Abstract
Gallibacterium anatis biovar haemolytica has been suggested to have a causal role in peritonitis and salpingitis in chickens. Therefore, the aim of this study was to investigate the occurrence of G. anatis biovar haemolytica in chickens with reproductive disorders. One hundred and forty one birds from 31 layer flocks were submitted for necropsy and the following organs were examined for bacteria: choana, trachea, lung, heart, liver, spleen, ovary, oviduct, duodenum and cloaca. Examination for Escherichia coli was included as it can induce reproductive disorders. G. anatis was isolated in pure culture from the reproductive tract of affected birds in six of the 31 flocks while E. coli was obtained in pure culture from 10 of them. Both G. anatis and E. coli were isolated from the reproductive tract of 14 of the 31 flocks. The genetic diversity of the Gallibacterium isolates was assessed by amplified fragment length polymorphism on a subset of 83 isolates. Generally, each flock was infected with a single clone, which could be isolated from various sites in the birds. However, in two flocks, the majority of birds yielded positive samples from the internal organs, indicating that these particular clones may be more invasive. The findings support previous suggestions that G. anatis biovar haemolytica is associated with infection of the reproductive tract of chickens, making it a likely cause of lowered productivity and an animal welfare concern.
Introduction
The genus Gallibacterium was recently established within the family of Pasteurellaceae Pohl 1981 (Christensen et al., Citation2003). The genus contains avian bacteria formerly known as Pasteurella haemolytica, Actinobacillus salpingitidis or Pasteurella anatis, and currently includes the species Gallibacterium anatis and Gallibacterium genomospecies 1 and 2. Two biovars are described within G. anatis, a haemolytic biovar haemolytica and a non-haemolytic, biovar anatis. Gallibacterium spp. can be isolated from a great variety of birds, such as chickens, turkeys, ducks, geese, psittacine birds, partridges and guinea fowl (Harbourne, Citation1962; Hacking & Pettit, Citation1974; Mushin et al., Citation1979; Addo & Mohan, Citation1984; Bisgaard, Citation1993).
Members of the genus Gallibacterium have been isolated from birds with various clinical conditions. In fact, Gallibacterium spp. can be isolated from healthy birds and it has been suggested that these bacteria may be part of the normal microbiota in the upper respiratory and the lower genital tracts (Bisgaard, Citation1977; Mushin et al., Citation1979). Contrary to this, Gallibacterium spp. have been obtained from layers with lesions affecting the reproductive organs including salpingitis and severe drops in egg production (Kohlert, Citation1968; Matthes et al., Citation1969; Hacking & Pettit, Citation1974; Gerlach, Citation1977; Bisgaard & Dam, Citation1981; Mirle et al., Citation1991). Furthermore, a mortality of 73% was noted in experimentally immunosuppressed 15-week-old brown layers after intravenous infection, emphasizing the importance of Gallibacterium spp. as a pathogen (Bojesen et al., Citation2004). In a field study performed in Denmark, Bojesen et al. (Citation2003b) demonstrated that the biosecurity level influenced the prevalence of Gallibacterium spp., with a lower level of biosecurity resulting in a more frequent detection of these pathogens.
In spite of all the experimental and epidemiological data so far reported, no data have been published on the presence of G. anatis in different organs of laying birds showing reproductive disorders. The present study was designed to investigate the prevalence of haemolytic G. anatis in individual birds kept in alternative husbandry systems and suffering from reproductive disorders.
Materials and Methods
Flocks investigated
In total 31 layer flocks were investigated: 14 organic free range flocks (54 birds), six conventional free range flocks (32 birds) and 11 conventional deep litter flocks (55 birds). Data on the size and the age of these flocks are presented in . The flocks were selected on the following criteria: an increase of mortality reported by the farmer (compared with the normal baseline mortality of the flock), a drop in egg production (up to 10% within 1 week), apathy of birds, pasting around the vent, and pathological signs of salpingitis/peritonitis noted during necropsy performed on site. During the site visit the clinical status of the flocks was recorded.
