-restriction fragment length polymorphism analysis and identified as Borrelia garinii species (either B/B′ or R/R′ pattern). Mycobacteria were detected in 42% and 26% of waders captured at Senianske Ponds and marsh-dwelling passerines captured in the Parizske Wetlands, respectively. Interestingly, forest-dwelling passerine species caught in the Trnava Ponds region were tested negative for Mycobacteria.
Introduction
There is ongoing surveillance of the infections carried by wild birds across Europe, with birds known to harbour numerous pathogens including influenza A virus, Lyme disease and paratuberculosis. It is important to gain an understanding of the natural reservoirs of these infections, considering their potential impact on human health—with interspecies transmission events into mammals and domestic birds well known. Many migratory birds fly across Slovakia and may be carrying pathogens with them, as well as dispersing arthropod vectors.
Influenza A has been isolated from a wide variety of mammalian hosts (including humans, pigs, horses, cats, mink and marine mammals) as well as from birds. All 16 haemagglutinin (HA) and nine neuraminidase (NA) subtypes of influenza A are known to infect wild waterfowl, resulting in an extensive reservoir of influenza viruses circulating in bird populations. While all birds are thought to be susceptible to infection with avian influenza viruses (AIV), many wild bird species carry these viruses with no apparent signs of disease (Webster et al., Citation1992). Transmission of AIV between susceptible birds mainly occurs through direct contact with infective secretions and excretions, particularly faeces. Ecological studies have established that all known influenza A viruses are derived from strains circulating within wild birds, where they usually appear to be non-pathogenic (Webster et al., Citation1992). Transmission of AIV from wild birds can cause disease in domestic poultry. Transfer of gene segments from avian to human viruses by reassortment has resulted in at least two human pandemics (Alexander, Citation2007).
Lyme borreliosis, or Lyme disease, is a multiphase, multisystem disease characterized by dermatological, musculoskeletal, and neurological manifestations. This illness is caused by spirochete bacteria, Borrelia burgdorferi sensu lato, which are transmitted to humans through the bite of Ixodes ticks. Small rodents, insectivores, hares, and birds are all known reservoirs for these bacteria. Eleven Borrelia species have been described within the B. burgdorferi s.l. complex worldwide (Baranton et al., Citation1992; Canica et al., Citation1993; Kawabata et al., Citation1994; Le Fleche et al., Citation1997) and, of these, five (B. burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii, Borrelia valaisiana, and Borrelia lusitaniae) have been recognized in Europe (Wang et al., Citation1999). Birds play an important role in the transmission of Lyme borreliosis, as carriers of the arthropod vectors of Borrelia (Gern & Humair, Citation2002; Hubalek, 2005).
Mycobacterium is a genus of Actinobacteria, classified into the family Mycobacteriaceae. Genus Mycobacterium includes a number of pathogens known to cause serious disease in mammals, including both tuberculosis and leprosy. Mycobacteria are widespread organisms, found in water (they are known to contaminate drinking water treated with chlorine), food sources (including milk) and soil. Mycobacterium avium subspecies paratuberculosis (MAP) is a pathogenic subspecies of this genus (Corn et al., Citation2005)—a multi-host pathogen capable of initiating and maintaining systemic infection and chronic inflammation of the intestine in many animal species including domestic ruminants, primates and birds. This subspecies causes animal paratuberculosis and may have a role in the aetiology of Crohn's disease.
The aim of the present study was to assess the prevalence and patterns of distribution of AIV, B. burgdorferi s.l., and Mycobacteria in commonly occurring wild waterfowl and terrestrial birds in eastern and western areas of Slovakia.
Materials and Methods
Collection of samples
In April 2006, cloacal and oropharyngeal swabs were collected from migratory waterfowl from the Senianske rybniky (Senianske ponds) National Nature Reserve in Eastern Slovakia (48°42′ N, 22°02′ E). This site is designated as an Important Bird Area. In June and October 2006 the swabs were collected from birds trapped in the Parizske Mociare (Parizske wetlands) and Trnava ponds (48°32′ N, 17°51′ E) areas of western Slovakia. The Parizske wetlands National Nature Reserve is one of the largest marshes in West Slovakia, and is located near the villages of Gbelce and Nova Vieska (47°51′ N, 18°29′ E). All samples were transported to the laboratory and immediately frozen at −70°C until analysis. Most of the birds looked healthy, without any sign of disease. No birds were harmed or killed during trapping and sampling.
