Abstract
Two experiments were performed to evaluate the protective effect of various vaccination combinations given at 5 and 9 weeks of age against experimental challenge with Salmonella enterica serovar Enteritidis (SE) phage type 4 at 12 weeks of age. In Experiment 1, groups of commercial layers were vaccinated by one of the following programmes: Group 1, two doses of a SE bacterin (Layermune SE); Group 2, one dose of a live Salmonella enterica serovar Gallinarum vaccine (Cevac SG9R) followed by one dose of the SE bacterin; Group 3, one dose of each of two different multivalent inactivated vaccines containing SE cells (Corymune 4K and Corymune 7K; and Group 4, unvaccinated, challenged controls. In Experiment 2, groups of broiler breeders were vaccinated by the same programmes as Groups 1 and 2 above while Group 3 was an unvaccinated, challenged control group. All vaccination programmes and the challenge induced significant (P < 0.05) seroconversion as measured by enzyme-linked immunosorbent assay. Overall, in both experiments, all vaccination schemes were significantly effective in reducing organ (spleen, liver and caeca) colonization by the challenge strain as well as reducing faecal excretion for at least 3 weeks. Vaccinated layers in Groups 1 and 2 and broiler breeders in Group 2 showed the greatest reduction in organ colonization and the least faecal excretion. In Experiment 1, layers vaccinated with multivalent inactivated vaccines containing a SE component (Group 3) were only moderately protected, indicating that such a vaccination programme may be useful in farms with good husbandry and housing conditions and low environmental infectious pressure by Salmonella.
Introduction
Salmonella infections may be responsible for chronic and acute diseases in poultry, but in the field are usually subclinical, leading to a risk of food poisoning in consumers of contaminated poultry products. Infections occur frequently and are difficult to control, especially in countries with intensive large-scale industrial production (World Health Organization & Food and Agriculture Organization of the United Nations, Citation2002; Rosu et al., Citation2007).
Salmonella enterica serovar Enteritidis (SE) is considered to be a zoonotic agent commonly found in domestic poultry and has been responsible for many outbreaks of human salmonellosis through consumption of contaminated food, especially those prepared with raw eggs or other poultry products (Rodrigue et al., Citation1990; Duguid & North, Citation1991; Morse et al., Citation1994; Santos et al., Citation2000; Altekruse et al., Citation2006; Humphrey, Citation2006; Gast, Citation2007). Although some strains of SE phage type 4 (PT4) may cause increased morbidity and mortality in broilers (Barrow et al., Citation1987; Poppe, Citation2000), birds often have subclinical infection and the organism can be disseminated readily by vertical and horizontal routes (Guard-Petter, Citation2001). Therefore it is necessary to implement monitoring programmes based on bacteriological and serological testing to prevent the infection or to control dissemination of the organisms. Additional measures such as competitive exclusion, heat treatment of feed, incorporation of organic acids in feed, control of rodents, and so forth, can be taken as part of a comprehensive biosecurity programme (Iba & Berchieri Jr, Citation1995; Patterson & Bukholder, Citation2003).
Vaccination of layer and broiler breeder hens could contribute significantly to reducing Salmonella numbers in the table egg industry and broiler processing plants (Collard et al., Citation2008). Vaccination might afford protection for the entire productive life of the birds (Mastroeni et al., Citation2000), reduce the duration and severity of infection and help to prevent reinfection (Gast, Citation2007). Cogan & Humphrey (Citation2003) reported that cases of human salmonellosis due to food poisoning decreased significantly in the United Kingdom after the implementation of a widespread vaccination programme in commercial layers.
Gast et al. (Citation1992) observed a rapid primary humoral response after vaccination of specific pathogen free chickens with a killed oil-emulsion SE vaccine, and there was partial protection against experimental challenge with SE. The presence of the challenge strain in organs and egg contents was reduced, but the enteric tract was still susceptible to colonization. It seems that such colonization is more difficult to prevent than that of internal organs (Hassan & Curtiss, Citation1996; Adriaensen et al., Citation2007). Gast et al. (Citation1993) reported that two different oil-emulsion SE bacterins significantly reduced faecal shedding 1 week after SE challenge. However, no protective effect could be measured by the second week because intestinal colonization by the challenge strain had reached such low levels in all groups.
The efficacy of the bacterins depends upon several factors such as stress conditions (Nakamura et al., Citation1994) and the composition of the vaccine (Liu et al., Citation2001). Freitas Neto et al. (Citation2008) observed variable performance among three commercial SE killed vaccines. A recent study by Davies & Breslin (Citation2004) supports earlier observations that the maximum performance of a vaccination programme is obtained when associated with very strict on-farm hygiene measures. Several studies have shown that vaccines can reduce faecal shedding and systemic spread of SE in laying hens, and thus reduce egg contamination (Miyamoto et al., Citation1999; Woodward et al., Citation2002; Davies & Breslin, Citation2004; Gantois et al., Citation2006; Collard et al., Citation2008).