Table 1. Size and age of the flocks investigated
All flocks were vaccinated according to a standard vaccination schedule including vaccinations against coccidia, Salmonella enteritidis, avian metapneumovirus, infectious bronchitis virus, infectious bursal disease virus and Newcastle disease virus. In addition, some flocks were vaccinated against Escherichia coli (eight with the commercially available vaccine Nobilis® E. coli inac., and two with an autogenous vaccine), Pasteurella multocida (one flock with an autogenous vaccine) and Mycoplasma gallisepticum (one flock with the commercially available vaccine Poulvac® MG).
Prior to any therapeutic treatment, three to eight birds per flock were brought to the clinic and killed for necropsy followed by a very detailed sampling regime. The only exception was a group of birds from a single conventional deep litter flock, which were treated with neomycin directly before sampling.
Necropsy findings
The necropsy findings of the birds were recorded according to the following protocol: trachea: haemorrhages; lungs: airsacculitis and oedema/hyperaemia; heart: haemorrhages, hydropericardium and pericarditis; liver: hepatomegaly, haemorrhages, necrosis and perihepatitis; ovary: haemorrhages, atrophy, deformed follicles, broken follicles, regression; oviduct: haemorrhages, non-functional; kidney: renomegaly and haemorrhages; spleen: splenomegaly; and abdominal cavity: peritonitis and ascites.
Bacteriological investigations
Bacteriological investigations were carried out from the following 10 sites: choana, trachea, lung, heart, liver, spleen, ovary, oviduct, duodenum and cloaca. Each sample was plated out directly on a blood agar plate (Columbia agar with 5% sheep blood; BioMeriéux, Vienna, Austria) and a McConkey agar plate (BioMeriéux) for isolation of Gallibacterium spp. and E. coli, respectively. The plates were incubated aerobically at 37°C for 24 h.
Haemolytic Gallibacterium isolates were identified by their growth on blood agar within 24 h, which is characterized as follows: circular, raised colonies with an entire margin, shiny and semi-transparent with a β-haemolytic zone. Such colonies were regarded as suspicious of Gallibacterium. Suspect colonies were subcultured on blood agar to obtain pure cultures. Gram-staining and cytochrome oxidase tests were performed for all isolates.
Up to five E. coli colonies per plate were tested for avian pathogenic serovars O1:K1, O2:K2 and O78:K80 using slide agglutination (Veterinary Laboratories Agency, Surrey, UK). Briefly, one drop of the agglutination sera was mixed with a single E. coli colony on an object slide and swirled for 2 min. The absence/appearance of agglutination with each serovar test sera was recorded.
Extraction of DNA and polymerase chain reaction
The QIAGEN DNEasy Tissue Kit (Qiagen, Hilden, Germany) was used for DNA extraction following the manufacturer's protocol. All isolates suggestive of Gallibacterium spp. were investigated according to Bojesen et al. (Citation2007). Using this polymerase chain reaction (PCR), two fragments of approximately 790 base pairs (bp) and 1080 bp were expected for strains of the genus Gallibacterium. All PCR products were separated by electrophoresis on 2% agarose gels following a standard procedure (Sambrook & Russel, Citation2001).
Amplified fragment length polymorphisms
The genetic diversity of a subset of 83 isolates from 18 flocks was evaluated by amplified fragment length polymorphisms (AFLP) as reported previously (Christensen et al., Citation2003). For each flock, at least one isolate from the respiratory tract and one from the liver, heart, spleen, ovary, oviduct or intestinal tract was chosen. Briefly, the non-selective BglII primer (FAM-5′-GAGTACACTGTCGATCT-3′) and the non-selective BspDI primer (5′-GTGTACTCTAGTCCGAT-3′) were used to amplify the fragments subsequent to restriction digestion and ligation to their corresponding adaptors. All AFLP reactions were repeated at least twice to allow evaluation of the reproducibility of the method.
Amplification products were detected on an ABI 377 automated sequencer (PE Biosystems). Each lane included internal-lane size standards labelled with ROX dye (Applied Biosystems). GeneScan 3.1 fragment analysis software (Applied Biosystems) was used for fragment size determination and pattern analysis. AFLP profiles comprising fragments in the size range 50 to 500 bp were considered for numerical analysis with the software GelCompar II (Applied Maths, Kortrijk, Belgium). Normalized AFLP fingerprints were compared using the Dice similarity coefficient, and clustering analysis was performed by the unweighted pair group method with arithmetic averages.
