Abstract
Avian infectious bronchitis virus (IBV) is a major pathogen in commercial poultry flocks. We recently demonstrated that sialic acid serves as a receptor determinant for IBV on the tracheal epithelium. Here we compared the IBV strains Beaudette, 4/91, Italy02, and QX for their sialic acid-binding properties. We demonstrate that sialic acid binding is important for the infection of primary chicken kidney cells and the tracheal epithelium by all four strains. There were only slight differences between the four strains, indicating the universal usage of sialic acids as receptor determinants by IBV. In addition, we analysed the primary target cells in the respiratory epithelium of the four different strains and found that all of them infected ciliated cells and goblet cells.
Introduction
Avian infectious bronchitis virus (IBV) causes an economically important disease mainly in the chicken and is clinically associated with respiratory symptoms and/or egg drop including cystic degeneration of the oviduct. IBV uses the respiratory epithelium of the upper respiratory tract as primary target cells and during infection the virus can spread to several other organs including kidney and oviduct. Belonging to the family Coronaviridae, IBV virions comprise four structural proteins: the nucleoprotein N, and the membrane proteins M, E, and S. The S protein is composed of two subunits, the S1 “head” and the S2 “stalk”, and is responsible for binding to and fusion with the host cells membranes. The important viral binding domain appears to be located on the S1 subunit of the S protein. Viruses lacking S1 are not infectious, nor are they able to haemagglutinate (Cavanagh & Davis, Citation1986). We recently demonstrated that sialic acid serves as receptor determinant for infection of cells (Winter et al., Citation2006, 2008). In those studies, the importance of sialic acid was shown only for the apathogenic Beaudette strain, a pathogenic M41 and a nephropathogenic B1648 isolate. One major characteristic of IBV virions is the high variability of the S1 subunit and the occurrence of new strains. Most serotypes differ by 20% to 25% from each other but some serotypes show a difference of 50% in the S1 sequence (reviewed in Cavanagh, Citation2007).
Because of the high diversity in the different S1 sequences, we addressed the question of whether the sialic acid binding activity is a conserved feature of IBV to facilitate binding to host cells. We demonstrate here that the currently important IBV strains Italy02, 4/91, and a QX-like variant, which cause economic problems in large parts of Europe and other parts of the world (Worthington et al., Citation2008), all use sialic acids on the surface of the respiratory epithelium and on primary chicken kidney cells to initiate infection.
Materials and Methods
Viruses
IBV strains Beaudette, 4/91, Italy 02, and QX were propagated in specific pathogen-free 10-day-old embryonated chicken eggs. The allantoic fluid was harvested, clarified by low-speed centrifugation, frozen in liquid nitrogen and stored at –80°C. The virus titre was determined by titration in primary chicken embryo kidney cells. All strains were kindly provided by Hans Philipp (Lohmann Tierzucht, Cuxhaven, Germany) except for the Beaudette strain, which was kindly given by Dave Cavanagh (Institute for Animal Health, Compton, UK).
Cells
Primary chick embryo kidney cells were prepared from 20-day-old specific pathogen free chicken embryos as described previously (Winter et al., 2006).
Preparation of tracheal organ cell culture
The tracheal organ cell cultures (TOCs) were prepared as described previously (Winter et al., Citation2008). The rings were incubated at 37°C on a rotator, and on the next day the rings were screened for selection of TOCs with 100% ciliary activity.
Preparation of cryosections
As described recently (Winter et al., Citation2008), tracheas were prepared from 4-week-old to 6-week-old specific pathogen free chickens, cut into small pieces of 1 cm length, washed with phosphate-buffered saline (PBS) and infected by one of the following IBV inocula: strains Beaudette and QX were applied at a titre of 105 plaque-forming units/ring, and strains Italy 02 and 4/91 applied at 5 x 105 plaque-forming units/ring. The infected rings were incubated at 37°C on a rotator. After 24 h they were mounted on small filter papers with tissue-freezing medium (Jung, Heidelberg, Germany), frozen in liquid nitrogen and preserved at –80°C prior to cutting. Sections of 10 µm thickness were obtained with a cryostat machine (Reichert-Jung, Nußloch, Germany). The sections dried overnight at room temperature and were frozen at –20°C until staining.
