Abstract
Ocular opacity, associated with reluctance to move and inability to feed properly, was observed in approximately 1% of all newly hatched females from several related flocks of Mulard ducks. A 5-week follow-up study of 10 1-day-old affected females was performed, and they were compared with 10 control animals. Clinical, ocular and ultrasonographic examinations, and a complete necropsy of two animals per group with histological examination of the eye, were performed weekly. A bilateral immature cortical anterior cataract was diagnosed at ocular examination and confirmed by ultrasonography in affected ducks. Dyscoria was occasionally observed in affected animals. Severe cataract, with Morgagnian globules, severe anterior fibre liquefaction and disorganization were observed by photonic microscopy. No retinal or choroidal lesions were observed. No progression or repair of ultrasonographic and microscopic lesions could be detected during the 5 weeks of examination. The female predisposition for the ocular lesions suggests a congenital sex-linked recessive cataract.
Introduction
Cataract, an opacification of the ocular lens, occurs most commonly as senile alteration in non-domesticated aged birds, including those in the order Galliformes (Keymer, Citation1977). In domesticated birds, cataracts may develop as a secondary ocular lesion in infectious disease (Ferguson et al., Citation1956; Smith et al., Citation1974; Silva et al., Citation1980). In ducks, ocular lesions are rarely reported and concern only acquired disease (Gilger et al., Citation1995; Gordus et al., Citation2002). This is the first report describing ocular and histological observations of Mulard ducks affected by a congenital cataract during a 5-week study.
Materials and methods
Animals
In the facility, 42 breeder flocks were in lay. All the animals received the same vaccination programme (against goose parvovirus, duck hepatitis virus, and Pasteurella multocida) and the same commercial feed. No abnormalities were observed among the breeders. In multiple flocks of newly hatched animals, each composed of 9350 animals hatched on the same day, abnormal behaviour was detected in 1-day-old females of Mulard duck, with an incidence in the first eight affected flocks ranging from 0.24% to 1.64%.
Affected flocks came from the mating of two different female lines with one male line. The animals were reluctant to move and were unable to feed properly. A reduced rate of weight gain was observed among the affected animals, causing them to be culled early. Complete necropsy and histological examination of 30 1-day-old animals did not reveal any lesion except in the eyes.
Experimental design
A 5-week follow-up of 10 1-day-old affected females was performed, and these were compared with 10 control female ducks derived from the same breeder flock and from the same hatch of the affected animals. Clinical, ocular and ultrasonographic examinations and a complete necropsy of two animals per group were performed weekly.
Clinical and ocular examination
Ocular examinations included observation under dim light, assessment of dazzle and pupillary light reflexes with a Finoff transilluminator and Slit-lamp biomicroscopy (Kowa SL-14; Luneau, Chartres, France) without induced pupil dilation. B-mode ultrasonography (ATL HDI 3500; Société Polygone, Paris, France) using a 10 MHz linear probe was performed under general anaesthesia with Medetomidine (Domitor®, 200 µg/kg; Pfizer Santé Animale, Paris, France) and Ketamine (Kétamine 500®, 10 mg/kg intramuscularly; Virbac France, Carros, France). These procedures were approved by the Institutional Animal Care and Use Committee of the National Veterinary School of Alfort and were performed by authorized investigators.
Pathology
At necropsy, right eyes were collected, fixed in 4% paraformaldehyde, and routinely processed to paraffin. Four-micrometre sections were stained with haematoxylin–eosin–saffron and periodic acid Schiff. Samples of the left lens were fixed in 2% glutaraldehyde, post fixed in 1% osmium tetroxide, dehydrated and embedded in Epon. Semi-thin sections of 0.5 µm were stained with toluidine blue.