Results
Flocks investigated
In addition to the criteria applied for selecting flocks, the most frequent additional findings were lack of uniformity (17/31), cannibalism (13/31), shaking heads (10/31) and nasal discharge (5/31).
Necropsy findings
Three to eight birds per farm were investigated, totalling 141 birds. In addition to peritonitis (21%) the main pathological findings were lesions in the reproductive tract, as summarized in . A high percentage of birds demonstrated regression of the ovaries (40%) and a non-functional oviduct (31%). Furthermore, 28% of the birds had deformed follicles, and in 10 birds severe egg concretions were found within the oviduct.
Table 2. Main necropsy findings
Fibrinous perihepatitis and pericarditis were found in only three and two birds, respectively. During necropsy, gastrointestinal helminths (Ascaridia spp.) were found in the small intestine of birds from three organic free range flocks. Many birds (36/141) showed signs of fatty liver syndrome. Severe pecking wounds due to cannibalism, mainly localized around the cloaca, were observed in 63 birds. No lesions were found in the respiratory tract.
Bacteriological investigations
A total of 310 bacterial isolates suggestive of haemolytic G. anatis were obtained. All these demonstrated a wide β-haemolytic zone (1 to 2 mm) as reported by Bisgaard (Citation1982) and Christensen et al. (Citation2003). All were Gram-negative rods that were sometimes pleomorphic, and were cytochrome oxidase-positive.
Data for bacteriological isolation are summarized in . Most of the isolates (161/310) were obtained from the respiratory tract. From the heart, liver, spleen, intestine and reproductive tract, 16, 14, 19, 63 and 37 isolates were found, respectively. In addition to the intestinal tract (244/847), E. coli was obtained mainly from the respiratory tract (274/844) and the reproductive tract (167/844). From the heart, liver and spleen, 55, 53, and 54 isolates were recovered, respectively. Almost one-half of the isolates belonged to avian pathogenic E. coli (APEC) (405/847). In nine flocks E. coli strains with the antigenic profile O1:K1 were observed, while O2:K1 was found in flock numbers 14 and 24 and O78:K80 was found only once in flock number 10. Furthermore, mixed infections with E. coli O1:K1 and O2:K1 were noted in two flocks, and mixed infection of O1:K1 and O78:K80 in one further flock.
Table 3. Isolation of haemolytic G. anatis and E. coli from different organs displayed in connection with the housing system and the number of birds expressing lesions in either the ovaries and/or the oviduct
In the reproductive tract, co-infections of haemolytic G. anatis and E. coli were found in 18 birds from 14 flocks. Haemolytic G. anatis isolates were obtained from eight birds of six flocks and infection of the reproductive tract with only E. coli was seen in 39 birds from 10 flocks. Both E. coli and haemolytic G. anatis were isolated from birds expressing lesions in either the ovaries or the oviduct. However, these microorganisms were also found in birds without lesions in the reproductive tract.