Plaque reduction assay
Primary chick embryo kidney cells grown on coverslips were treated with 50 mU neuraminidase of Clostridium perfringens type 5 (Sigma-Aldrich, St Louis, Missouri, USA) diluted in medium 199, or mock-treated, for 1 h at 37°C. After washing, the cells were infected by the indicated IBV strains at a multiplicity of infection of 0.001. Methylcellulose was added after 1 h incubation at 37°C. After 24 h, the cells were fixed with 3% paraformaldehyde and permeabilized with 0.2% Triton X-100. Plaques were stained with an anti-IBV polyclonal serum raised in rabbits and a FITC-labelled secondary antibody. After counting the plaques, the outcome of the mock-treated cells were set as 100%. Data shown are the mean values of three experiments.
Neuraminidase treatment and infection of TOCs
Two groups of four TOCs were either treated with 50 mU neuraminidase of C. perfringens type 5 per ring or incubated with medium alone. After incubation at 37°C for 1 h, the rings were washed with PBS and infection with the indicated virus strain was performed using 1 x 104 per ring. Following three washes with PBS, the TOCs were incubated with medium at 37°C on a rotator. All experiments were performed three times. Ciliary activity was analysed as described recently (Winter et al., Citation2008). Briefly, each ring was observed at daily intervals under a microscope to monitor the ciliary activity. The ring was divided virtually into 10 portions and each portion was analysed to see whether ciliary activity was detectable. The number of portions with visible ciliary activity was multiplied by 10 to give the percentage ciliary activity of the respective TOC.
Immunofluorescence analysis of cryosections
The sections were fixed with 3% paraformaldehyde for 15 min and were permeabilized with 0.2% Triton X-100 for 5 min followed by three washing steps with PBS. All antibodies were diluted in 1% bovine serum albumin and incubated with the sections for 1 h at room temperature in an incubation chamber. For detection of infected cells, monoclonal antibody Ch/IBV 48.4 directed against the N protein of IBV (ID Lelystad, The Netherlands) or a polyclonal IBV antiserum raised in rabbits was used. Mucus-producing goblet cells were stained with anti-MUC-5AC antibody (Acris, Hiddenhausen, Germany). Cilia were detected by CY3 labelled