RNA extraction and detection of AIV by nested reverse transcriptase-polymerase chain reaction
Each swab was extracted in 3 ml phosphate-buffered saline, and RNA within 100 µl aliquots of these extracts was purified using an RNeasy Mini Kit (Qiagen). cDNA was synthesized from purified RNA by reverse transcription using random oligonucleotide primers. Nested polymerase chain reaction (PCR) was performed using primers against the conserved region of the M gene, as described previously by Betakova et al. (Citation2005) and Gronesova et al. (Citation2007).
Determination of HA and NA subtypes by nested PCR
AIV-positive samples were used in nested PCR reactions, employing primers specific to each HA and NA subtype. Primer sequences are available upon request.
DNA extraction
One hundred microlitres of each phosphate-buffered saline extract (mentioned above) was subjected to DNA purification, using a DNeasy Mini kit (Qiagen). Extracted DNA was stored at −20°C.
Detection of Borrelia and Mycobacteria by PCR
The 2×PCR Master Mix (Fermentas) was used for all PCR reactions. Primers specific for all Borrelia species, together with PCR conditions, have been described previously by Kurtenbach et al. (Citation1998). Primers specific for Mycobacteria were described previously by Telenti et al. (Citation1993). Birds were screened for MAP using primers IS900A and IS900B previously described by Overduin et al. (Citation2004). All specific PCR products were separated by electrophoresis through 2% agarose gels and were visualized by staining with ethidium bromide.
PCR and restriction fragment length polymorphism analysis
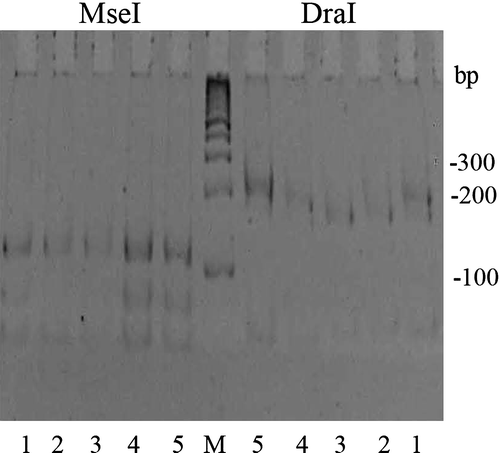
All samples testing positive for Borrelia were further characterized by 5S-23S rRNA integric spacer restriction fragment length polymorphism (RFLP) analysis. Primers and conditions have been described previously by Postic et al. (Citation1994) and Ishiguro et al. (Citation2000). PCR products were digested with MseI (pattern B or R) and DraI (pattern B′ or R′), according to the manufacturer's instructions (Fermentas, Merck). Digested DNA was electrophoresed through 16% polyacrylamide gels and stained with ethidium bromide. A 100-base-pair DNA ladder (Promega) was used as a molecular size marker.
Results
Bird sampling
A total of 109 waterfowl and terrestrial birds was caught in two designated Ramsar Sites (the National Nature Reserves at Senianske ponds and Parizske wetlands) and a third protected area (Trnava ponds).
In April 2006, we captured birds in the Senianske ponds area of eastern Slovakia, obtaining samples from 33 birds of 13 common species belonging to three distinct orders (). Of the Charadriiformes sampled, four species were strict migrants that do not nest in this territory (Ruff, Spotted Redshank, Greenshank, and Jack Snipe). The other species were migrants nesting in this locality.
Table 1. Detection of Borrelia, M. avium/MAP, and AIV in samples obtained from birds captured at Senianske ponds during April 2006
In June 2006 we caught 42 birds in Parizske wetlands, with representatives of 11 common species, all belonging to the order Passeriformes (). Nearly all were migrant's, aquatic birds (warblers), although some were forest-dwelling species (Chiffchaff, Blue Tit, and Starling) and swallows are synanthropic.
Table 2. Detection of Borrelia, M. avium/MAP, and AIV in samples obtained from birds captured at Parizske mociare during June 2006
In October 2006 a total of 34 birds were caught at Trnava ponds, all of which belonged to the order Passeriformes ( ). Of the 10 species sampled, only the European Tree Sparrow was local to the area, and over-winters there. The other species were forest-dwelling migrants, with the exception of the Black Redstart (a synanthropic migrant).
Table 3. Detection of Borrelia, M. avium/MAP, and AIV in samples from birds captured at Trnava ponds during October 2006
Detection of AIV
PCR screening of samples obtained from birds captured at all three localities in Slovakia revealed that 26% to 57% were positive for AIV (, 2, and 3), with the highest proportion of infected birds being seen at Senianske ponds and the lowest at Parizske wetlands. Infections were detected either in oropharyngeal or cloacal samples, or in both.