Live vaccines against Salmonella are able to induce cell-mediated and humoral immune responses (Babu et al., Citation2003; Rana & Kulshreshtha, Citation2006). Although killed SE bacterins do not promote a full immune response, they are considered safer because there is no risk of reversion to virulence, no spread in the environment and they are good enough, in general, to protect chickens when applied in large-scale poultry production (Barrow et al., Citation1991; De Buck et al., Citation2004). Alternatively, it has been suggested that the utilization of an attenuated rough strain of Salmonella enterica serovar Gallinarum (SG9R) may be a safe and very effective way of controlling SE infections in chickens (Tan et al., Citation2008a, Citationb; Witvliet et al., Citation2008). This strain has been shown to be non-pathogenic for chickens (Smith, Citation1956; Gordon & Luke, Citation1959; Gordon et al., Citation1959; Gupta & Mallick, Citation1976; Smith & Tucker, Citation1980) and has no public health significance as it cannot cause infection in humans. Lee et al. (Citation2005)) demonstrated that SG9R was able to control the systemic infection by Salmonella Gallinarum, drastically reducing the morbidity and mortality rates among vaccinated birds. In field studies, Feberwee et al. (Citation2001a, Citationb) observed that SG9R contributed to the reduction of SE in layer flocks and, since the vaccine strain could not be detected in the faeces or eggs, it could be considered that it offers minimum risk for the environment.
The present study assessed the efficacy of commercial killed SE vaccines alone or in combination with a commercial live SG9R vaccine in controlling an experimental SE challenge.
Materials and Methods
Birds
Chickens from a commercial genetic strain of table egg layers (Dekalb white; Granja Planalto, Uberlândia-MG, Brazil) were used in Experiment 1, and female birds from a commercial genetic strain of broiler breeders (Cobb 500; Cobb-Vantress Brasil, Guapiaçú-SP, Brazil) were used in Experiment 2. They were obtained at 1 day of age and were reared and fed as recommended in the respective production manuals.
On arrival, the Salmonella-free status of the birds was checked by sampling the transport boxes with drag swabs for detection of Salmonella spp. (Zancan et al., Citation2000). All samples tested were negative.
Experiment 1 was carried out first and then a downtime period of 4 weeks was allowed for cleaning and disinfection before the start of Experiment 2. The birds were reared in the same house until challenge. Each group consisted of 36 birds, equally divided into separate sets of six cages. Empty cages were placed in between the groups so there was no contact between birds of different groups, facilitating the daily husbandry, cleaning and disinfection of the house. Two weeks before challenge, the groups were moved to separate rooms with controlled ambient conditions.
Vaccines
Commercial vaccines produced by CEVA-Phylaxia (Cevac Corymune 4K and 7K; Budapest, Hungary), CEVA Biomune (Layermune SE; Lenexa, USA) and CEVA Campinas (Cevac SG9R; Campinas, SP Brazil) were used. Cevac Corymune 4K contains an inactivated combination of Avibacterium paragallinarum serotypes A, B and C, an SE strain, homogenized with aluminium hydroxide adjuvant and thiomersal as a preservative. Cevac Corymune 7K is as above but with the addition of Newcastle disease virus (La Sota strain), infectious bronchitis virus (Massachusetts strain) and egg drop syndrome virus (B8/78 strain). Layermune SE is an inactivated bacterial vaccine (bacterin) containing multiple selected strains of SE in oil adjuvant. Cevac SG9R contains live Salmonella Gallinarum strain 9R in freeze-dried form. The vaccine is at a concentration of at least 107 colony-forming units per dose and is naturally attenuated and non-pathogenic for chickens.
Vaccines were administered either intramuscularly in the breast muscle (Cevac Corymune 4K and 7K and Layermune SE) or subcutaneously in the dorsal lower part of the neck (Cevac SG9R) as recommended by the manufacturer.
Challenge strain and preparation of inocula
The inocula were prepared from a strain of Salmonella Enteritidis PT4, kindly donated by Professor P.A. Barrow (University of Nottingham, Leicestershire, UK) and previously shown to be invasive in laying hens (Barrow & Lovell, Citation1991; Freitas Neto et al., Citation2008; Inoue et al., Citation2008). For ease of enumeration in organs and faeces, a spontaneous nalidixic acid and spectinomycin-resistant mutant of this strain (SENalrSpecr) was used. The inocula consisted of a culture of SENalrSpecr in Luria-Bertani (LB) broth (CM0395 and LP0121A; Oxoid) grown for 24 h in a shaking incubator (100 rev/min ) at 37°C. This culture contained approximately 8×108 colony-forming units/ml. At 12 weeks of age all birds were inoculated orally, directly into the crop, with 2 ml (Experiment 1) or 3 ml (Experiment 2) of the SENalrSpecr culture. The different volumes allowed for differences in body weight of the two types of bird and had been established by earlier pilot experiments.
Experimental design
The designs of both experiments are shown in .
Table 1. Vaccination programmes and SE challenge
Experiment 1
One hundred and forty-four commercial table egg layers were divided into four groups of 36 birds. Groups 1, 2 and 3 were immunized at 5 weeks (first dose) and 9 weeks (second dose) of age. Group 1 received two doses of Layermune SE, intramuscularly; Group 2 received one dose of CEVAC SG9R subcutaneously in the nape of the neck, and a second vaccination with Layermune SE (intramuscularly); Group 3 received one dose of Corymune 4K (intramuscularly) and a second vaccination with Corymune 7K (intramuscularly); and Group 4 was kept as non-immunized, Salmonella-challenged positive controls.
Experiment 2
One hundred and eight broiler breeders were divided into three different groups (36 birds per group). Groups 1 and 2 received the same vaccination treatments as the corresponding groups in Experiment 1, and Group 3 birds were kept as non-immunized, Salmonella-challenged positive controls.
In both experiments all birds were challenged with a culture of SENalrSpecr at the age of 12 weeks, and in both experiments five birds from each group were killed by cervical dislocation at each of 2, 5, 7, 14 and 21 days post challenge. Approximately 1g samples of spleen, liver, and caecal contents were removed from each bird for isolation and determination of bacterial count of the challenge strain.