Table 4. E. coli obtained from different organs
Identification of Gallibacterium by PCR
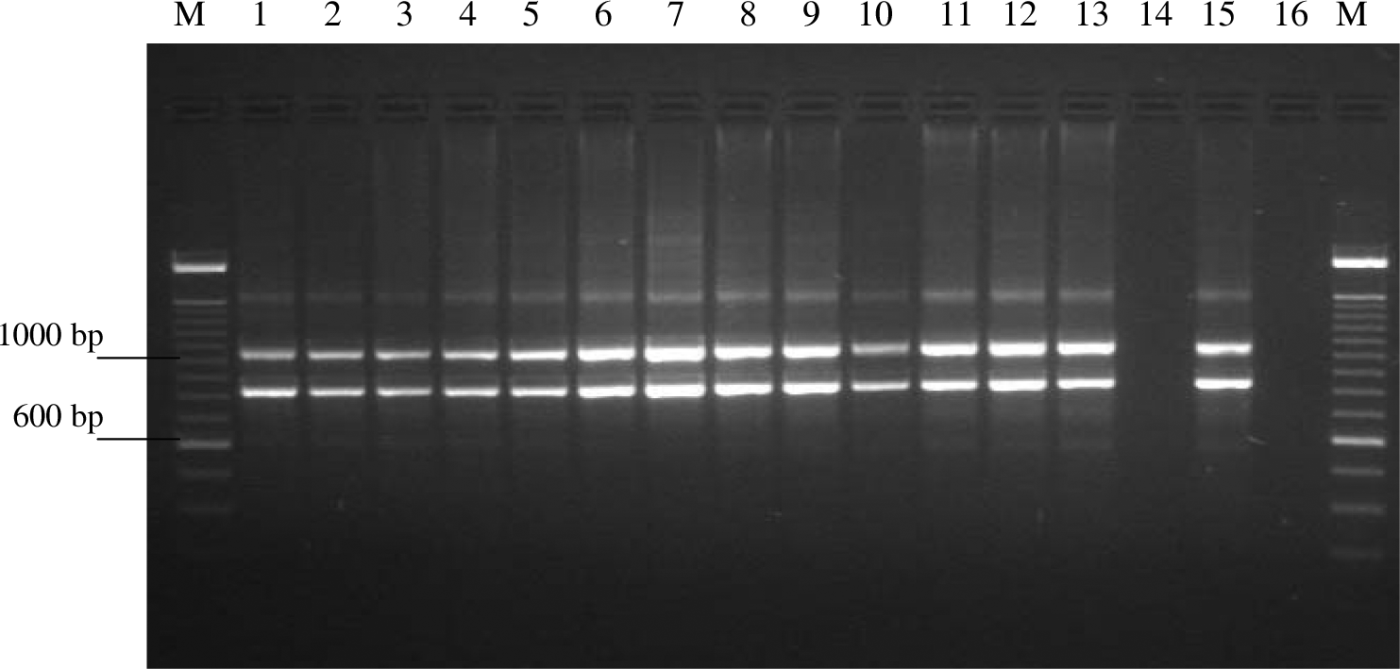
All 310 isolates that were tentatively identified as Gallibacterium were tested with a Gallibacterium-specific PCR, and from all isolates amplicons of approximately 790 bp and 1080 bp were obtained as exemplified in .
Figure 1. Agarose gel electrophoresis of amplification products from haemolytic G. anatis field isolates. Lane M, 100 bp DNA ladder (Invitrogen); lanes 1 and 10, isolates from cloaca; lanes 2, 6, 11 and 12, isolates from choana; lanes 3 and 8, isolates from intestine; lanes 4 and 7, isolates from trachea; lane 5, isolate from ovary; lanes 9 and 13, isolates from lungs; lanes 14 and 16, negative controls; lane 15, positive control.
Amplified fragment length polymorphisms
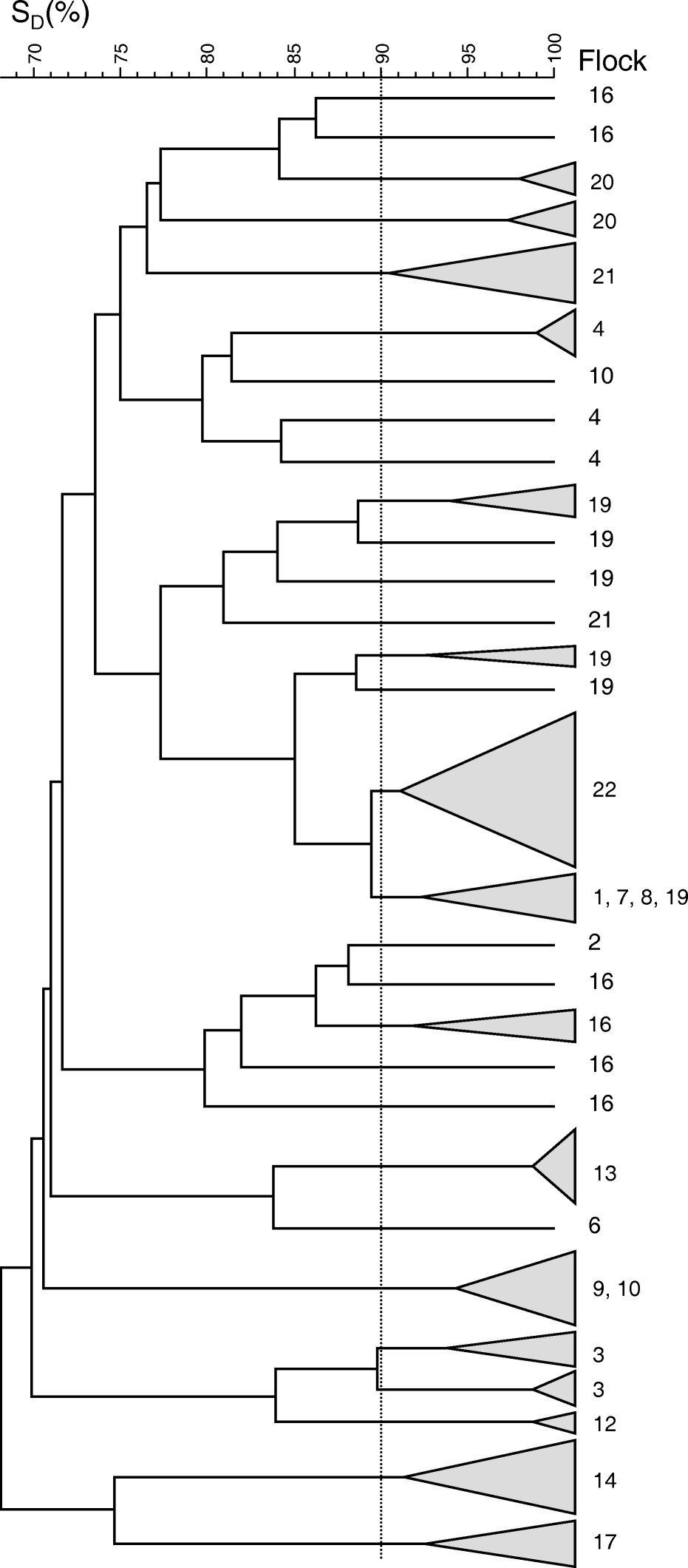
Eighty-three isolates from 18 flocks were investigated. Using a cut-off level of 90% to define a genotype (clone), 34 individual clones were identified. Generally, a high genetic similarity was demonstrated both between and within flocks. In 11 flocks only one clone was present, this includes the three flocks (numbers 6, 7, 8) where only one isolate was obtained. In four flocks two clones were demonstrated. One flock (flock number 4) had three clones and two flocks (flock numbers 16 and 19) had six clones (). One clone was present in four different flocks.
Figure 2. Dendrogram (unweighted pair group method using arithmetic averages) of AFLP similarities of 83 Gallibacterium isolates. SD, band-based Dice similarity coefficient (%). Flocks, the flock number from which the isolates originate. Isolates grouping at a 90% similarity level or higher were pooled as indicated by grey triangles. Dotted line, clonal cut-off level at 90% similarity.
Discussion
The present study aimed to investigate the association between the presence of haemolytic G. anatis and reproductive disorders in laying birds kept in alternative husbandry systems as birds kept in these systems are more likely to be infected with G. anatis (Bojesen et al., Citation2003b).
E. coli is considered the most common bacterium associated with reproductive disorders in chickens (Bisgaard & Dam, Citation1981; Jones & Owen, Citation1981; Jordan et al., Citation2005). However, other reports suggest that Gallibacterium spp. may cause these disorders and their importance is likely to be underestimated due to overgrowth by other bacterial species and the difficulties of identification (Mirle et al., Citation1991; Bojesen et al., Citation2003a).
Based on specific signs in the flocks, birds were selected for further investigations in accordance with Vanderkerchove et al. (Citation2004). A drop in egg production, apathy of birds, pasting around the vent and increased mortality were all signs used by the farmer to initiate further investigations as reported in the present study. The results of the postmortem investigations performed on the farms and the notification of salpingitis/peritonitis were additional criteria to include the farm in the study. In total, 141 birds were killed and the samples were taken from fresh carcasses. Except the five birds from flock number 15, all birds (136/141) were investigated prior to any antibiotic treatment to increase the recovery of bacteria from the different organs.