anti-β-tubulin antibody (Sigma-Aldrich). Bound antibodies were visualized by FITC-labelled and Cy3-labelled anti-rabbit antibodies (Sigma-Aldrich), or anti-mouse antibodies (Acris). For lectin staining, biotinylated Maackia amurensis Lectin II (MAAII) was used after preincubation of sections with the Avidin/Biotin Blocking kit (both from Vector Laboratories, USA). Detection of lectin was carried out with streptavidin Cy3 (Sigma-Aldrich). Fluorescence microscopy was performed with a Leica inverted-2 confocal microscope.
Results
Plaque reduction after neuraminidase treatment
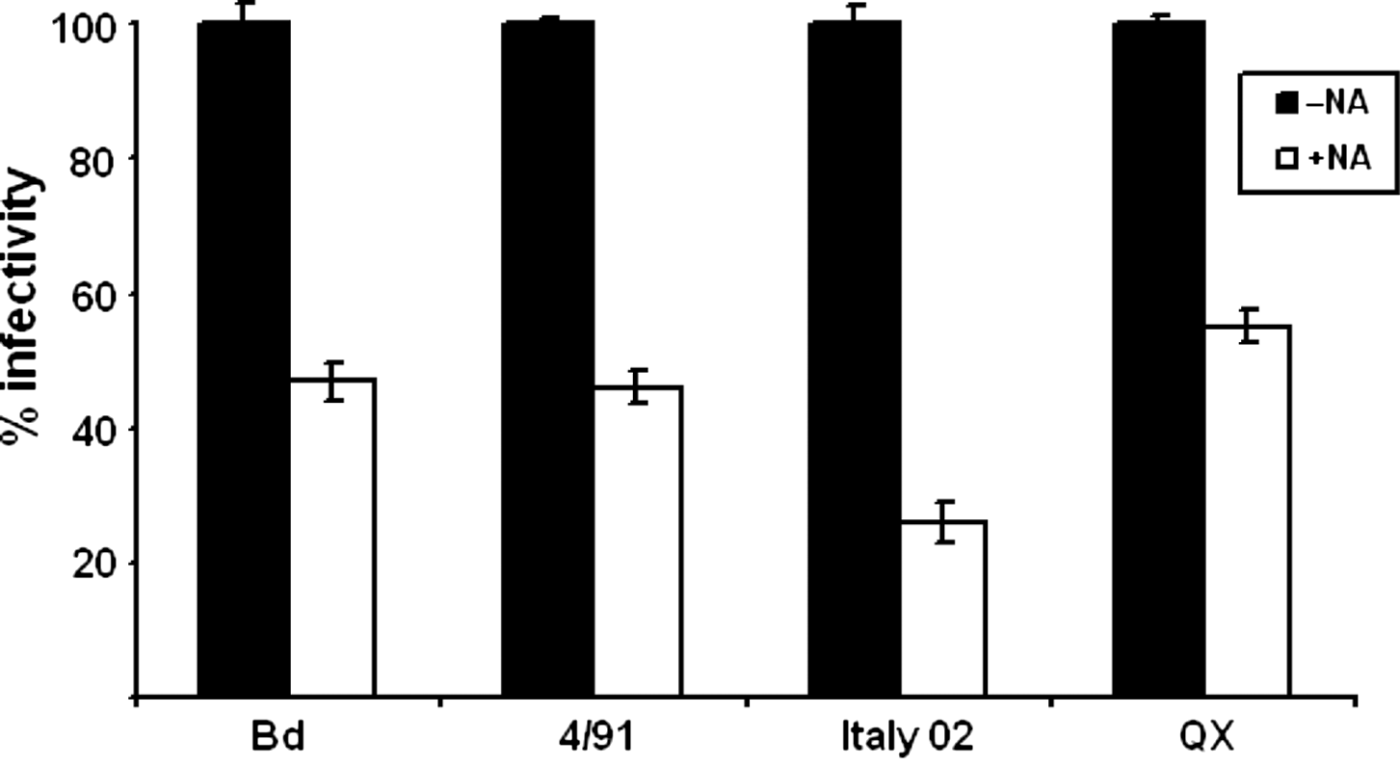
Most IBV strains do not grow in permanent cell cultures. To analyse the importance of sialic acids for the infection of cultured cells for the strains Italy02, 4/91 and QX, we chose primary embryo chick kidney cells because they are sensitive to infection with all strains including the Beaudette strain, which has a broader tropism on cultured cells and was used as a reference strain. For this strain the importance of sialic acid for infection of embryo chick kidney cells has been described recently (Winter et al., 2006). As shown in , desialylation of the cells with 50 mU neuraminidase prior to infection by either of the different strains decreased the number of infected cells by about 50% for Beaudette, 4/91 and QX. After infection with Italy 02, the decrease in the plaque number was about 75%.
Figure 1. Effect of pretreatment of cells with neuraminidase on the infection by different strains of IBV. Chick embryo kidney cells were incubated in the presence (open boxes) or absence (black boxes) of neuraminidase (NA) of C. perfringens and then infected by one of the four IBV strains Beaudette (Bd), 4/91, Italy 02, and QX at a multiplicity suitable for a plaque assay. The reduction in the plaque number was used to determine the effect of the enzyme treatment on the infectivity of IBV.
Importance of sialic acids for the infection of TOCs
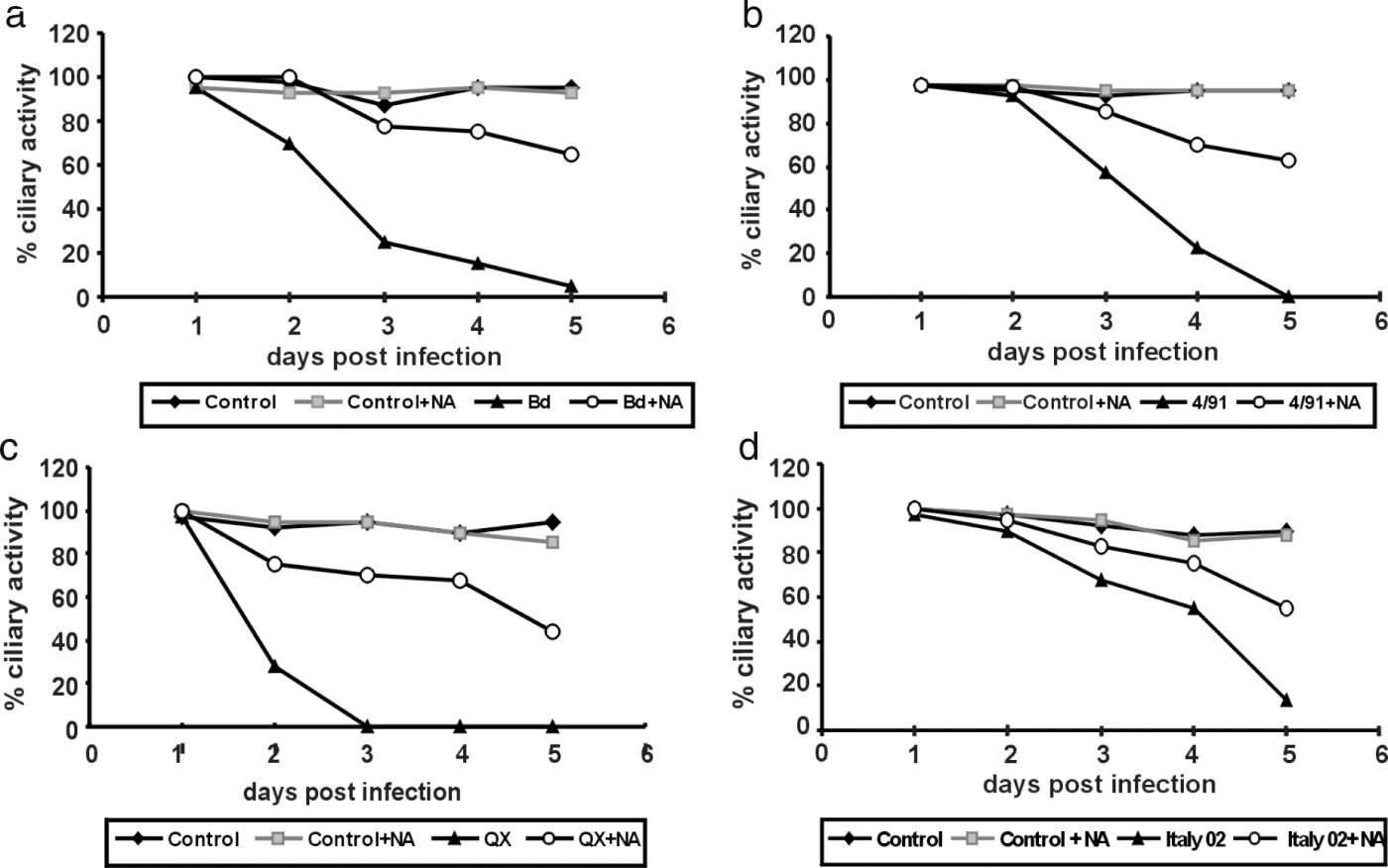