Results
Clinical and ocular examination
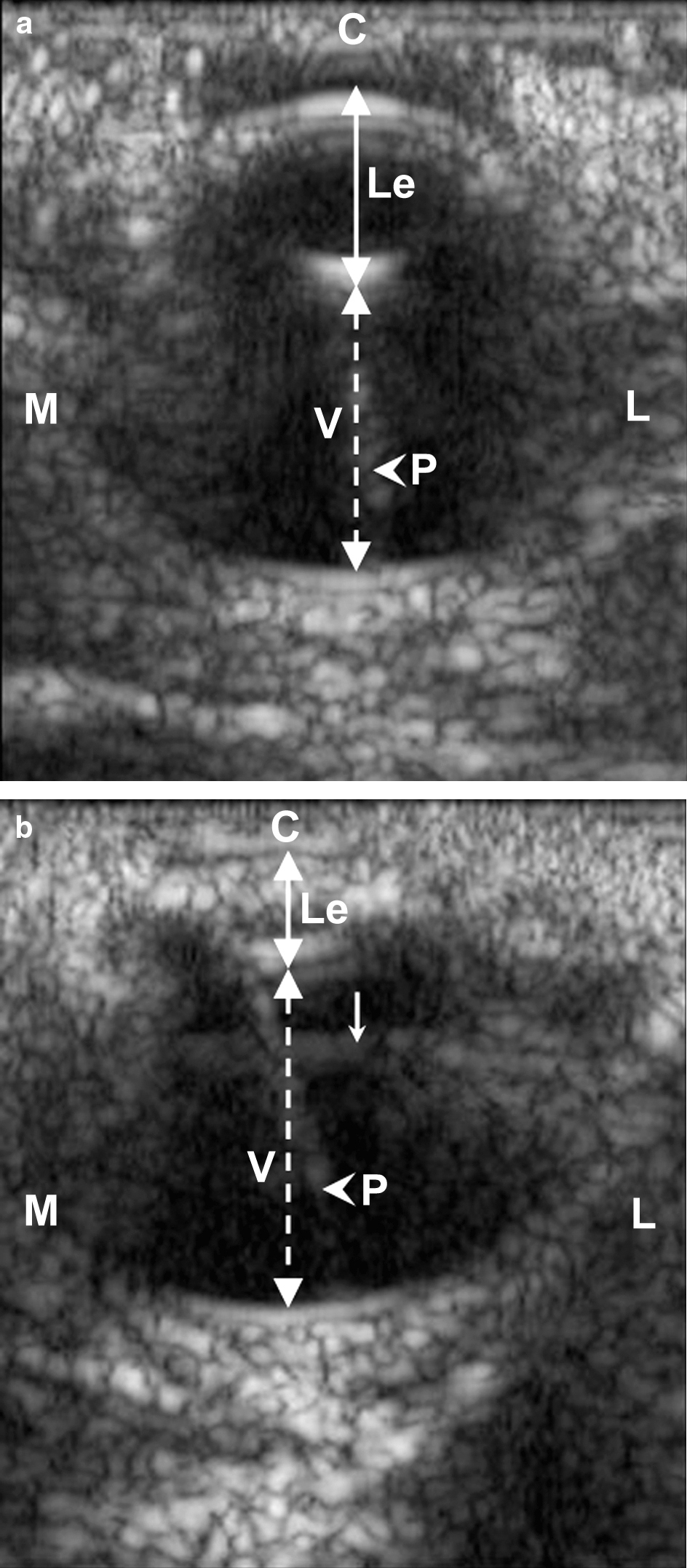
All of the affected animals were female. Weekly, at ocular examination, dazzle and pupillary reflexes were normal in all animals. Unilateral or bilateral dyscoria was occasionally seen in affected animals, with the pupil being pear-shaped in all cases. All of the affected animals presented a bilateral immature cataract involving the anterior cortex. Examination of the lens was limited due to the lack of induced pupil dilation. However, the location and the degree of the opacification were similar in all affected animals. An ophthalmoscopic examination of the posterior compartment could not be performed because of lens opacity. At ultrasonographic examination, affected animals had microphakia with a flattened shape of the lens, compared with control birds. Hyperechogenicity was observed in the anterior cortex, as well as in the nucleus. No major difference in the severity of the lens opacity was noted at the ultrasonographic examination. The ultrasonographic appearance of the posterior compartment was similar in control animals and in affected animals ().
Figure 1. Ultrasonographic photography of the eye of a 3-week-old affected animal (1b) and its control (1a). In the affected animal, an abnormal hyperechogenicity in the lens cortex and a microphakia are detectable when compared with the control. C, cornea. Le, lens; V, vitreous, Continuous doubleheaded arrow, antero-posterior axis of the lens; discontinuous doubleheaded arrow, antero-posterior axis of the vitreous; arrowhead, with pecten (P); thin arrow, probe-related artefact. M, medial; L, lateral. B-scan/10 MHz.