In the present study most of the haemolytic G. anatis isolates were obtained from the respiratory tract, especially from the choana and trachea. This is not surprising considering previous reports by Bisgaard (Citation1977), Mushin et al. (Citation1979) and Bojesen et al. (Citation2003b) indicating that Gallibacterium spp. constitute part of the normal tracheal microbiota in chickens and that under certain conditions they might act as opportunists and cause respiratory disease. In the case of E. coli, the natural route of infection is generally thought to be via the respiratory tract (Gross, Citation1961; Carlson & Whenham, Citation1968) and a similar route might exist for Gallibacterium. The present investigation supports this assumption for both bacteria, as 52% of the G. anatis and 32% of the E. coli isolates were recovered either from the choana, trachea or the lungs of the birds. Penetration of Gallibacterium organisms from the mucosa of the respiratory tract into the systemic circulation may be due to the influence of an impaired host immunity, co-infections and environmental factors (Matthes & Löliger, Citation1976; Gerlach, Citation1977; Nagaraja et al., Citation1984; Shaw et al., Citation1990; Leitner & Heller, Citation1992).
From the case history of the flocks it was known that cannibalism occurred in nearly one-half of the flocks (13/31) and that numerous birds showed extensive pecking wounds around the cloaca. This is in contrast to the findings reported by Jones & Owen (Citation1981) and Jordan et al. (Citation2005), who observed only a few cases of vent cannibalism in birds suffering from salpingitis/peritonitis. However, Cumming (Citation2001) attributed considerable importance to such injuries from which the bacteria may spread into the blood stream resulting in bacteraemia with bacteria to be isolated from the heart, liver, spleen and the reproductive tract. In the present investigation, ascending infections may have contributed to the finding of G. anatis and E. coli in the reproductive tract, in addition to the heart, liver and spleen. Bojesen et al. (Citation2004) used fluorescence in situ hybridization to demonstrate bacteria in the spleen and liver following intraperitoneal infection of chickens with Gallibacterium, indicating that ascending infections might be of relevance.
The gross pathological lesions of reproductive disorders due to Gallibacterium infection cannot be distinguished from infections with E. coli, and for this reason all samples were also investigated for E. coli. With an increase of birds/flock showing pathological signs in the ovaries or the oviduct, E. coli was isolated more frequently. This might have been because G. anatis was overgrown in such cases as already mentioned above. E. coli are present in the normal microbiota of the intestinal tract in poultry, but certain strains of APEC possess specific virulence factors and are able to cause severe infections. The APEC serogroups O1, O2 and O78 are most frequently recovered from infected chickens (Sojka & Carnaghan, Citation1961; Glantz et al., Citation1962; Cloud et al., Citation1985; Dozois et al., Citation1992). In the present investigation, E. coli was isolated from birds of each flock and APECs (O1:K1, O2:K1 and O78:K80) were found in more than 50% of the flocks. The bacteria were very often isolated from the respiratory tract (274/844) and from the reproductive tract (166/844). Bisgaard & Dam (Citation1981) investigated 150 salpinx samples from layer carcasses condemned at slaughterhouses due to salpingitis, and found that E. coli was isolated most frequently. Their findings were confirmed by Jones & Owen (Citation1981) investigating 292 birds in which salpingitis/peritonitis was diagnosed over a period of 2 years. Furthermore, these authors also found mixed infections of E. coli and haemolytic Gallibacterium spp., as shown in nearly one-half of the cases in the present study.
According to the existing literature, detection of members of Pasteurellaceae by traditional methods often results in difficulties at isolation, cultivation and identification. The weak and unreliable reactions observed for some of the phenotypic identification tests add to these uncertainties (Christensen et al., Citation2003). Diagnostic PCR assays were therefore developed to complement these diagnostic methods (Bojesen et al., Citation2007) and were found very useful in confirming the classification of the isolates. The genetic diversity assessed by AFLP analysis of 83 isolates confirmed previous indications of high genetic relatedness among isolates from the same flock (Bojesen et al., Citation2003a). Furthermore, the fact that the same clone could be isolated from the mucosal lining of the upper respiratory tract and from lesions in the reproductive tract, heart, liver and spleen, suggests that, given the right opportunity, Gallibacterium isolates residing in the upper respiratory or lower genital tract may gain access to the systemic circulation and/or the upper reproductive tract and cause disease.