We have recently shown that desialylation of TOCs results in a delay of ciliostasis after infection by IBV. Here we confirmed this effect with the isolates Italy02, 4/91 and QX (). All strains induced ciliostasis after infection of TOCs with 104 plaque-forming units. In the case of QX, the epithelium had completely lost ciliary activity by 2 days post infection, whereas infection with the other strains resulted in complete ciliostasis at around 5 days post infection. Prior desialylation of the TOCs prevented complete ciliostasis. At 5 days post infection, more than 50% of the ciliary activity was retained for all strains. This shows the universal importance of sialic acids for the infection of the tracheal epithelium by all analysed IBV strains.
Figure 2. Effect of neuraminidase treatment on the infection of TOCs by different strains of IBV. TOCs were incubated in the presence or absence of neuraminidase (NA) from C. perfringens and then infected by one of the four IBV strains (2a) Beaudette (Bd), (2b) 4/91, (2c) QX, and (2d) Italy 02, or mock-infected. Up to 5 days post inoculation, TOCs were analysed for ciliary activity at daily intervals.
Infection of the epithelial cells in TOCs
We were interested to analyse whether there are strain-dependent differences in the infection of primary target cells by IBV. For this purpose cryosections were prepared from infected TOCs and were stained for virus antigen. In parallel, the sections were immunostained for tubulin (), mucin (), and for reactivity with the lectin MAA () to visualize ciliated cells, mucus-producing cells, or cells expressing 2,3-linked sialic acids, respectively. Co-staining of cells with antibodies against IBV N protein and β-tubulin indicated that all strains were able to infect ciliated cells (). Co-staining of cryosections for virus antigen and MUC5Ac demonstrated that all strains were able to infect mucus-producing cells (). However, the strains differed in their efficiency to infect the tracheal epithelium. Infection by Beaudette and QX resulted in a larger number of infected cells compared with Italy 02 and 4/91 when TOCs were infected with the same amount of virus, which was determined by titration on primary chick kidney cells. To achieve comparable pictures, TOCs infected with Italy 02 and 4/91 were infected with an infectious dose that was five times higher compared with the other strains.
Figure 3. Immunofluorescence analysis of cryosections prepared from IBV-infected tracheal organ cultures. At 24 h post infection, sections were stained with an anti-β-tubulin antibody to detect cilia (red) and with a monoclonal anti-N antibody to vizualize virus antigen (green). Bd, Beaudette.