The highest diversity of HA–NA subtypes was found in birds sampled from Senianske ponds in eastern Slovakia. A total of 13 different viral subtypes were identified in 19 AIV-positive samples from this location, with 13 (39%) oropharyngeal samples and 11 (33%) cloacal samples determined as AIV-positive (). Samples from both the oropharynx and cloaca were positive in only five birds (15%). In most cases, the same virus was identified in both the oropharynx and cloaca. However, there were two examples where different viruses were found in the two anatomical sites of one bird. One of the 11 captured Black-Headed Gulls was demonstrated to harbour subtype H13N6 in its oropharynx and H1N2 in its cloaca, while the only Spotted Redshank captured at this location tested positive for H10N5 in its oropharynx and H12N6 in its cloaca.
Looking at the samples from all 33 infected birds captured at Senianske ponds, AIV subtype H3N2 was detected in four birds (Ruff [two individuals], Jack Snipe, and Cuckoo), while H7N6 and H9N5 were both detected in three birds (Ruff [two individuals] and Black-Headed Gull; and Ruff, European Curlew, and Black-Headed Gull, respectively), and subtype H12N6 was identified in two birds (Ruff and Spotted Redshank). Nine further AIV subtypes were detected in only one individual bird: H1N2 (Black-Headed Gull), H2N2 (Black-Headed Gull), H6N6 (Ruff), H9N6 (Greenshank), H10N5 (Spotted Redshank), H10N6 (European Curlew), H12N6 (Spotted Redshank), H13N6 (Black-Headed Gull), and H16N6 (Black-Headed Gull).
AIV subtype H3N5 was detected in nearly 50% of all positive samples derived from birds caught in western Slovakia (Parizske wetlands) during June 2006 (). Only 11 of 42 birds (26%) were positive for AIV—the lowest proportion of infections in any of the three locations. Nine oropharyngeal samples (21%) and six cloacal samples (14%) were observed to be AIV-positive. There was one example of where different viruses were found in the two anatomical sites of one bird at this location: a Reed Bunting tested positive for subtype H9N2 from its oropharyngeal swab and for H3N5 from the cloacal swab. H9N5 was detected in two birds (Reed Warbler and Swallow), while four other subtypes were recorded in only one individual bird: H7N2 (Reed Bunting), H9N2 (Reed Bunting), H12N1 (Moustached Warbler), and H13N2 (Blue Tit).
A larger number of AIV-positive passerines were observed in the Trnava ponds area of western Slovakia in October 2006. A total of 44% of birds (15 of the 34 captured at this location) tested AIV-positive, with 32% of oropharyngeal swabs testing positive and 26% of cloacal swabs. Six of the birds (40%) testing AIV-positive were infected with subgroup H9N5 (Blackcap [two individuals], Robin, Blue Tit, Chiffchaff, and Black Redstart; ). Subtype H10N6 was detected in two birds (Dunnock and European Tree Sparrow), while five subtypes were detected in just one individual bird: H1N6 (European Tree Sparrow), H7N2 (European Tree Sparrow), H7N6 (Dunnock), H10N3 (Blackcap), and H12N5 (Blackcap). In this location, two birds were shown to harbour different AIV subtypes in the two anatomical sites sampled. The only captured Chiffchaff had an H9N5-positive oropharyngeal swab and an H6N5-positive cloacal swab, while one Dunnock tested positive for H10N6 in its oropharynx and H7N6 in its cloaca. Despite all efforts, the HA or NA subtypes of viruses detected in three birds could not be identified from this locality.
Detection of Borrelia
Oropharyngeal and cloacal samples were analysed by PCR using two sets of primers (refer to Materials and Methods). The first set of primers was used for PCR detection of Borrelia (Kurtenbach et al., Citation1998). The PCR products obtained from positive samples with a second set of primers were used in RFLP analyses (Postic et al., Citation1994; Ishiguro et al., Citation2000). A total of seven birds (21%) captured at Senianske ponds (eastern Slovakia) were positive for Borrelia (). The prevalence of Borrelia was higher in western Slovakia, with 35% to 52% of samples positive for this spirochete in the Parizske wetlands and Trnava ponds areas, respectively ( and 3).
All PCR products were sequenced, and our samples were found to contain sequences showing very high nucleotide identity to one another (95% to 98%). The sequenced fragments were small (377 base pairs), and it proved impossible to identify the species of Borrelia within each sample from this amount of sequence. To characterize the genospecies present, we performed RFLP analyses.