In conclusion, we have shown that haemolytic G. anatis isolates are prevalent in layers with reproductive disorders. The results from the current study provide strong support for the theory that Gallibacterium plays a role in reproductive disorders. Furthermore, we have demonstrated that Gallibacterium isolates from different locations (i.e. heart, spleen, upper respiratory and lower genital tract) were highly similar, indicating that isolates residing in their natural habitat may cause reproductive disorders and/or systemic disease under certain conditions.
Acknowledgements
The authors would like to thank Boehringer Ingelheim Vetmedica for cooperation and financial support of the project.
References
- Addo , P.B. and Mohan , K. 1984 . Atypical Pasteurella haemolytica type A from poultry . Avian Diseases , 29 : 214 – 217 .
- Bisgaard , M. 1977 . Incidence of Pasteurella haemolytica in the respiratory tract of apparently healthy chickens and chickens with infectious bronchitis. Characterisation of 213 strains . Avian Pathology , 6 : 285 – 292 .
- Bisgaard , M. 1982 . Isolation and characterization of some previously unreported taxa from poultry with penotypical characters related to Actinbacillus- and Pasteurella species . Acta Pathologica Microbiologica et Immunologica Scandinavica (B) , 90 : 59 – 67 .
- Bisgaard , M. 1993 . Ecology and significance of Pasteurellaceae in animals . Zentralblatt für Bakteriologie , 229 : 7 – 26 .
- Bisgaard , M. and Dam , A. 1981 . Salpingitis in poultry. II. Prevalence, bacteriology, and possible pathogenesis in egg-laying chickens . Nordisk Veterinaer Medicin , 33 : 81 – 89 .
- Bojesen , A.M. , Torpdahl , M. , Christensen , H. , Olsen , J.E. and Bisgaard , M. 2003a . Genetic diversity of Gallibacterium anatis in chicken flocks representing different production systems . Journal of Clinical Microbiology , 41 : 2737 – 2740 .
- Bojesen , A.M. , Nielsen , S.S. and Bisgaard , M. 2003b . Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels . Avian Pathology , 32 : 503 – 510 .
- Bojesen , A.M. , Nielsen , O.L. , Christensen , J.P. and Bisgaard , M. 2004 . In vivo studies of Gallibacterium anatis infection in chickens . Avian Pathology , 33 : 145 – 152 .
- Bojesen , A.M. , Vazquez , M.E. , Robles , F. , Gonzalez , C. , Soriano , E.V. , Olsen , J.E. and Christensen , H. 2007 . Specific identification of Gallibacterium by a PCR using primers targeting the 16S rRNA and 23S rRNA genes . Veterinary Microbiology , 123 : 262 – 268 .
- Carlson , H.C. and Whenham , G.R. 1968 . Coliform bacteria in chicken broiler house dust and their possible relationship to coli-septicemia . Avian Diseases , 12 : 297 – 302 .
- Christensen , H. , Bisgaard , M. , Bojesen , A.M. , Mutters , R. and Olsen , J.E. 2003 . Genetic relationships among avian isolates classified as Pasteurella haemolytica, Actinobacillus salpingitidis or Pasteurella anatis with proposal of Gallibacterium anatis gen. nov., comb. nov. and description of additional genomospecies within Gallibacterium gen. nov . International Journal of Systematic and Evolutionary Microbiology , 53 : 275 – 287 .
- Cloud , S.S. , Rosenberger , J.K. , Fries , P.A. , Wilson , R.A. and Odor , E.M. 1985 . In vitro and in vivo characterization of avian Escherichia coli. I. Serotypes, metabolic activity, and antibiotic sensitivity . Avian Diseases , 29 : 1084 – 1093 .