Twice a week, at 3, 6, 10, 13, 17 and 20 days post challenge cloacal swabs were taken from all remaining birds and assayed for faecal shedding of SE. Blood samples from six designated birds in each group were collected weekly for 5 weeks, beginning one week after the second vaccination.
Bacteriology
Bacteriological analyses were carried out as described by Barrow & Lovell (Citation1991) with some modification. Swabs were placed in selenite broth (CM0395 and LP0121A; Oxoid) containing novobiocin (40 µg/ml) (SN) and directly plated onto Brilliant Green Agar (CM0263; Oxoid) containing nalidixic acid (100 µg/ml) and spectinomycin (100 µg/ml) (BGA Nal/Spec). Incubation was at 37°C for 24 h and, in the absence of growth, the appropriate enriched swab cultures were inoculated onto fresh plates.
After the harvesting of spleen, liver and caecal contents, the samples were diluted immediately (1:10) in phosphate-buffered saline, pH 7.4 and homogenized with a pestle and mortar. The viable count of SENalrSpecr in the samples was measured by plating aliquots of decimal dilutions on BGA Nal/Spec incubated overnight at 37°C. The first dilution of the sample was added to an equal volume of double-strength SN. This was incubated at 37°C overnight and plated on BGA Nal/Spec agar when there was no growth from the viable count assay.
Serology
Sera collected from the blood samples were stored at −20°C until the end of both experiments, when enzyme-linked immunosorbent assays (ELISAs) were carried out on all samples using a single commercial ELISA kit (FlockChek Salmonella Enteritidis Antibody Test Kit; IDEXX Laboratories, Westbrook, Maine, USA). Sera were diluted 1:2 and antibody titres were expressed as the sample value/negative control value (S/N) ratio of optical density (OD) values (sample OD value divided by the average OD value for the negative control samples of the ELISA kit). The S/N ratio presents a high correlation with circulating antibody titres; the higher the S/N ratio, the lower the antibody titre (a positive sample must have an S/N ratio < 0.6).
Statistical analysis
SE antibody titres were analysed and compared within and between groups by the Students t test (Sigmaplot 8.0 software; http://www.sigmaplot.com). Mean viable numbers of SENalrSpecr in organ samples taken from the spleen, liver and caeca and cumulative numbers of birds SENalrSpecr positive on cloacal swabs were analysed and compared between groups by the Tukey test and the chi-square test, respectively. Statistical differences were set at the probability level of P < 0.05.
Results
Bacteriology
Experiment 1
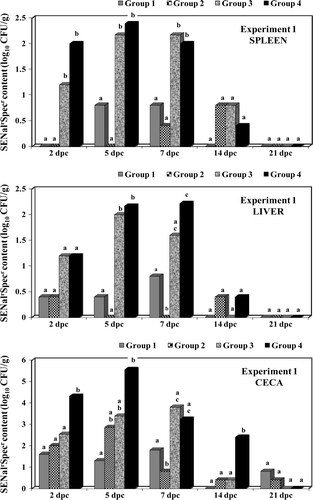
shows the results of bacteriological cultures for samples of spleen, liver and caecal content. A significant (P < 0.05) reduction in numbers of SENalrSpecr was seen in vaccinated birds in Groups 1 and 2 at the earlier time points. Birds in Group 3 showed a numerical reduction when compared with unvaccinated birds in Group 4 but the differences were significant only in caecal contents and only on two occasions.
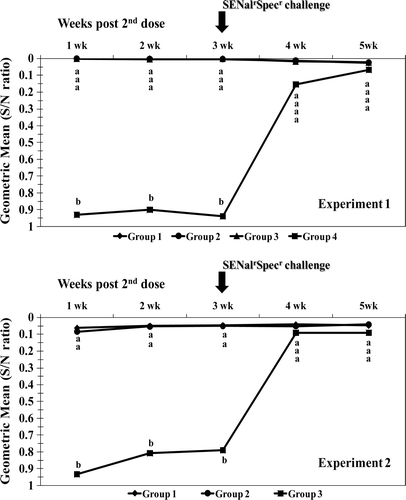
Figure 1. Experiments 1 and 2. Circulating SE antibody titres measured by ELISA in serum taken weekly for 5 weeks after the second dose of vaccine. Titres are shown as geometric means of the S/N ratio; the higher the S/N ratio value, the lower the antibody titre. Samples presenting S/N ratio < 0.6 are positive. Group means with different letters at the same sampling time are statistically different (P<0.05).
shows the results for faecal excretion (cloacal swab samples) in the different treatment groups as expressed by the cumulative number of birds testing positive for SENalrSpecr isolation at different days post challenge. Birds in Groups 1 and 2 always excreted significantly less (P < 0.05) SENalrSpecr when compared with birds in Groups 3 and 4. Vaccinated birds in Group 3 also presented a significant reduction in faecal excretion when compared with control birds in Group 4 and overall, Group 3 showed an intermediate reduction in SENalrSpecr faecal excretion.
Experiment 2
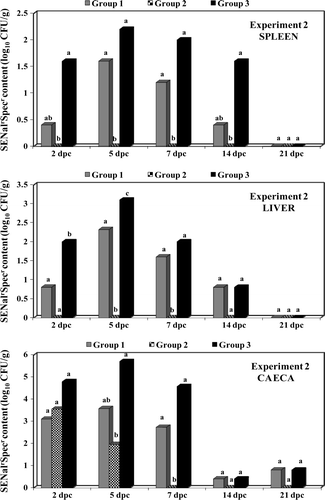
shows the bacteriology results of the experimental groups in Experiment 2 for samples from caecal contents and liver and spleen tissue. As in Experiment 1, vaccinated birds in Experiment 2 showed an evident protection against organ colonization by the SENalrSpecr challenge strain. The most pronounced and significant (P < 0.05) protective effect from SE vaccination was seen in birds in Group 2, which were vaccinated with live SG9R vaccine followed by an inactivated SE bacterin.