Digestion with restriction enzymes MseI and DraI yielded patterns corresponding to genospecies B. garinii (), according to the patterning illustrated by Ishiguro et al. (Citation2000). MseI/DraI digestion of DNA from spirochetes harboured by birds captured in this study revealed both B/B′ and R/R′ patterns. Most samples gave an RFLP pattern identical to the B/B′ pattern seen for a French isolate (20047) presented in the Ishiguro et al. study (, samples 2 and 3). However, RFLP analysis of spirochetes from two birds caught at Senianske ponds (Black-Headed Gull, Ruff) yielded the R/R′ pattern seen previously in a Chinese isolate (ChY13p) (Ishiguro et al., Citation2000) (, samples 4 and 5). One Black-Headed Gull was co-infected with both strains, such that RFLP for this sample yielded a mix of the B/B′ and R/R′ patterns (, sample 1).
Figure 1. Representative RFLP patterns of the 5S to 23S rRNA integric spacer observed among Borrelia samples. PCR products were digested with MseI or DraI. Numbers, samples 1 to 5; M, molecular size standard with the sizes (base pairs) indicated on the right of the gel. Sample 1, mixed patterns B/B′ (•) and R/R′ (*); samples 2 and 3, pattern B/B′; and samples 4 and 5, pattern R/R′.
Detection of Mycobacteria
Neither M. avium nor MAP was detected in any of the samples obtained from birds captured in the Trnava ponds region of western Slovakia in autumn 2006 (). However, a high prevalence of M. avium was noted in birds caught in Senianske ponds in East Slovakia 6 months earlier (). A total of 42% of samples from birds caught at this location in April 2006 were positive for M. avium and 18% were positive for MAP (). In the Parizske wetlands area of western Slovakia, 26% of birds captured in June 2006 were M. avium-positive and 5% were MAP-positive (). The PCR products obtained were not further investigated.
Discussion
The present study is part of ongoing wild bird surveillance across Europe. Recent improvements in molecular diagnostic tests have facilitated high-throughput screening of wild birds for AIV and other pathogens.
We confirmed a high prevalence of AIV in waterfowl and terrestrial birds in Slovakia. Our results are not consistent with most AIV studies. AIV and subtypes were detected with a nested PCR, a method that is highly prone to laboratory contamination issues. However, we can exclude the contamination of our samples for several reasons: the samples were collected in three different localities, the samples were collected at different times, the samples were not tested in one laboratory at one time, the negative controls were always negative, and the determined subtypes of HA and NA were not the same. It has been already published that the molecular screening detected positive birds at a much higher rate than viral isolation (Runstadler et al., Citation2007). The prevalence of AIV in passerines (based on virus isolation) has previously been reported as being particularly low (Morishita et al., Citation1999; Fouchier et al., Citation2003; Schnebel et al., Citation2005; Lebarbenchon et al., Citation2007), although use of nested reverse transcriptase-PCR may have increased the sensitivity of virus detection (Gronesova et al., Citation2007; Mizakova et al., Citation2008). The prevalence of virus was clearly highest in samples derived from aquatic bird species captured at Senianske ponds in spring 2006 (57%), and lowest in samples obtained from passerines in the Parizske wetlands area in early summer (26%). However, a reasonably high prevalence was noted in passerines caught at Trnava ponds in the autumn of that year (44% harboured one or more AIV infections). While we confirmed previous findings that the prevalence of AIV was higher in waterfowl than in terrestrial birds, AIV was still detected in quite a large number of passerine species. While we detected an unusually high AIV prevalence in these passerines, van der Goot et al. (Citation2007) pointed out that there are many factors that can affect detection, including incidence of concurrent infections, density of birds, species and age heterogeneity of birds at a site, climate, sensitivity of methods used to screen samples, and number and type of samples taken. Our finding could not, therefore, simply be interpreted as a rise in AIV prevalence in passerines.
The highest diversity of AIV isolates was identified in waterfowl from the Senianske ponds area. Ten different subtypes of HA (H1, H2, H3, H6, H7, H9, H10, H12, H13, and H16) and three different subtypes of NA (N2, N5, and N6) were detected in samples from this location. Viruses detected in passerines did not show such high diversity, with subtype H3N5 predominating in samples from passerines captured at Parizske marsh and subtype H9N5 predominating in samples from species caught at Trnava ponds.