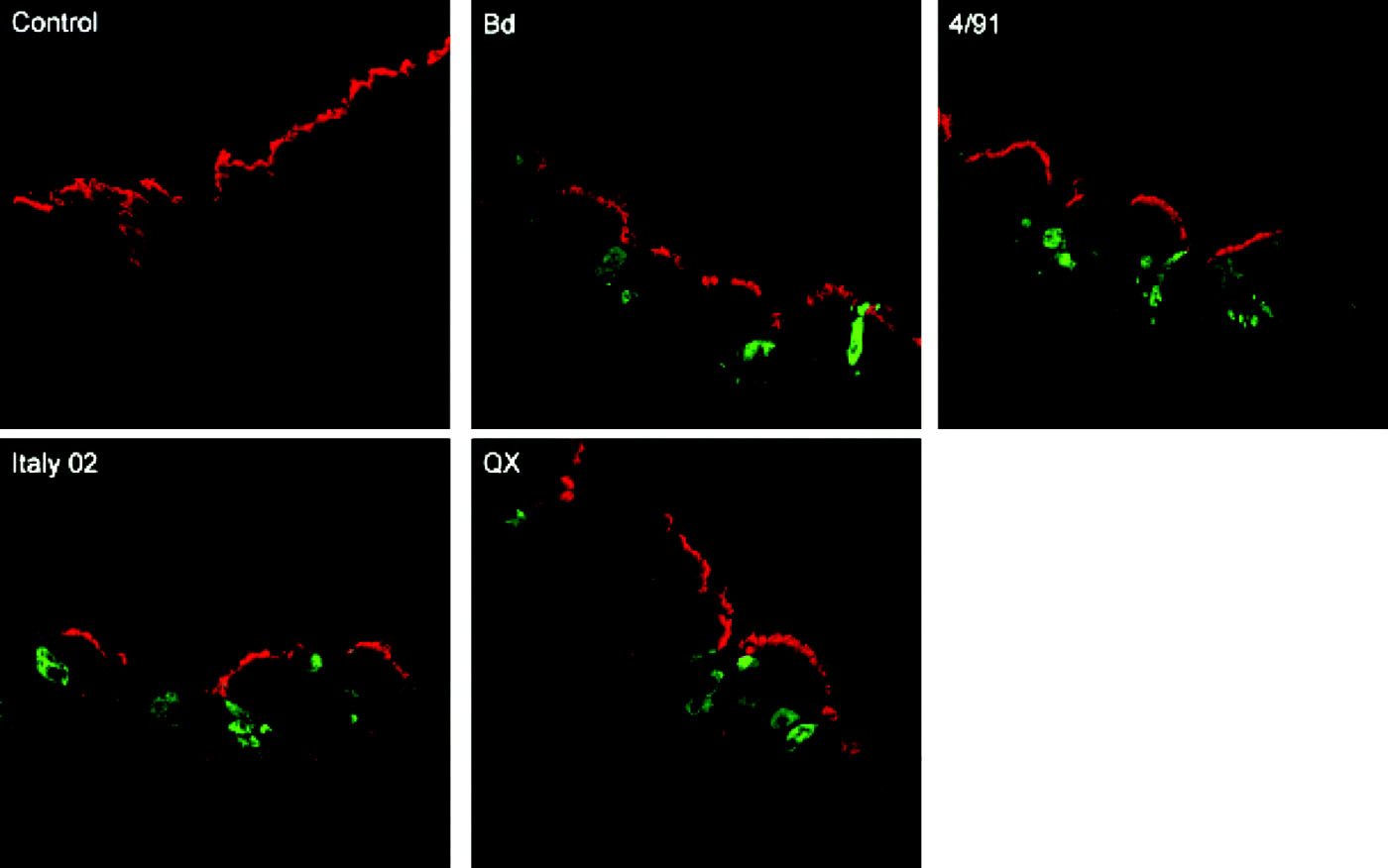
Figure 4. Immunofluorescence analysis of cryosections prepared from IBV-infected tracheal organ cultures. At 24 h post inoculation, sections were stained for goblet cells using an anti-Muc5AC antibody (red) and for virus antigen using a polyclonal IBV serum (green). Areas of co-staining are indicated by white arrows. Bd, Beaudette.