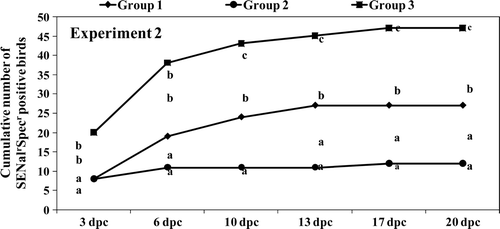
Figure 3. Experiment 1. Cumulative numbers of birds positive for SENalrSpcr isolation at each sampling day post challenge (dpc). Different letters at each sampling dpc indicate statistically different totals (P<0.05).
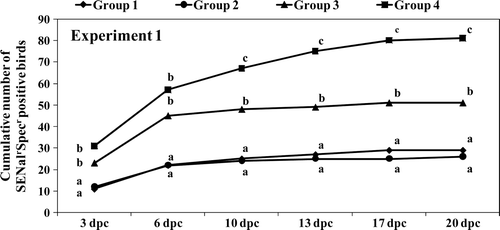
Bacteriology results from the cloacal swab samples also showed a significant (P < 0.05) protective effect of vaccination against faecal excretion of the SENalrSpecr challenge strain (). Birds in both vaccinated groups excreted significantly less Salmonella in faeces after the heavy experimental challenge.
Serology (Experiments 1 and 2)
Levels of circulating antibodies against SE in vaccinated and control birds in both experiments are shown in . All vaccinated birds in both experiments were strongly positive for SE antibodies throughout the sampling period and the SENalrSpecr challenge did not induce any detectable change in their antibody titres. All control birds remained antibody negative for SE until the time of the experimental challenge after which all birds seroconverted within 1 week to levels similar (P > 0.05) to those of vaccinated birds. For the ELISA assay, serum samples were diluted 1:2 as per the manufacturer's instructions and samples were simply considered either positive or negative. Due to the low sample dilution and qualitative measurement it was impossible to detect a measurable variation in SE antibody positivity among birds in the vaccinated groups. In both experiments, all vaccinated groups presented very low S/N ratios and a very low coefficient of variation (range 15 to 31) throughout. Also, no variation in SE antibody positivity was observed in vaccinated birds even after the SE challenge, possibly due to already high levels of circulating antibody. A similar picture was observed in both control groups, which were negative until challenge and then showed a significant seroconversion at 1 and 2 weeks after challenge (coefficient of variation range for the S/N ratios at those 2 weeks were 60 to 78 and 51 to 89, respectively). The positive and negative control OD values for the ELISA run were 0.247/0.261 (positive controls) and 1.219/1.257 (negative controls).
Discussion
SE has been disseminated worldwide by poultry through vertical transmission (Wall & Ward, Citation1999; Zancan et al., Citation2000; Gast & Holt, Citation2001). The emergence of this pathogen in the commercial poultry production and the risks to public health posed by Salmonella infection (Rodrigue et al., Citation1990) increased interest in its control (Mead & Barrow, Citation1990; Zhang-Barber et al., Citation1999). Once SE is introduced on a poultry farm it can remain in the environment for several months (Gama et al., Citation2003; Maciorowski et al., Citation2004), being easily disseminated by vertebrate and invertebrate vectors (Henzler & Opitz, Citation1992; Davies & Wray, Citation1996; Davies & Breslin, Citation2003; Hazeleger et al., Citation2008).
In view of the complex epidemiology of SE it is economically impractical to eliminate the pathogen from farms. Establishing immunity in commercial flocks would be the most effective way to control infections, but, as with other specific control measures, vaccination alone only produces partial protection and/or control. Although Davies & Breslin (Citation2004) found SE in vaccinated flocks, its presence can be reduced by vaccination (Gast et al., Citation1993; Woodward et al., Citation2002), which reduces the duration and the severity of the infection and helps to prevent re-infection (Gast, Citation2007). This indirectly decreases cases of human food-borne salmonellosis (Collard et al., Citation2008).
Environmental conditions (stress, poor hygiene, etc.) and poor management practices may interfere with the success of vaccination. Kanashiro et al. (Citation2008) demonstrated the extent of such interferences when they assessed the serological response of broiler breeder from commercial flocks and from experimentally vaccinated chickens. All samples from the experimentally vaccinated birds were seropositive while no antibody was detected in many broiler breeders vaccinated under field conditions, indicating vaccination failure due to poor administration procedure. The composition of the vaccine is also a matter of concern. The reduction in the systemic infection and faecal excretion are very variable (Barbour et al., Citation1993; Freitas Neto et al., Citation2008).