The relatively low diversity of influenza viruses observed in passerines can be explained partly by the low number of birds captured and screened, and partly by the fact that these bird species were actually nesting in the area. Birds nesting at the same locality would be exposed to any viruses circulating within local bird populations sharing their environment. Thus many birds could be predicted to share common, local infections.
The absence of subtypes H14 and H15 in our samples was probably due to geographical separation of the birds captured in this study and the Australian and Russian ducks and pelagic birds known to carry these infections (Kawaoka et al., Citation1990; Rohm et al., Citation1996). The isolation of H13 and H16 subtypes from the only gull species we sampled (and from none of the other species captured) lends support to the common notion that these subtypes belong to a “gull lineage”. H13 and H16 viruses are genetically distinct from other AIV viruses and seem to be particularly adapted to replication within gull hosts (Kawaoka et al., Citation1988; Hinshaw et al., Citation1982; Munster et al., Citation2007). It should be noted, however, that we also detected subtypes H1N2, H2N2, H7N6, and H9N6 in samples derived from Black-Headed Gulls, meaning that gulls can be infected by, and spread, the other subtypes that co-circulate in environments they occupy.
Most interestingly, we found a number of cases where two different influenza subtypes were present within one bird. This was not uncommon, with five of 109 birds screened displaying this phenomenon, including one or more case at each of the three locations surveyed. Co-infection of this type is perhaps not surprising, since long-term shedding of virus from the gastrointestinal and respiratory tracts of infected birds is well documented (Humberd et al., Citation2006) and birds living/nesting close together are likely to be exposed to many subtypes. Birds shed AIV for many days post infection (up to 45 days in rare cases)—even while lacking overt clinical signs—and aquatic birds are prone to the high concentrations of virus excreted, through faeces, into lake water (Humberd et al., Citation2006; van der Goot et al., Citation2007).
Harbouring one AIV infection does not seem to preclude infection with another AIV, with a reservoir of different subtypes co-circulating at high titres in the environment. With co-infected birds identified in this study harbouring different AIV subtypes in separate anatomical sites (one being excreted from the oropharynx and one from the cloaca), we assume that these birds were infected with the two subtypes at different times. Co-infected birds serve as a mixing vessel for AIV, providing optimal conditions for reassortment of virus, such that novel influenza viruses can arise (antigenic shift).
The majority of studies on Lyme Borreliosis focus on the detection and characterization of Borrelia genospecies present within ticks. Arthropod-borne B. burgdorferi s.l. species have been linked with human disease (neuroborreliosis) acquired from wild birds (Rand et al., Citation1998; Daszak et al., Citation2000; Humair, Citation2002). In most parts of Central Europe, B. garinii, B. valaisiana and B. afzelii are the most frequently encountered genospecies, with B. garinii and B. valaisiana frequently associated with songbirds, seabirds and pheasants, while some data indicate that B. afzelii does not use these avian reservoir hosts but is typically associated with rodents (Kurtenbach et al., Citation2002). However, in 2004 Hubalek observed that B. afzelii appeared to be one of the most abundant pathogens carried by migratory birds in continental Europe. Strains of B. burgdorferi s.s. appear to be much less specialized than strains of the other genospecies.
RFLP analysis is a commonly used method for distinguishing between different genospecies of Borrelia spirochetes (Postic et al., Citation1994; Ishiguro et al., Citation2000; Derdakova et al., Citation2003; Pejchalova et al., Citation2007). We located a number of sequences from different species of Borrelia in the GenBank database, and alignments and analyses were carried out using Vector NTI software (Invitrogen). The virtual image patterns obtained from analysis using B. garinii sequences corresponded to the actual patterns observed with our samples. We also compared the patterns from our samples with those published by other authors and confirmed that they all corresponded to B. garinii (Ishiguro et al., Citation2000).
Digestion with MseI and DraI revealed that our B. garinii spirochetes yielded patterns B/B′ and R/R′. RFLP pattern B is common in Borrelia from adult ixodid ticks of the species Ixodes persulcatus in Euroasia, and Ixodes ricinus in Europe (Postic et al., Citation1994; Masuzawa et al., Citation1996) as well as in juvenile ticks (I. persulcatus) removed from birds surveyed in Europe and far-Eastern Asia (Nakao et al., Citation1994). With this distribution already established, we were not surprised to see that this strain was prevalent in Slovakia. However, pattern R B. garinii has been observed previously in samples from Korea, China, and Japan (Ishiguro et al., Citation2000), but has never been described in Europe.
