Abstract
Intravenous administration of lipopolysaccharide (LPS) from Escherichia coli O127:B8 at a dose of 1,500,000 u/kg body weight evoked a hypothermic response followed by a fever phase in 5-week-old broiler chickens. The hypothermic phase coincided with a severe decrease in blood pressure. We assume that this decrease in blood pressure is, at least partly, responsible for the hypothermic phase of the body temperature curve. LPS administration also caused a decrease in circulating white blood cells. The heterophils were predominantly sequestered in the lungs. In LPS-treated chickens, far more apoptotic leukocytes were present in the circulation, compared with control chickens. The molecular players responsible for the LPS-induced inflammatory response could be TL1A, IL-1β and IL-6, since a slight increase in their mRNA levels in white blood cells was already seen 1 h after LPS administration. In accordance with these observations, the levels of secreted IL-6 were maximal 3 h after LPS administration. These parameters characterize this LPS-induced inflammation model in broiler chickens.
Introduction
Broilers come into contact with large amounts of lipopolysaccharide (LPS) by inhalation of dust and in cases of airsacculitis, after colonization of the air sacs with Escherichia coli (Lorenzoni & Wideman, Citation2008; Dwars et al., Citation2009). LPS is a major component of the Gram-negative bacterial cell wall, and is capable of eliciting a plethora of effects including changes in body temperature, blood pressure and circulating leukocytes. Exposure of leukocytes and other cell types to a pathogen is followed by the appearance of pyrogenic cytokines or endogenous pyrogens in the circulation, among which tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 are considered the most important. These cytokines signal to the brain, where the level of prostaglandin E2 is elevated, which is recognized as a key mediator to induce fever (Coceani & Akarsu, Citation1998; Netea et al., Citation2000). In chickens, TNF-α has not been identified but TNF-like ligand 1A (TL1A) produces effects similar to TNF-α such as production of IL-1β and IL-6. TL1A also promotes inflammation by raising α1 acid glycoprotein, ceruloplasmin and nitric oxide (NO) (Takimoto et al., Citation2008). After intravenous (i.v.) LPS administration in chickens, the IL-6 plasma concentration peaks 3 h after treatment and precedes the febrile phase (De Boever et al., Citation2008a). In the spleen, mRNA levels of IL-1β were significantly elevated 1 h after LPS administration (Leshchinsky & Klasing, Citation2003).
High doses of LPS tend to evoke hypothermia instead of fever, and both behaviours are reported to be adaptive (Romanovsky et al., Citation1996; Almeida et al., Citation2006b; Akarsu & Mamuk, Citation2007). Hypothermia can potentially lower tissue hypoxia and could play a defensive role after LPS administration (Romanovsky et al., Citation1997). Another hallmark of LPS administration is cardiovascular collapse resulting in hypotension through upregulation of inducible NO synthase and subsequent NO production (Jordan & Hinshaw, Citation1964; Wakabayashi et al., Citation1991; Leturcq et al., Citation1996; Miyamoto et al., Citation1996; Romanovsky et al., Citation1997; Thiemermann, Citation1997; Mayr et al., Citation2008). Furthermore, LPS induces the adhesion of circulating leukocytes to the endothelial surface in postcapillary venules in rabbits, rats and mice. The accumulation of these activated leukocytes is described primarily in the lung (Haslett et al., Citation1987; Cardozo et al. Citation1991; Walther et al., Citation2000; Yipp et al., Citation2002).
The current study was performed to further elucidate which players and cell types are involved in the inflammatory effects seen after LPS administration in broiler chickens. In this acute inflammation model, body temperature and blood pressure were measured as primary clinical parameters. Pro-inflammatory cytokines were measured on three levels: assessment of mRNA expression of TL1A, IL-1β and IL-6 by polymerase chain reaction (PCR), intracellular protein expression using a flow cytometric method for IL-1β and IL-6, and secreted protein in plasma using an IL-6 bio-assay. The characterization of this intravenous LPS inflammation model will enable us to study the influence of non-steroidal anti-inflammatory drug administration on the evaluated parameters in future experiments.
Materials and Methods
Animals
Twenty-five 1-day-old, unvaccinated, Ross chicks of both sexes were obtained from a commercial hatchery. Temperature and relative humidity accorded with the requirements of young chickens, and temperature was adjusted to prevent huddling (Anonymous, Citation2004). The concrete floor was covered with wood shavings. Normal hygienic measures were taken to avoid contact with pathogens as much as possible. The lighting schedule consisted of 12 h dark and 12 h light. Commercial pullet feed and tap water were available ad libitum. All experiments and procedures were approved by the Ethics Committee of the Faculty of Veterinary Medicine (EC 2008/006 and 008).