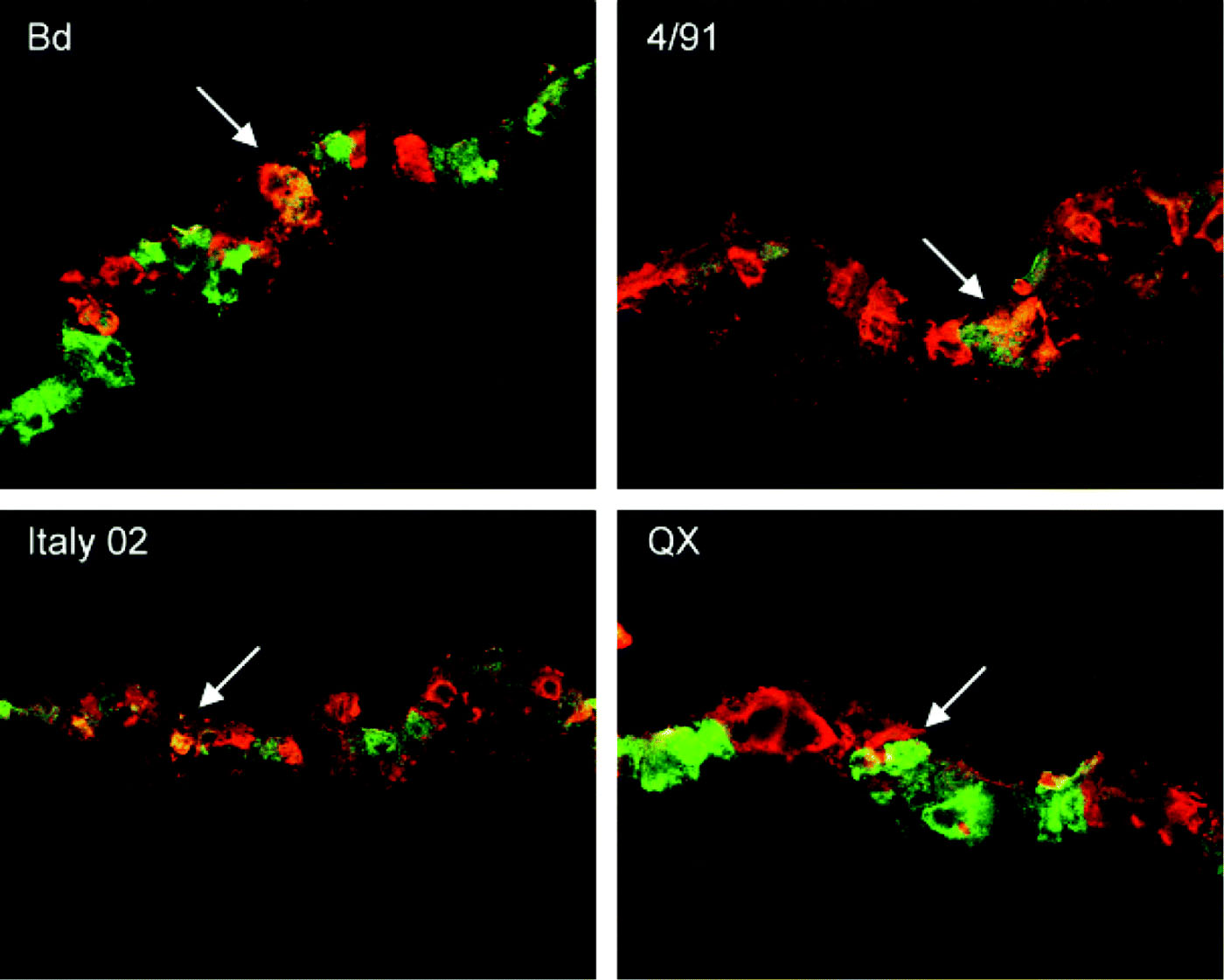
Figure 5. Immunofluorescence analysis of cryosections prepared from IBV-infected tracheal organ cultures. At 24 h post infection, sections were incubated with the lectin MAAII to detect α2,3-linked sialic acid (red). The apical side of the ciliated epithelium is indicated by an arrow. Virus antigen was detected with a monoclonal anti-protein N antibody (green). Interestingly, the apical staining with MAAII was always reduced in infected tissues. Bd, Beaudette.