Screening of the 109 birds captured during this study identified three birds from eastern Slovakia carrying spirochetes with the R/R′ pattern (Black-Headed Gull [tw o individuals] and Ruff). One Gull was co-infected with both B/B′ and R/R′ pattern B. garinii. Black-Headed Gulls are local to Senianske ponds, while Ruffs are migratory, not nesting in our territory but often visiting this area of eastern Slovakia. Migratory birds carrying new strains of Borrelia could transmit them to species local to this territory (and vice versa), which may be how Black-Headed Gulls came to be carrying R/R′ pattern B. garinii. Because of the small number of samples screened in this study, further investigation of ticks and birds in this area must be undertaken in order to establish the full extent of the diversity of Borrelia strains present.
M. avium subsp. paratuberculosis has been found in a number of species of free-ranging wild birds that live in close association with domestic stock, including sparrows, starlings, and also scavenging birds such as corvids, pigeons and gulls (Bougiouklis et al., Citation2005; Corn et al., Citation2005). A decade-long study of nearly 12 000 wild birds necropsied in the Netherlands revealed that 0.7% had tuberculosis (Smith et al., Citation1987). The birds sampled included waterfowl, birds of prey, songbirds and pheasants.
Our study shows a relatively high prevalence of M. avium and MAP among migratory birds, especially waterfowl, resting or living in eastern Slovakia (). We found that over 40% of birds captured in the Senianske ponds area carried M. avium, while nearly 20% tested positive for M. avium subspecies paratuberculosis. The six birds carrying tuberculosis were two Black-Headed Gulls, two European Curlews, a Cuckoo and a Ruff. While no birds were found to carry M. avium or MAP at Trnava ponds, 26% of those captured in the Parizske wetlands area of western Slovakia carried M. avium, while 5% (two passerines: Starling and Savi's Warbler) screened positive for subspecies paratuberculosis (). These results suggest that waterfowl quite commonly carry tuberculosis in Slovakia, and this will need to be monitored as it could present a serious problem in the future.
Surveillance is a useful tool for detecting the occurrence of pathogens in wild birds. However, it has limitations. All studies are prone to sample sizes that are small in relation to the true wild bird population and therefore unlikely to provide an accurate estimate of prevalence. While our results provide an interesting indication of the levels at which wild birds carry pathogenic infections, further research at these—and other—Slovak locations will be necessary to explore geographic variations in the infections birds carry, as well as the relative roles of passerines and waterfowl as reservoirs for these pathogens.
Acknowledgements
The authors wish to express special thanks to Dipl. Ing. Jan Liptak and Dipl. Ing. Darina Svetlikova for excellent assistance, and to Dr Joanne Martin for editing of this manuscript. This research was supported by the Slovak Research and Development Agency (Grant No. APVV-51-004105).
References
- Alexander , D.J. 2007 . Summary of avian influenza activity in Europe, Asia, Africa, and Australia, 2002–2006 . Avian Diseases , 51 : 161 – 166 .
- Baranton , G. , Postic , D. , Saint Girons , I. , Boerlin , P. , Piffaretti , J.C. , Assous , M. and Grimont , P.A. 1992 . Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis . International Journal of Systematic Bacteriology , 42 : 378 – 383 .
- Betakova , T. , Marcin , J. , Kollerova , E. , Molcanyi , T. , Dravecky , M. , Nemeth , J. and Mizakova , A. 2005 . Detection of an influenza virus in wild waterbirds migrating through Slovakia in autumn 2004 . Acta Virologica , 49 : 287 – 289 .
- Bougiouklis , P. , Brellou , G. , Fragkiadaki , E. , Iordanidis , P. , Vlemmas , I. and Georgopoulou , I. 2005 . Outbreak of avian mycobacteriosis in a flock of two-year-old domestic pigeons (Columba livia f. domestica) . Avian Diseases , 49 : 442 – 445 .
- Canica , M.M. , Nato , F. , DuMerle , L. , Mazie , J.C. , Baranton , G. and Postic , D. 1993 . Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis . Scandinavian Journal of Infectious Diseases , 25 : 441 – 448 .
- Corn , J.L. , Manning , E.J. , Sreevatsan , S. and Fischer , J.R. 2005 . Isolation of Mycobacterium avium subsp. paratuberculosis from free-ranging birds and mammals on livestock premises . Applied and Environmental Microbiology , 71 : 6963 – 6967 .
- Daszak , P. , Cunningham , A.A. and Hyatt , A.D. 2000 . Emerging infectious diseases of wildlife-threats to biodiversity and human health . Science , 287 : 443 – 449 .