Experiment 1
Body temperature and cytokine expression
For the first experiment, chickens were divided into two groups of six LPS-treated chickens and six control chickens. LPS-treated chickens were injected intravenously in the wing vein with LPS of E. coli O127:B8 (Sigma-Aldrich, Bornem, Belgium) dissolved in aqua ad injectabilia at a dose of 1 500 000 u/kg body weight (BW) at the age of 5 weeks. In this batch of LPS, 1 500 000 u/kg BW corresponded to a dose of 2.5 mg/kg BW. For all experiments, LPS from the same batch was used. Control chickens were injected with the same amount of aqua ad injectabilia.
Body temperature was measured by inserting a thermocouple probe (Physitemp Instruments Inc., Clifton, New Jersey, USA) 5 cm into the cloaca, before and at 1, 2, 3.5, 5, 6.5, 8, 12 and 24 h after LPS administration. The area under the body temperature versus time curve from 0 to 5 h and from 0 to 24 h post administration (AUC0→5 h and AUC0→24 h) was calculated to evaluate differences between treatment groups (α = 0.05).
One millilitre of blood was withdrawn in citrate-containing tubes before and 1, 2 and 3 h after LPS administration for PCR analysis. Blood was transferred into two Eppendorf cups (Novolab, Geraardsbergen, Belgium) containing 1 ml RNA/DNA stabilization reagent for blood/bone marrow (Roche Applied Sciences, Vilvoorde, Belgium) and frozen at −20°C until RNA extraction.
One-half of a millilitre of blood was withdrawn in heparin-containing tubes (Venoject®; Terumo Corp., Tokyo, Japan) from the leg vein before and 1, 2, 3, 4, 5, 6, 8 and 12 h after LPS administration for flow cytometric analysis. From three LPS-treated chickens and three control chickens, a drop of these blood samples was also used to prepare a blood smear.
For determination of IL-6 in plasma using a bio-assay, 0.4 ml blood was withdrawn in heparin-containing tubes before and 1, 2, 3, 4, 5, 6, 8 and 12 h after LPS administration.
PCR analysis
Blood samples were analysed for chicken TL1A, IL-1β, IL-6 and two housekeeping genes (i.e. ubiquitin (UB) and glucose-6-phosphate dehydrogenase (G6PDH)) and prepared as described previously (De Boever et al., Citation2008b). Briefly, total RNA was extracted from 1 ml citrate-treated blood using a High Pure RNA Isolation Kit (Roche Applied Science, Vilvoorde, Belgium) according to the manufacturer's instructions. The reverse transcription was performed using the Reverse Transcriptase Core kit (Eurogentec, Seraing, Belgium), following the manufacturer's instructions. A fixed amount of RNA (600 ng) from each sample was converted into cDNA, using random nonamers as the priming method in the PTC-100 DNA engine (Bio-Rad Laboratories, Nazareth Eke, Belgium). The primers were designed with the LightCycler Probe Design Software 2.0 (Roche Applied Sciences) using the GenBank sequences for TL1A, IL-1β and IL-6 and G6PDH. The specific primer sequences for TL1A, IL-1β and IL-6 are presented in . The primer for UB was based on the sequences used by Li et al. (Citation2007).
Table 1. Primer sequences used for real-time reverse transcriptase PCR analysis
Real-time reverse transcriptase PCR was performed using a LightCycler 2.0 System (Roche Applied Science). All reactions were run in duplicate and were quantified by including a standard curve (five points in duplicate) in the real-time reverse transcriptase PCR analysis. The concentration of IL-1β, IL-6 and TL1A was divided by the normalization factor, calculated as the square root of the multiplication of the concentration of both housekeeping genes.
Flow cytometric analysis
Intracellular IL-1β and IL-6 were analysed in chicken leukocytes using a flow cytometric method (De Boever et al., manuscript submitted).
Reagents and antibodies
Polyclonal antibodies, raised in rabbits and directed against chicken IL-1β and IL-6, were used as primary antibodies (AHP941Z and AHP942Z; AbD Serotec, Cergy Saint-Christophe, France). Since no antibodies against TL1A are commercially available, this analysis could not be included.
Allophycocyanin-conjugated F(ab′)2 goat antibodies to rabbit immunoglobulin were used as secondary antibodies (Santa Cruz Biotechnology Inc., Heidelberg, Germany).
RPMI 1640, goat serum and bovine serum albumin were purchased at Invitrogen (Merelbeke, Belgium). BD FACS Lysing solution, BD FACS Permeabilizing solution 2 and BD CellFIX were purchased from Beckton Dickinson Biosciences (Erembodegem, Belgium).