Pathology
At necropsy, bilateral cataract was confirmed. No other macroscopic findings were observed. Microscopically, ocular lesions were restricted to the lens in all affected animals, and the appearance of the lesions remained unchanged during the 5 weeks of study. In sections stained with haematoxylin–eosin–saffron, lens fibres were haphazardly arranged. Multifocally, they were replaced by an amorphous eosinophilic material (fibre liquefaction), which occasionally involved the anterior cortex and the nucleus (). At the periphery of the liquefied fibres, globules of degenerating protein (Morgagnian globules) were evident. Ingrowing epithelium, derived from the annular pad, and the most peripheral anterior epithelium exhibited cytoplasmic vacuolation and ballooning degeneration (bladder cells) (). The lens capsule was intact and there was no difference in its thickness as seen on slides stained with periodic acid Schiff when compared with control animals. The lesions were consistent with a cortical anterior cataract. The degenerative changes were not associated with any inflammatory process. No evidence of progression or more advanced lens and ocular changes were observed in 5-week-old birds.
Figure 2. Section of the eye from a 1-day-old duck: (a) low magnification and (b) high magnification. The anterior cortex and the nucleus of the lens (Le) were replaced by an amorphous eosinophilic material (fibre liquefaction), forming a cavity (*). Lenticular epithelial cells showed cytoplasm vacuolation and ballooning degeneration. C, cornea; Ci, ciliary bodies; Ir, iris; V, vitreous. Haematoxylin–eosin–saffron staining.