A similar situation was seen in the present study. The vaccination programmes with Layermune SE (two doses) and Cevac SG9R plus Layermune SE were superior to Corymune 4K and Corymune 7K, although the latter also reduced faecal excretion, demonstrating the beneficial effect of the vaccination with those multivalent inactivated vaccines containing a SE fraction. Previous studies (Gast et al., Citation1992; Cooper et al., Citation1994; Betancor et al., Citation2005) have reported that vaccines against SE were more effective in reducing systemic infection and had only a poor action on the digestive tract. It seems likely that cell-mediated immunity is responsible for tissue clearance, but how it could be responsible for intestinal clearance remains unclear (Zhang-Barber et al., Citation1999). Resistance to intestinal Salmonella infections may depend more on the humoral response, particularly IgA, and polymorphonuclear cells, although this has not been proven experimentally (Nagaraja & Rajashekara, Citation1999; Barrow & Wallis, Citation2000). In Experiment 2, Corymune 4K and 7K vaccines were not used as they were developed for use in commercial layers only. The combination of the live Cevac SG9R plus Layermune SE was more effective than Layermune SE (two doses) in broiler breeders and equally effective in commercial layers. It appears that the use of a live SG9R vaccine in the present study induced a stronger immune response than use of inactivated vaccines only. It is widely accepted that cell-mediated immunity is more important than humoral responses in protection against Salmonella infections (Collins, Citation1974; Mastroeni et al., Citation1993); therefore, vaccinating the birds with the live SG9R vaccine may have been responsible for the more effective protection against colonization and faecal excretion seen in birds from Group 2 in both our experiments.
The live vaccine (Cevac SG9R) used here contains an apathogenic rough strain of Salmonella Gallinarum. Both Salmonella Gallinarum and SE belong to the Salmonella serogroup Dl, and share the same immunodominant (Ochoa-Repáraz et al., Citation2004) somatic “O” antigen formula (1,9,12; Ewing, Citation1986), and therefore reasonable cross-protection between the two serotypes is expected. This theory was confirmed in a large field trial in the Netherlands in which 80 commercial flocks were vaccinated with the SG9R vaccine strain and the flock level of occurrence of SE infections was 2.5% (2/80 ?ocks), which was significantly less than that in unvaccinated flocks (214 out of 1854 flocks, 11.5%; Feberwee et al., Citation2001a). No vaccine strain bacteria were detected in 4500 eggs derived from five SG9R vaccinated ?ocks, while in another study no evidence was found for the faecal spread of the vaccine strain (Feberwee et al., Citation2000, Citation2001b).
In the present study, birds with two very different genetic backgrounds were used in Experiments 1 and 2. Although the vaccination programme consisting of the live Cevac SG9R plus the inactivated Layermune SE vaccines was effective in both experiments and the most effective as far as faecal excretion was concerned, the results from organ colonization indicate that a more consistent protection against systemic infection occurred in the broiler breeders. This suggests that besides environmental factors, vaccine composition and bird management, the genetic background of the birds might be influencing the immune response. It is well known that genetic resistance to Salmonella infections varies among different lines of domestic chicken (Kaiser et al., Citation2002).
In general, white lines of layers are much more resistant than heavy broiler breeder lines (Bumstead & Barrow, Citation1993; Girard-Santosuosso et al., Citation1998). In addition, there is some evidence indicating that white layer lines may excrete SE for longer than semi-heavy and heavy lines after experimental challenge (Berchieri Jr et al., Citation2001; A. Berchieri Jr, unpublished data). Genetic selection work carried out on commercial birds does not yet include resistance to Salmonella infections. Thus, the best way to prevent or control Salmonella infection in poultry flocks is still through the use of sound biosecurity measures. The present study clearly showed that vaccines can also be a useful tool to help decrease SE infection.
Finally, commercial layers in Group 3 in Experiment 1 (vaccinated with Corymune 4K and Corymune 7K vaccines) showed intermediate protection against the experimental SENalrSpecr challenge. This was most evident in the reduction of faecal excretion of the challenge strain. Such results could be expected because the vaccine is a multivalent inactivated formulation that contains lower SE-antigen concentration. However, such a product could be a very good choice for use in farms with good biosecurity and low environmental infectious pressure by SE.
In conclusion, results from the current experiments indicate that vaccination against Salmonella can be an important and effective tool within a comprehensive biosecurity programme designed for successful Salmonella control in industrial poultry farms.
Acknowledgements
The authors would like to express their sincere thanks to Miss Adriana Almeida for her invaluable technical assistance in the laboratory and research facilities.
References
- Adriaensen , C. , De Greve , H. , Tian , J.Q. , De Craeye , S. , Gubbels , E. Eeckaut , V. 2007 . A live Salmonella enterica serovar Enteritidis vaccine allows serological differentiation between vaccinated and infected animals . Infection Immunity , 75 : 2461 – 2468 .
- Altekruse , S.F. , Bauer , N. , Chanlongbutra , A. , DeSagun , R. , Naugle , A. Schlosser , W. 2006 . Salmonella Enteritidis in broiler chickens, United States, 2000–2005 . Emerging Infectious Diseases , 12 : 1848 – 1852 .
- Babu , U. , Scott , M. , Myers , M.J. , Okamura , M. , Gaines , D. Yancy , H.F. 2003 . Effects of live attenuated and killed Salmonella vaccine on T-lymphocyte mediated immunity in laying hens . Veterinary Immunology and Immunopathology , 91 : 39 – 44 .
- Barbour , E.K. , Wayne , W.M. , Nabbut , N.H. , Poss , P.E. and Brinton , M.K. 1993 . Evaluation of bacterins containing three predominant phage types of Salmonella Enteritidis for prevention of infection in egg laying chickens . American Journal of Veterinary Research , 54 : 1306 – 1309 .
- Barrow , P.A. and Lovell , M.A. 1991 . Experimental infection of egg-laying hens with Salmonella enteritidis phage type 4 . Avian Pathology , 20 : 335 – 348 .
- Barrow , P.A. and Wallis , T.S. 2000 . “ Vaccination against Salmonella infections in food animals: rationale, theoretical basis and practical application ” . In Salmonella in Domestic Animals , 1st edn , Edited by: Wray , C. 323 – 339 . Oxford : CAB International .