- Derdakova , M. , Beati , L. , Petko , B. , Stanko , M. and Fish , D. 2003 . Genetic variability within Borrelia burgdorferi sensu lato genospecies established by PCR-single-strand conformation polymorphism analysis of the rrfA-rrlB intergenic spacer in Ixodes ricinus ticks from the Czech Republic . Applied and Environmental Microbiology , 69 : 509 – 516 .
- Fouchier , R.A. , Olsen , B. , Bestebroer , T.M. , Herfst , S. , van der Kemp , L. , Rimmelzwaan , G.F. and Osterhaus , A. 2003 . Influenza A virus surveillance in wild birds in Northern Europe . Avian Diseases , 47 : 857 – 860 .
- Gern , L. and Humair , P.F. 2002 . “ Ecology of Borrelia burgdorferi sensu lato in Europe ” . In Lyme Borreliosis: Biology, Epidemiology and Control , Edited by: Gray , J. 149 – 174 . Wallingford , Oxon : CAB International .
- Gronesova , P. , Mizakova , A. , Kabat , P. , Trnka , A. , Svetlikova , D. and Betakova , T. 2007 . Detection of influenza A virus in wild birds in West Slovakia by nested PCR . Acta Virologica , 51 : 63 – 65 .
- Hinshaw , V.S. , Air , G.M. , Gibbs , A.J. , Graves , L. , Prescott , B. and Karunakaran , D. 1982 . Antigenic and genetic characterization of a novel hemagglutinin subtype of influenza A viruses from gulls . Journal of Virology , 42 : 865 – 872 .
- Hubalek , Z. 2004 . An annotated checklist of pathogenic microorganisms associated with migratory birds . Journal of Wildlife Diseases , 40 : 639 – 659 .
- Humair , P.F. 2002 . Birds and Borrelia . International Journal of Medical Microbiology , 291 : 70 – 74 .
- Humberd , J. , Guan , Y. and Webster , R.G. 2006 . Comparison in the replication of influenza A viruses in Chinese ring-necked pheasants and chukar partridges . Journal of Virology , 80 : 2151 – 2161 .
- Ishiguro , F. , Takada , N. , Masuzawa , T. and Fukui , T. 2000 . Prevalence of Lyme disease Borrelia spp. in ticks from migratory birds on the Japanese mainland . Applied and Environmental Microbiology , 66 : 982 – 986 .
- Kawabata , H. , Tashibu , H. , Yamada , K. , Masuzawa , T. and Yanagihara , Y. 1994 . Polymerase chain reaction analysis of Borrelia species isolated in Japan . Microbiology and Immunology , 38 : 591 – 598 .
- Kawaoka , Y. , Chambers , T.M. , Sladen , W.L. and Webster , R.G. 1988 . Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? . Virology , 163 : 247 – 250 .
- Kawaoka , Y. , Yamnikova , S. , Chambers , T.M. , Lvov , D.K. and Webster , R.G. 1990 . Molecular characterization of a new hemagglutinin, subtype H14, of influenza A virus . Virology , 179 : 759 – 767 .
- Kurtenbach , K. , Peacey , M. , Rijpkema , S.G. , Hoodless , A.N. , Nuttall , P.A. and Randolph , S.E. 1998 . Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England . Applied and Environmental Microbiology , 64 : 1169 – 1174 .
- Kurtenbach , K. , De Michelis , S. , Etti , S. , Schafer , S.M. , Sewell , H.S. , Brade , V. and Kraiczy , P. 2002 . Host association of Borrelia burgdorferi sensu lato-the key role of host complement . Trends in Microbiology , 10 : 74 – 79 .
- Lebarbenchon , C. , Chang Ch , M. , van der Werf , S. , Aubin , J.T. , Kayser , Y. , Bellesteros , M. , Renaud , F. , Thomas , F. and Gauthier-Clerc , M. 2007 . Influenza A virus in birds during spring migration in the Camargue, France . Journal of Wildlife Diseases , 43 ( 4 ) : 789 – 793 .
- Le Fleche , A. , Postic , D. , Girardet , K. , Peter , O. and Baranton , G. 1997 . Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis . International Journal of Systematic Bacteriology , 47 : 921 – 925 .
- Masuzawa , T. , Komikado , T. , Iwaki , A. , Suzuki , H. and Yanagihara , Y. 1996 . Characterization of Borrelia sp. isolated from Ixodes tanuki, I. turdus, and I. columnae in Japan by restriction fragment length polymorphism of rrf(5S)-rrl(23S) intergenic spacer amplicon . FEMS Microbiology letters , 142 : 77 – 83 .