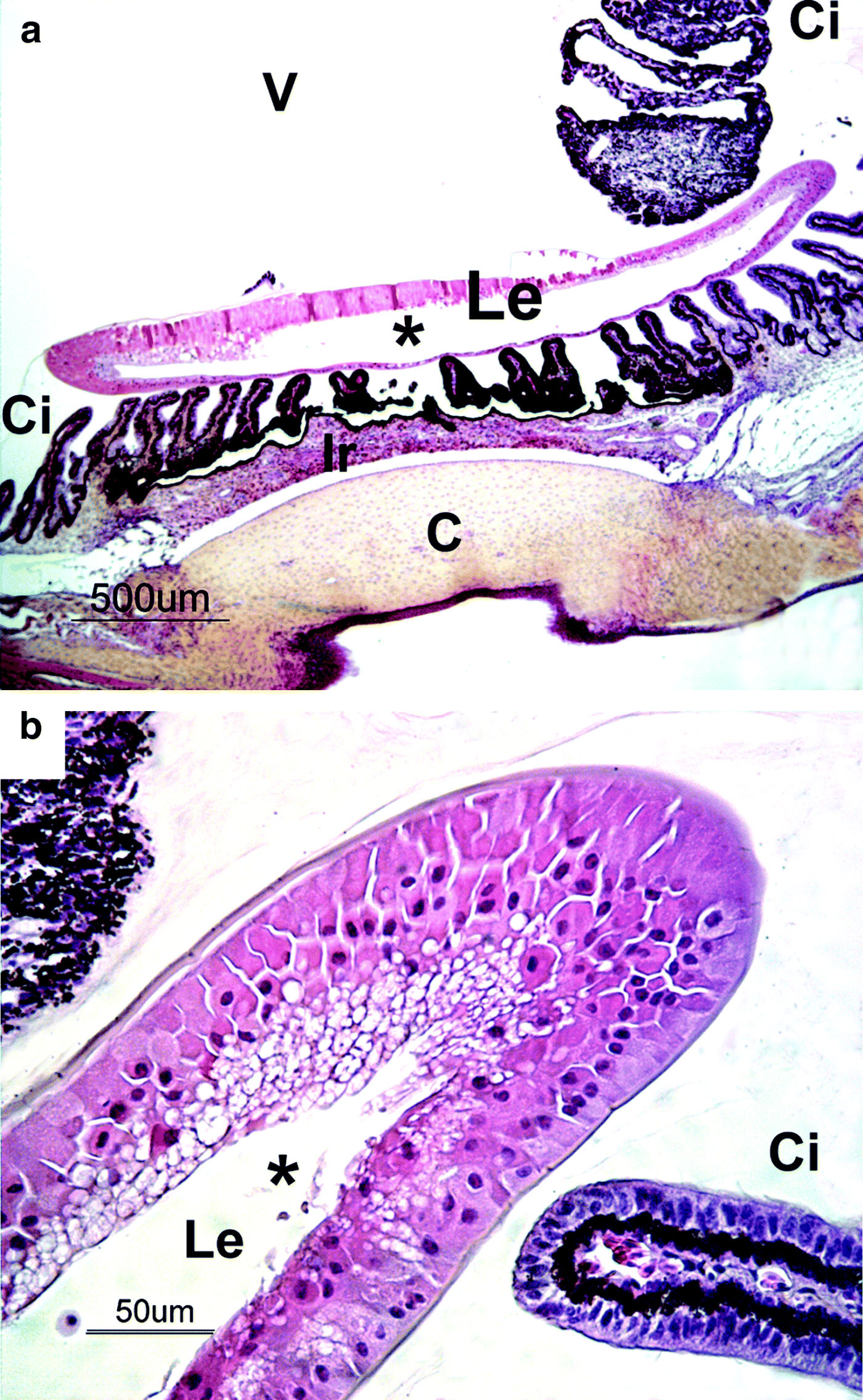
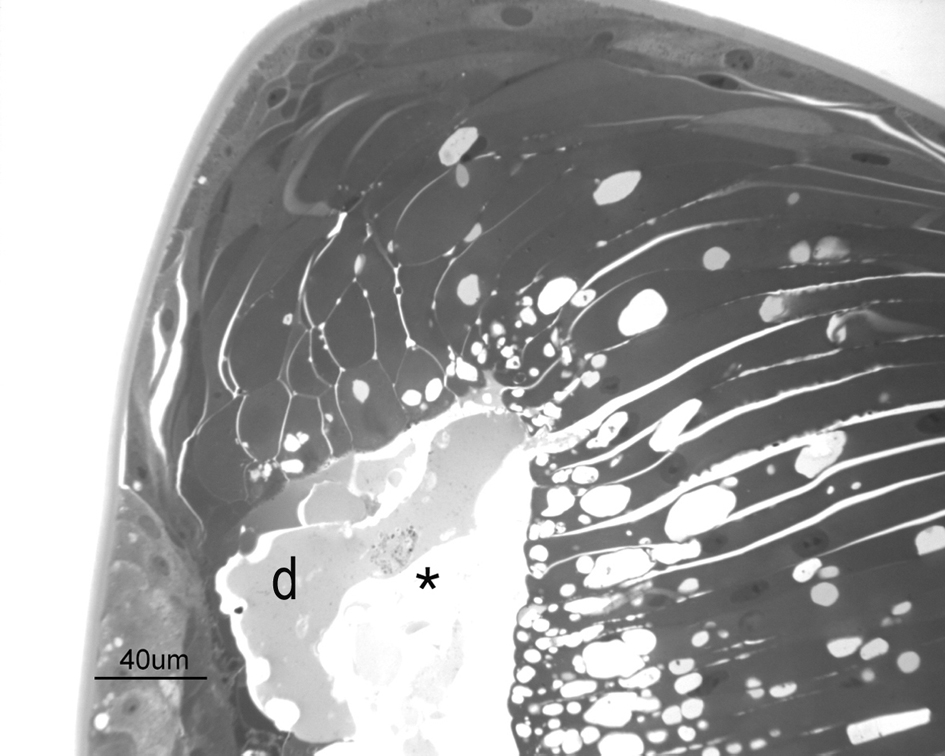
Figure 3. Pre-equatorial semi-thin section of the lens from a 4-week-old duck. Vacuolated cells lined a cavity (*) in the anterior part of the lens containing liquefactive material. Lens fibres were irregular in diameter and dishomogeneous. A degenerated cell (d) is present within the cavity. Toluidine blue staining.
Discussion
In poultry, cataract generally occurs as a secondary ocular lesion, following uveitis. It has been described in association with Marek's disease (Smith et al., Citation1974), avian encephalomyelitis or Salmonella arizonae infection (Silva et al., Citation1980). Cataracts have also been associated with vitamin E deficiency in turkeys (Ferguson et al., Citation1956) or as a result of light-induced oxidative stress (Lauber & McGinnis, Citation1966). In wild ducks, a report of salt toxicosis, characterized by cataract, has been described (Gordus et al., Citation2002). In that case, ocular lesions consisted of cellular swelling and fibre liquefaction of the cortical pad, without involvement of the capsule; changes resembling those observed in our case. Nevertheless, those ducks were all adults and had other pathological changes, such as conspicuous cutaneous salt encrustations.