- Barrow , P.A. , Huggins , M.B. , Lovell , M.A. and Simpson , J.M. 1987 . Observations of the pathogenesis of experimental Salmonella typhimurium infection of chickens . Research in Veterinary Science , 42 : 239 – 252 .
- Barrow , P.A. , Lovell , M.A. and Berchieri , A. Jr . 1991 . The use of two live attenuated vaccines to immunize egg-laying hens against Salmonella enteritidis phage type 4 . Avian Pathology , 20 : 681 – 692 .
- Berchieri , A. Jr , Wigley , P. , Page , K. , Murphy , C.K. and Barrow , P.A. 2001 . Further studies on vertical transmission and persistence of Salmonella enterica serovar Enteritidis phage type 4 in chicken . Avian Pathology , 30 : 297 – 310 .
- Betancor , L. , Schelotto , F. , Fernandez , M. , Pereira , M. , Rial , A. and Chabalgoity , J.A. 2005 . An attenuated Salmonella Enteritidis strain derivative of the main genotype circulating in Uruguay is an effective vaccine for chickens . Veterinary Microbiology , 107 : 81 – 89 .
- Bumstead , N. and Barrow , P. 1993 . Resistance to Salmonella gallinarum, S. pullorum, and S. enteritidis in inbred lines of chickens . Avian Diseases , 37 : 189 – 193 .
- Cogan , T.A. and Humphrey , T.J. 2003 . The rise and fall of Salmonella Enteritidis in the UK . Journal of Applied Microbiology , 94 : 114 – 119 .
- Collard , J.M. , Bertrand , S. , Dierick , K. , Godard , C. , Wildemauwe , C. Kermeersh , K. 2008 . Drastic decrease of Salmonella Enteritidis isolated from humans in Belgium in 2005, shift in phage types and influence on foodborne outbreaks . Epidemiology and Infection , 136 : 771 – 781 .
- Collins , F.M. 1974 . Vaccines and cell-mediated immunity . Bacteriological Reviews , 38 : 371 – 374 .
- Cooper , G.L. , Venables , L.M. , Woodward , M.J. and Hormaeche , C.E. 1994 . Vaccination of chickens with strain CVL30, a genetically defined Salmonella enteritidis aroA live oral vaccine candidate . Infection and Immunity , 62 : 4747 – 4754 .
- Davies , R. and Breslin , M. 2004 . Observations on Salmonella contamination of eggs from infected commercial laying flocks where vaccination for Salmonella enterica serovar Enteritidis has been used . Avian Pathology , 33 : 133 – 144 .
- Davies , R.H. and Breslin , M. 2003 . Persistence of Salmonella Enteritidis Phage Type 4 in the environment and arthropod vectors on an empty free-range chicken farm . Environmental Microbiology , 5 : 79 – 84 .
- Davies , R.H. and Wray , C. 1996 . Studies of contamination of three broiler breeder houses with Salmonella enteritidis before and after cleaning and disinfection . Avian Diseases , 40 : 626 – 633 .
- De Buck , J. , Van Immerseel , F. , Haeseberouck , F. and Ducatelle , R. 2004 . Effect of type fimbriae of Salmonella enterica serotype Enteritidis on bacteraemia and reproductive tract infection in laying hens . Avian Pathology , 33 : 314 – 320 .
- Duguid , J.P. and North , R.A.E. 1991 . Eggs and Salmonella food-poisoning: an evaluation . Journal of Medical Microbiology , 34 : 65 – 72 .
- Ewing , W.H. 1986 . Edwards and Ewing's Identification of Enterobacteriaceae , 4th edn , New York : Elsevier .
- Feberwee , A. , de Vries , T.S. , Elbers , A.R. and de Jong , W.A. 2000 . Results of a Salmonella enteritidis vaccination field trial in broiler-breeder flocks in The Netherlands . Avian Diseases , 44 : 249 – 255 .
- Feberwee , A. , de Vries , T.S. , Hartman , E.G. , De Wit , J.J. , Elbers , A.R.W. and De Jong , W.A. 2001a . Vaccination against Salmonella Enteritidis in Dutch commercial layer flocks with a vaccine based on a live Salmonella Gallinarum 9R strain: evaluation of efficacy, safety, and performance of serologic Salmonella tests . Avian Diseases , 45 : 83 – 91 .
- Feberwee , A. , Hartman , E.G. , de Wit , J.J. and de Vries , T.S. 2001b . The spread of Salmonella Gallinarum 9R vaccine strain under field conditions . Avian Diseases , 45 : 1024 – 1029 .
- Freitas Neto , O.C. , Mesquita , A.L. , Paiva , J.B. , Zotesso , F. and Berchieri , A. Jr . 2008 . Control of Salmonella enterica serovar Enteritidis in laying hens by inactivated Salmonella Enteritidis vaccine . Brazilian Journal of Microbiology , 39 : 390 – 396 .
- Gama , N.M.S.Q. , Berchieri , A. Jr and Fernandes , S.A. 2003 . Salmonella sp. in laying hens . Revista Brasileira de Ciência Avícola , 5 : 15 – 21 .
- Gantois , I. , Ducatelle , R. , Timbermont , L. , Boyen , F. , Bohez , L. Haesebrouck , F. 2006 . Oral immunisation of laying hens with the live vaccine strains of TAD Salmonella vac® E and TAD Salmonella vac® T reduces internal egg contamination with Salmonella Enteritidis . Vaccine , 24 : 6250 – 6255 .
- Gast , R.K. 2007 . Invited Review. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry . Avian Diseases , 51 : 817 – 828 .