- Cumming 2001 . The aetiology and importance of salpingitis in laying hens . In Proceedings of the Australian Poultry Science Symposium (pp. 194 – 196 ). Sydney, Australia
- Dozois , C.M. , Fairbrother , J.M. , Harel , J. and Bossé , M. 1992 . Pap- and pil-related DNA sequences and other virulence determinants associated with Escherichia coli isolated from septicemic chickens and turkeys . Infection and Immunity , 60 : 2648 – 2656 .
- Gerlach , H. 1977 . Significance of Pasteurella haemolytica in poultry . Der Praktische Tierarzt , 58 : 324 – 328 .
- Glantz , P.J. , Narotzky , S. and Bubash , G. 1962 . Escherichia coli serotypes isolated from salpingitis and chronic respiratory disease in poultry . Avian Diseases , 6 : 322 – 328 .
- Gross , W.B . 1961 . The development of “air sac disease” . Avian Diseases , 5 : 431 – 436 .
- Hacking , W.C. and Pettit , J.R. 1974 . Pasteurella hemolytica in pullets and laying hens . Avian Diseases , 18 : 483 – 486 .
- Harbourne , J.F. 1962 . A haemolytic cocco-bacillus recovered from poultry . The Veterinary Record , 74 : 566 – 567 .
- Jones , H.G.R. and Owen , D.M. 1981 . Reproductive tract lesions of the laying fowl with particular reference to bacterial infection . The Veterinary Record , 108 : 36 – 37 .
- Jordan , F.T.W. , Williams , N.J. , Wattret , A. and Jones , T. 2005 . Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock . The Veterinary Record , 157 : 573 – 577 .
- Kohlert , R. 1968 . Untersuchungen zur Ätiologie der Eileiterentzündung beim Huhn . Monatshefte der Veterinärmedizin , 23 : 392 – 395 .
- Leitner , G. and Heller , E.D. 1992 . Colonization of Escherichia coli in young turkeys and chickens . Avian Diseases , 36 : 211 – 220 .
- Matthes , S. and Löliger , H.Ch. 1976 . Beitrag zur Kinetik bakterieller Infektionen beim Huhn . Berliner und Münchener Tierärztliche Wochenschrift , 89 : 98 – 102 .
- Matthes , S , Lölinger , H.Ch. and Schubert , H.J. 1969 . Enzootisches Auftreten der Pasteurella haemolytica beim Huhn . Deutsche Tierärztliche Wochenschrift , 76 : 94 – 95 .
- Mirle , C. , Schöngarth , M. , Meinhart , H. and Olm , U. 1991 . Studies into the incidence of Pasteurella haemolytica infections and their relevance to hens, with particular reference to diseases of the egg-laying apparatus . Monatshefte für Veterinärmedizin , 45 : 545 – 549 .
- Mushin , R. , Weisman , Y. and Singer , N. 1979 . Pasteurella haemolytica found in the respiratory tract of fowl . Avian Diseases , 24 : 162 – 168 .
- Nagaraja , K.V. , Emery , D.A. , Jordan , K.A. , Sivanandan , V. , Newman , J.A. and Pomeroy , B.S. 1984 . Effect of ammonia on the quantitative clearance of Escherichia coli from lungs, air sacs, and livers of turkeys aerosol vaccinated against Escherichia coli . American Journal of Veterinary Research , 45 : 392 – 395 .
- Sambrook , J. and Russel , D.W. 2001 . “ Detection of DNA in agarose gels ” . In Molecular Cloning. A Laboratory Manual , 3rd edn , Edited by: Sambrook , J. and Russel , D.W. 5 New York : Cold Spring Harbor Laboratory Press .
- Shaw , D.P. , Cook , D.B. , Maheswaran , S.K. , Lindeman , C.J. and Halvorson , D.A. 1990 . Pasteurella haemolytica as a co-pathogen in pullets and laying hens . Avian Diseases , 34 : 1005 – 1008 .
- Sojka , W.J. and Carnaghan , R.B.A . 1961 . Escherichia coli infection in poultry . Research in Veterinary Science , 2 : 340 – 352 .
- Vanderkerchove , D. , De Herdt , P. , Laevens , H. and Pasmans , F. 2004 . Colibacillosis in caged layer hens: characteristics of the disease and the aetiological agent . Avian Pathology , 33 : 117 – 125 .