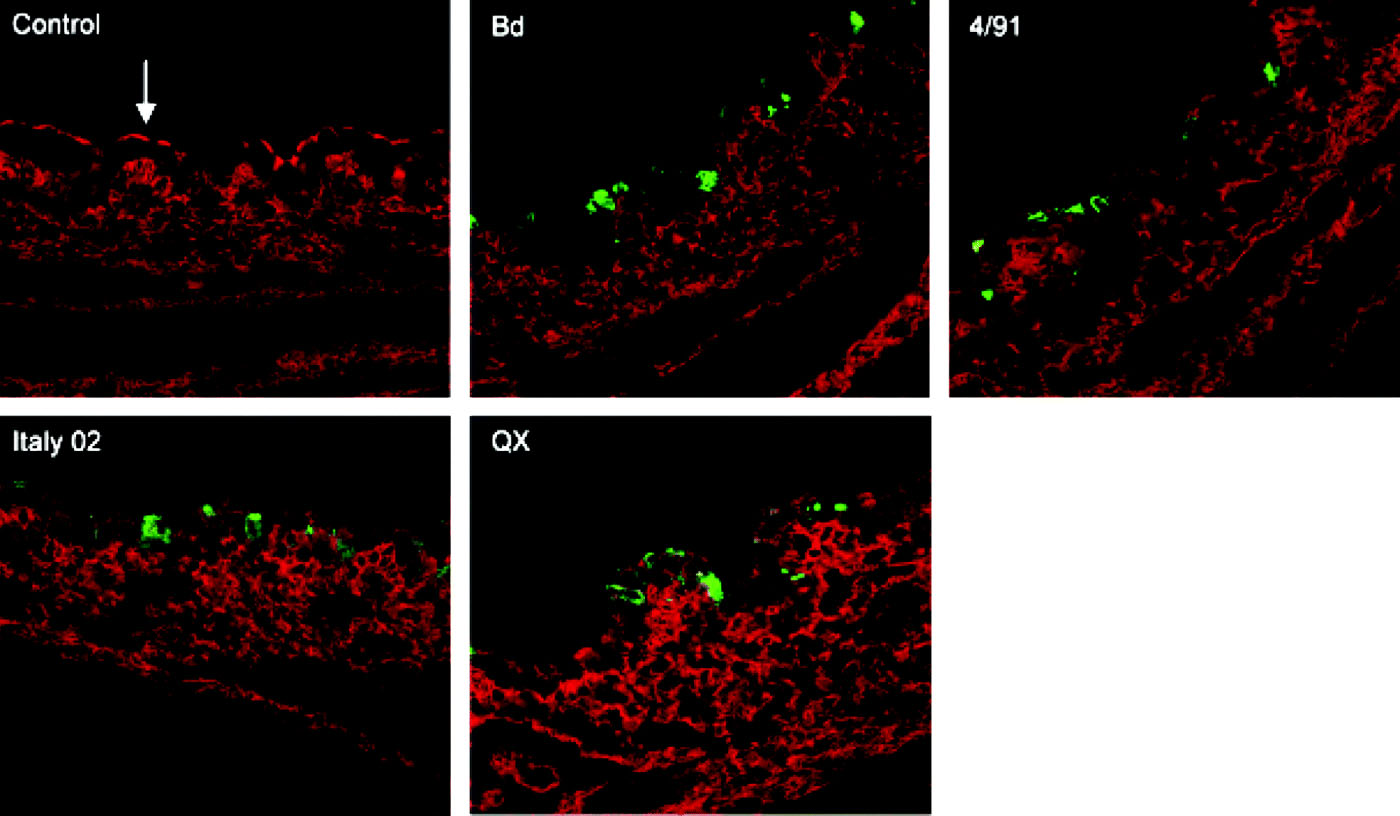
Sialic acid expression of infected cells
When TOCs were analysed for reactivity with lectins, we found an intensive staining of the tracheal epithelium with the lectin MAAII that recognized α2,3-linked sialic acids (). We observed staining of the basal cell layer as well as mucus in goblet cells and a clear staining of the apical membrane. This result showed that in the tracheal epithelium there were many cells expressing α2,3-linked sialic acids. These are potential receptor determinants for the different IBV strains. Interestingly, a co-staining of infected TOCs for virus antigen and sialic acids revealed a weaker MAAII binding in infected tissues, especially on the apical membrane.
Discussion
Infection of poultry with IBV is still a matter of economic concern, mainly due to the occurrence of new serotypes with low cross-protection by conventional vaccines. We analysed three major serotypes, which show a high incidence in Europe, for their ability to use sialic acids as a receptor determinant.