- Gast , R.K. and Holt , P.S. 2001 . Assessing the frequency and consequences of Salmonella Enteritidis deposition on the egg yolk membrane . Poultry Science , 80 : 997 – 1007 .
- Gast , R.K. , Stone , H.D. , Holt , P.S. and Beard , C.W. 1992 . Evaluation of the efficacy of oil–emulsion bacterin for protecting chickens against Salmonella enteritidis . Avian Diseases , 36 : 992 – 999 .
- Gast , R.K. , Stone , H.D. and Holt , P.S. 1993 . Evaluation of the efficacy of oil–emulsion bacterin for reducing faecal shedding of Salmonella enteritidis by laying hens . Avian Diseases , 37 : 1085 – 1091 .
- Girard-Santosuosso , O. , Menanteau , P. , Duchet-Suchaux , M. , Berthelot , F. , Mompart , F. Protais , J. 1998 . Variability in the resistance of four chicken lines to experimental intravenous infection with Salmonella enteritidis Phage Type 4 . Avian Diseases , 42 : 462 – 469 .
- Gordon , R.F. , Garside , J.S. and Tucker , J.F. 1959 . The use of living attenuated vaccines in the control of fowl typhoid . The Veterinary Record , 71 : 300 – 305 .
- Gordon , W.A.M. and Luke , D. 1959 . A note on the use of the 9R fowl typhoid vaccine in poultry breeding flocks . The Veterinary Record , 71 : 926 – 927 .
- Guard-Petter , J. 2001 . The chicken, the egg and Salmonella enteritidis . Environmental Microbiology , 3 : 421 – 430 .
- Gupta , B.R. and Mallick , B.B. 1976 . Immunization against fowl typhoid. 1. Live oral vaccine . Indian Journal of Animal Science , 46 : 502 – 505 .
- Hassan , J.O. and Curtiss , R. 1996 . Effect of vaccination of hens with an avirulent strain of Salmonella typhimurium on immunity of progeny challenged with wild-type Salmonella strains . Infection and Immunity , 64 : 938 – 944 .
- Hazeleger , W.C. , Bolder , N.M. , Beumer , R.R. and Jacobs-Reitsma , W.F. 2008 . Darkling beetles (Alphitobius diapernus) and their larvae as potential vectors for the transfer of Campylobacter jejuni and Salmonella enterica serovar Paratyphi B variant Java between successive broiler flocks . Applied and Environmental Microbiology , 74 : 6887 – 6891 .
- Henzler , D.J. and Opitiz , H.M. 1992 . The role of mice in the epizootiology of Salmonella Enteritidis infection on chicken layer farms . Avian Diseases , 36 : 625 – 631 .
- Humphrey , T.J. 2006 . Are happy chickens safer chickens? Poultry welfare and disease susceptibility . Brazilian Poultry Science , 47 : 379 – 391 .
- Iba , A.M. and Berchieri , A. Jr . 1995 . Studies on the use of a formic acid-propionic acid mixture (Bio-add™) to control experimental Salmonella infection in broiler chickens . Avian Pathology , 24 : 303 – 311 .
- Inoue , A.Y. , Berchieri , A. Jr. , Bernardino , A. , Paiva , J.B. and Sterzo , E.V. 2008 . Passive immunity of progeny from broiler breeders vaccinated with oil-emulsion bacterin against Salmonella Enteritidis . Avian Diseases , 52 : 567 – 571 .
- Kaiser , M.G. , Lakshmanan , N. , Wing , T. and Lamont , S.J. 2002 . Salmonella enterica serovar Enteritidis burden in broiler breeder chicks genetically associated with vaccine antibody response . Avian Diseases , 46 : 25 – 31 .
- Kanashiro , A.M.I. , Stoppa , G.F.Z. , Luciano , R.L. , Freitas Neto , O.C. and Berchieri , A. Jr . 2008 . Resposta sorológica em aves vacinadas contra Salmonella . Brazilian Journal of Poultry Science , 10 : 253
- Lee , Y.J. , Mo , I.P. and Kang , M.S. 2005 . Safety and efficacy of Salmonella gallinarum 9R vaccine in young laying chickens . Avian Pathology , 34 : 362 – 366 .
- Liu , W. , Yang , Y. , Chung , N. and Kwang , J. 2001 . Induction of humoral immune response and protective immunity in chickens against Salmonella enteritidis after a single dose of killed bacterium-loaded microspheres . Avian Diseases , 45 : 797 – 806 .
- Maciorowski , K.G. , Jones , F.T. , Pillai , S.D. and Ricke , S.C. 2004 . Incidence, sources, and control of foodborne Salmonella spp. in poultry feeds . World's Poultry Science Journal , 60 : 446 – 457 .
- Mastroeni , P. , Villareal-Ramos , B. , Demarco de Hormaeche , D. and Hormaeche , C.E. 1993 . Delayed (footpad) hypersensitivity and Arthus reactivity using protein rich antigens and LPS in mice immunized with live attenuated aroA Salmonella vaccines . Microbial Pathogenesis , 14 : 369 – 379 .
- Mastroeni , P. , Chabalgoity , J.A. , Dunstan , S.J. , Maskell , D.J. and Dougan , G. 2000 . Salmonella: immune responses and vaccines . The Veterinary Journal , 161 : 132 – 164 .
- Mead , G.C. and Barrow , P.A. 1990 . Salmonella control in poultry by ‘competitive exclusion’ or immunization . Letters in Applied Microbiology , 10 : 221 – 227 .