- Mizakova , A. , Gronesova , P. and Betakova , T. 2008 . Monitoring of avian influenza viruses in waterfowls and terrestrial birds in eastern Slovakia . Acta Virologica , 52 : 71 – 73 .
- Morishita , T.Y. , Aye , P.P. , Ley , E.C. and Harr , B.S. 1999 . Survey of pathogens and blood parasites in free living passerines . Avian Diseases , 43 : 549 – 552 .
- Munster , V.J. , Baas , C.H. , Lexmond , P. , Waldenstrom , J. , Wallensten , A. , Fransson , T. , Rimmelzwaan , G.F. , Beyer , W.E.P. , Schutten , M. , Olsen , B. and Osterhaus , A.D.M.E. 2007 . Spatial, temporal, and species variation in prevalence of influenza A virus in wild migratory birds . PLOS Pathogens , 3 : 630 – 638 .
- Nakao , M. , Miyamoto , K. and Fukunada , M. 1994 . Lyme disease spirochetes in Japan: enzootic transmission cycles in birds, rodents, and Ixodes persulcatus ticks . Journal of Infectious Diseases , 170 : 878 – 882 .
- Overduin , P. , Schouls , L. , Roholl , P. , van der Zanden , A. , Mahmmod , N. , Herrewegh , A. and van Soolingen , D. 2004 . Use of multilocus variable-number tandem-repeat analysis for typing Mycobacterium avium subsp. Paratuberculosis . Journal of Clinical Microbiology , 42 : 5022 – 5028 .
- Pejchalova , K. , Zakovska , A. , Mejzlikova , M. , Halouzka , J. and Dendis , M. 2007 . Isolation, cultivation and identification of Borrelia burgdorferi genospecies from Ixodes ricinus ticks from the city of Brno, Czech Republic . Annals of Agricultural and Environmental Medicine , 14 : 75 – 79 .
- Postic , D. , Assous , M.V. , Grimont , P.A. and Baranton , G. 1994 . Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons . International Journal of Systematical Bacteriology , 44 : 743 – 752 .
- Rand , P. W. , Lacombe , E.H. , Smith , R.P. Jr and Ficker , J. 1998 . Participation of birds (Aves) in the emergence of Lyme disease in southern Maine . Journal of Medical Entomology , 35 : 270 – 276 .
- Rohm , C. , Zhou , N. , Suss , J. , Mackenzie , J. and Webster , R.G. 1996 . Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes . Virology , 217 : 508 – 516 .
- Runstadler , J.A. , Happ , G.M. , Slemons , R.D. , Sheng , Z.M. , Gundlach , N. , Petrula , M. , Senne , D. , Nolting , J. , Evers , D.L. , Modrell , A. , Huson , H. , Hills , S. , Rothe , T. , Marr , T. and Taubenberger , J.K. 2007 . Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks at Minto Flats State Game Refuge, Alaska, during August 2005 . Archives of Virology , 152 : 1901 – 1910 .
- Schnebel , B. , Dierschke , V. , Rautenschlein , S. and Ryll , M. 2005 . No detection of avian influenza a viruses of the subtypes H5 and H7 and isolation of lentogenic avian paramyxovirus serotype in passerine birds during stopover in the year 2001 on the island Helgoland (North Sea) . Deutche Tierzliche Wochenschrift , 112 : 456 – 460 .
- Smith , T. , Eger , A. , Haagsma , J. and Bakhuisen , T. 1987 . Avian tuberculosis in wild birds in the Netherlands . Journal of Wildlife Diseases , 23 : 485 – 487 .
- Telenti , A. , Imboden , P. , Marchesi , F. , Schmidheini , T. and Bodmer , T. 1993 . Direct, automated detection of rifampin-resistant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis . Antimicrobial Agents and Chemotherapy , 37 : 2054 – 2058 .
- van der Goot , J.A. , van Boven , M. , Koch , G. and de Jong , M.C.M. 2007 . Variable effect of vaccination against highly pathogenic avian influenza (H7N7) virus on disease and transmission in pheasant and teals . Vaccine , 25 : 8318 – 8325 .
- Wang , G. , van Dam , A.P , Schwartz , I. and Dankert , J. 1999 . Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications . Clinical Microbiology Reviews , 12 : 633 – 653 .
- Webster , R.G. , Bean , W.J. , Gorman , O.T. , Chambers , T.M. and Kawaoka , Y. 1992 . Evolution and ecology of influenza A viruses . Microbiological Reviews , 56 : 152 – 179 .