In the ducks of the present study, lesions were confined to the lens. The absence of uveitis and of gross findings in other organs at necropsy led us to exclude an infectious origin of the outbreak, as also supported by the flock's vaccinal status and the lack of lesions in other animals of the facility. No nutritional disease or toxicosis were suspected, since the animals in the flocks were bred in the same conditions and had the same commercial feed, which had not been changed recently.
Cataract was present at hatching, and only in females. These findings, together with the exclusion of infectious or nutritional causes, suggest a primary hereditary defect. In the literature, primary cataract in avian species has been rarely described. A case was reported in Brahma chickens (Chmielewski et al., Citation1993). Ocular lesions appeared by 4 months of age, followed by the development of crooked toes. In White Leghorn chickens, an inherited ocular disease, present at hatching, has been described (Salter et al., Citation1997). It was characterized by several ocular malformations, including cataract, related to an autosomal recessive mutation associated with the pigmentary epithelium. In turkey poults, a form of congenital cataract associated with an optic nerve hypoplasia has been reported (Barr et al., Citation1988). In the present case, cataract was present at hatch and was not associated with other malformations. The fact that affected animals were females suggests a recessive mutation carried on the Z chromosome. In contrast to mammals, female birds are the heterogametic sex, carrying one copy each of the Z and W sex chromosomes, while males are homogametic (ZZ). A recessive gene present on the chromosome Z would be expressed in the females of the first generation and in none of the males. Since all of the animals in the progeny flocks were slaughtered for meat, and as they are infertile, no further studies on following generations could be performed. The incidence of approximately 1% of all females leads us to speculate that the mutation is sex-linked and transmitted by males.
Acknowledgements
The authors would like to thank Jérôme Amiaud for his technical assistance, Marie-Thérèse Masson for her expertise in electron microscopy and the Department of Medical Imaging, Ecole Nationale Vétérinaire d'Alfort, for the ultrasonographic images.
References
- Barr , B.C. , Murphy , C.J. , Ghazikhanian , G.Y. and Bellhorn , R.W. 1988 . Cataracts and optic nerve hypoplasia in turkey poults . Avian Disease , 32 : 469 – 477 .
- Chmielewski , N.T. , Render , J.A. , Schwartz , L.D. , Keller , W.F. and Perry , R.F. 1993 . Cataracts and crooked toes in Brahma chickens . Avian Disease , 37 : 1151 – 1157 .
- Ferguson , T.M. , Rigdon , R.H. and Couch , J.R. 1956 . Cataracts in vitamin E deficiency; an experimental study in the turkey embryo . AMA Archives of Ophthalmology , 55 : 346 – 355 .
- Gilger , B.C. , McLaughlin , S.A. and Smith , P. 1995 . Uveal malignant melanoma in a duck . Journal of the American Veterinary Medical Association , 206 ( 10 ) : 1580 – 1582 .
- Gordus , A.G. , Shivaprasad , H.L. and Swift , P.K. 2002 . Salt toxicosis in ruddy ducks that winter on an agricultural evaporation basin in California . Journal of Wildlife Disease , 38 : 124 – 131 .
- Keymer , I.F. 1977 . Cataracts in birds . Avian Pathology , 6 : 335 – 341 .
- Lauber , J.K. and McGinnis , J. 1966 . Eye lesions in domestic fowl reared under continuous light . Vision Research , 6 : 619 – 626 .
- Salter , D.W. , Payne , W.S. , Ramsey , D.T. , Blair , M. and Render , J.A. 1997 . A new inherited ocular anomaly in pigmented white Leghorn chickens . Journal of Veterinary Diagnostic Investigation , 9 : 407 – 409 .
- Silva , E.N. , Hipolito , O. and Grecchi , R. 1980 . Natural and experimental Salmonella arizonae 18:z4,z32 (Ar. 7:1,7,8) infection in broilers. Bacteriological and histopathological survey of eye and brain lesions . Avian Disease , 24 : 631 – 636 .
- Smith , T.W. , Albert , D.M. , Robinson , N. , Calnek , B.W. and Schwabe , O. 1974 . Ocular manifestations of Marek's disease . Investigative Ophthalmology , 13 : 586 – 592 .