One characteristic of IBV is that most strains grow only in primary cells derived from their natural host, the chicken. A major factor determining this tropism might be the binding to structures on the cell surface that allow internalization of the virions. In a previous work (Winter et al., 2006) we demonstrated that primary chicken kidney cells became resistant to infection by the two IBV strains Beaudette and M41 after sialic acids had been removed from the cell surface by neuraminidase treatment. Here we show for the strains 4/91 and QX that desialylation of chick embryo kidney cells decreases the number of infected cells to the same extent as for the control strain Beaudette. For the strain Italy 02 we observed an even stronger effect. This may be explained by a different efficiency of this strain in the recognition of sialic acid molecules on the cell surface. Interestingly, the protective effect of the neuraminidase treatment observed in Italy 02-infected TOCs was not more powerful than that of TOCs infected by the other strains. However, the ciliostasis induced by this strain was slower compared with the other strains, indicating that Italy 02 does not replicate in TOCs as efficient as the three other strains. A weaker binding of strain Italy 02 to the sialoglycoconjugates on the tracheal epithelium may explain our results. The most rapidly damaging virus in TOCs appeared to be the QX isolate followed by the Beaudette strain. This finding is in accordance with the fact that, with these two viruses, a five times lower amount of virus was required for infection of TOCs to get a similar number of infected cells, compared with Italy 02 and 4/91. This difference makes it all the more astonishing that, for both strains QX and Beaudette, at least 50% of the ciliary activity was retained at 5 days post infection of desialylated cells, similar to the values determined for Italy 02 and 4/91. The finding that neuraminidase treatment could not prevent ciliostasis completely can be explained by incomplete removal of sialic acids from the epithelial cells, so that some cells still became infected.
We could not observe any differences in the choice of the primary target cells by the analysed strains. Antigen of all strains could be detected in ciliated cells as well as in goblet cells. It appears that there is no preference for one cell type that could explain the differences in the increased infectivity of QX and Beaudette. The analysis of the tracheal epithelium with the lectin MAAII showed that α2,3-linked sialic acids are abundantly expressed in the epithelial cells. The interesting finding that the MAAII binding to the apical membrane was always reduced after infection by the different IBV strains might be a consequence of infection. Spike proteins expressed on the cell surface may interact with α2,3-linked sialic acids and thus prevent the lectin from binding to this sugar molecule. The interaction of S protein with α2,3-linked sialic acids may also result in down-regulation of the respective sialoglycoconjugates from the cell surface. Such a phenomenon could also explain the observed effect of virus interference after vaccination with different attenuated strains at the same time point (Winterfield & Fadly, Citation1975).
Sialic acids are used by a broad range of pathogenic organisms to attach to cells. IBV appears to follow a similar strategy as, for example, avian influenza viruses to bind to epithelial cells. The fact that all analysed IBV strains use sialic acids to bind to epithelial cells leads to the conclusion that, despite the high variation among the different S proteins, the sialic acid binding activity is a conserved feature of IBV. This does not necessarily mean that all strains bind with the same strength to specific glycans. It could well be that some strains prefer different sialoglycoconjugates than others, and hence have a slightly different organ tropism within the host. The analysis of the recognized sialylated glycans and the identification of the sialic acid binding site in the S protein will be interesting in future work.
Acknowledgements
The authors thank Hans Philipp and Dave Cavanagh for providing the different IBV strains. They also thank Sonja Bernhardt for technical assistance. This work was supported by the Arab republic of Egypt with a scholarship for Sahar Abd El Rahman and Deutsche Forschungsgemeinschaft (NE221/5-1)
References
- Cavanagh , D. 2007 . Coronavirus avian infectious bronchitis virus . Veterinary Research , 38 : 281 – 297 .
- Cavanagh , D. and Davis , P.J. 1986 . Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells . Journal of General Virology , 67 : 1443 – 1448 .
- Winter , C. , Schwegmann-Wessels , C. , Cavanagh , D. , Neumann , U. and Herrler , G. 2006 . Sialic acid is a receptor determinant for infection of cells by avian Infectious bronchitis virus . Journal of General Virology , 87 : 1209 – 1216 .
- Winter , C. , Herrler , G. and Neumann , U. 2008 . Infection of the tracheal epithelium by infectious bronchitis virus is sialic acid dependent . Microbes and Infection , 10 : 367 – 373 .
- Winterfield , R.W. and Fadly , A.M. 1975 . Potential for polyvalent infectious bronchitis vaccines . American Journal of Veterinary Research , 36 : 524 – 526 .
- Worthington , K.J. , Currie , R.J. and Jones , R.C. 2008 . A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006 . Avian Pathology , 37 : 247 – 257 .