- Miyamoto , T. , Kitaoka , D. , Withanage , G.S. , Fukata , T. , Sakai , K. and Baba , E. 1999 . Evaluation of the efficacy of Salmonella Enteritidis oil-emulsion bacterin in an intravaginal challenge model in hens . Avian Diseases , 43 : 497 – 505 .
- Morse , D.L. , Birkhead , G.S. , Guardino , J. , Kondracki , S.F. and Guzewich , J.J. 1994 . Outbreak and sporadic egg-associated cases of Salmonella Enteritidis: New York's experience . American Journal of Public Health , 84 : 859 – 860 .
- Nagaraja , K.V. and Rajashekara , G. 1999 . “ Vaccination against Salmonella enterica serovar Enteritidis infection: dilemma and realities ” . In Salmonella enterica serovar Enteritidis in humans and animals , 1st edn , Edited by: Saeed , A.M. 397 – 404 . Ames : Iowa State University Press .
- Nakamura , M. , Nagamine , N. , Takahashi , T. , Suzuki , S. and Sato , S. 1994 . Evaluation of the efficacy of a bacterin against Salmonella enteritidis infection and the effect of stress after vaccination . Avian Diseases , 38 : 717 – 724 .
- Ochoa-Repáraz , J. , Sesma , B. , Álvarez , M. , Renedo , M.J. , Irache , J.M. and Gamazo , C. 2004 . Humoral immune response in hens naturally infected with Salmonella Enteritidis against outer membrane proteins and other surface structural antigens . Veterinary Research , 35 : 291 – 298 .
- Patterson , J.A. and Bukholder , K.M. 2003 . Application of prebiotics and probiotics in poultry production . Poultry Science , 82 : 627 – 631 .
- Poppe , C. 2000 . “ Salmonella infections in domestic fowl ” . In Salmonella in Domestic Animals , 1st edn , Edited by: Wray , C. and Wray , A. 107 – 132 . Wallingford : Oxford University Press .
- Rana , N. and Kulshreshtha , R.C. 2006 . Cell-mediated and humoral immune responses to a virulent plasmid-cured mutant strain of Salmonella enterica serotype Gallinarum in broiler chickens . Veterinary Microbiology , 115 : 159 – 162 .
- Rodrigue , D.C. , Tauxe , R.V. and Rowe , B. 1990 . International increase in Salmonella enteritidis: a new pandemic? . Epidemiology and Infection , 105 : 21 – 27 .
- Rosu , V. , Chadfield , M.S. , Santona , A. , Christensen , J.P. , Thomsen , L.E. , Rubino , S. and Olsen , J.E. 2007 . Effects of crp deletion in Salmonella enterica serotype Gallinarum . Acta Veterinaria Scandinavica , 49 : 14 – 20 .
- Santos , D.M.S. , Berchieri , A Jr , Fernandes , S.A. , Tavechio , S.A. and Amaral , L.A. 2000 . Salmonella em carcaças de frango congeladas . Pesquisa Veterinária Brasileira , 20 : 39 – 42 .
- Smith , H.W. 1956 . The use of live vaccines in experimental Salmonella gallinarum infections in chickens with observations on their interference effect . Journal of Hygiene , 54 : 419 – 432 .
- Smith , H.W. and Tucker , J.F. 1980 . The virulence of Salmonella strains for chickens; their excretion by infected chicken . Journal of Hygiene , 84 : 479 – 488 .
- Tan , T.Z. , Nay , B. , Bricker , J.M. , Hughes , H. , Sterner , F. & Hein , R. 2008a An attenuated Salmonella Gallinarum live vaccine induces long term protection against Salmonella enteritidis challenge in chickens . Available online at http://www.safe-poultry.com/publications.asp (accessed 30 December 2008) .
- Tan , T.Z. , Nay , B. , Bricker , J.M. , Witvliet , M. , Hughes , H. , Sterner , F. & Hein , R. 2008b Safety studies and risk analysis of an attenuated Salmonella Gallinarum live vaccine for layer chickens . Available online at http://www.safe-poultry.com/publications.asp (accessed 30 December 2008) .
- Wall , P.G. and Ward , R.L. 1999 . “ Epidemiology of Salmonella enterica Serovar Enteritidis Phage Type 4 in England and Wales ” . In Salmonella enterica serovar Enteritidis in Humans and Animals , 1st edn , Edited by: Saeed , A. M. 107 – 132 . Ames : Iowa State University Press .
- Witvliet , M. , Mols-Vorstermans , T. & Wijnhoven , L. 2008 Vaccination of chickens against S. Enteritidis with the S. Gallinarum 9R strain . Available online at http://www.safe-poultry.com/publications.asp (accessed 30 December 2008) .
- Woodward , M.J. , Gettingby , G. , Breslin , M.F. , Corkish , J.D. and Houghton , S. 2002 . The efficacy of Salenvac, a Salmonella enterica subsp. enterica serotype Enteritidis iron-restricted bacterin vaccine, in laying chickens . Avian Pathology , 31 : 383 – 392 .
- World Health Organization & Food and Agriculture Organization of the United Nations 2002 Risk assessments of Salmonella in eggs and broiler chickens . Interpretative summary. Available online at http://www.who.int/foodsafety/publications/micro/en/salm_summary.pdf (accessed 22 May 2008) .
- Zancan , F.B. , Berchieri , A. Jr , Fernandes , S.A. and Gama , N.M.S.Q. 2000 . Salmonella spp. investigation in transport boxes of day-old birds . Brazilian Journal of Microbiology , 31 : 230 – 232 .
- Zhang-Barber , L. , Turner , A.K. and Barrow , P.A. 1999 . Vaccination for control of Salmonella in poultry . Vaccine , 17 : 2538 – 2545 .