Staining procedure and flow cytometric analysis
Chicken leukocytes were obtained after lysis of the erythrocytes with FACS Lysing solution: 4.5 ml FACS Lysing solution (1/10 diluted with distilled water) was added to 0.5 ml heparin-treated blood in a Falcon tube and kept for 10 min at room temperature. After washing, the pellet was resuspended in 500 µl RPMI and 50 µl was pipetted into a flow cytometer tube. Leukocytes were separated from the remaining red blood cell nuclei, using phycoerythrin-conjugated Cell Lab mouse anti-chicken CD45 antibodies (733074; Beckman Coulter), which were added in a concentration of 6 µg/ml. Samples were incubated for 30 min at 4°C. Permeabilization was performed using 500 µl FACS Permeabilizing solution 2 (1/10 diluted with distilled water) for 10 min, again at room temperature. Primary antibodies were added in a concentration of 50 µg/ml and samples were incubated for 30 min at room temperature. Next, goat serum at a concentration of 2% in RPMI was added for 30 min and samples were incubated at room temperature. Finally, the corresponding allophycocyanin-labelled secondary antibody was added at a concentration of 6 µg/ml.
The cells were resuspended in 450 µl CellFIX (1/10 diluted with distilled water) and pipetted into Trucount Tubes (BD Biosciences). Samples were analysed using a FACSCanto flow cytometer (BD Biosciences). The different cell populations were demarcated in an FL-2/Side Scatter dot plot, based on a positive staining for CD45 and their granularity. The phycoerythrin fluorescence dot plots of the gated cells were then plotted and all data were corrected for autofluorescence. Cytokine-specific fluorescence was defined as events to the right of the autofluorescence marker, and the mean fluorescence intensity (MFI) and proportion of cells staining positive for the cytokine in question was recorded.
IL-6 measurement by bio-assay
Concentrations of secreted IL-6 were measured in plasma using IL-6-dependent murine 7TD1 hybridoma cells (Van Snick et al., Citation1986; De Boever et al., Citation2008a). Briefly, cells were cultured in 96-well microtitre plates for 72 h in medium with different dilutions of the plasma samples. The number of living cells was measured using a colorimetric hexosaminidase enzyme reaction (Landegren, Citation1984). The concentration of IL-6 in plasma samples was calculated from a standard curve obtained using a dilution of recombinant chicken IL-6.
Microscopic analysis of blood smears
Blood smears were prepared with heparinized blood and coloured with Hemacolor® (Merck, Darmstadt, Germany) according to the manufacturer's guidelines. Slides were evaluated using a light microscope (Zeiss, Zaventem, Belgium) equipped with a 100x objective. On each slide, 100 leukocytes were evaluated.
To demonstrate the presence of apoptotic cells in circulation after LPS administration, a blood sample of a LPS-treated chicken was incubated with Annexin-V-FLUOS labelling reagent (Annexin-V-FLUOS Staining Kit; Roche Applied Science) after lysis of the red blood cells as described above. Leukocyte nuclei were stained using DAPI (1/100 diluted) (Roche Applied Science). After this double staining, cells were mounted on microscope slides using glycerin–PBS solution (0.9/0.1, v/v) with 2.5% 1,4-diazabicyclo(2,2,2)octane (Janssen Chimica, Beerse, Belgium) and analysed using a Leica DM RBE fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Experiment 2
Blood pressure
Blood pressure was measured before and every hour until 12 h after LPS administration using a non-invasive Doppler technique in six LPS-treated chickens and two control chickens at the age of 5 weeks (Christensen et al., Citation1987). A Doppler crystal probe coated with ultrasound gel was placed over the plantar arcuate artery on the ventral side of the foot web (Ultrasonic Doppler flow detector, model 811-B; Parks Medical Electronics Inc., Oregon, USA). The crystal was connected to a medium-gain amplifier. The bird was positioned on its side and an inflatable cuff, connected to a sphygmomanometer, was placed around the upper part of the hind limb. Chickens were gently handled, and adapted to this procedure to avoid stress and treatment-related effects on the measurement as much as possible. The pulse in the foot web was located as an audio signal. The cuff was inflated until the sound was extinguished. Return of the pulse sound during deflation of the cuff indicated systolic blood pressure. The AUC0→5 h and AUC0→24 h values were calculated to evaluate differences between treatment groups.
Experiment 3
Histology
Sequestration of heterophils in tissues of LPS-treated chickens was demonstrated by histological examination of the lung after Giemsa staining. Twenty-four 1-day-old, unvaccinated, Ross chicks of both sexes were obtained from a commercial hatchery and raised as described in the earlier “Animals” section. At the age of 5 weeks, chickens were divided into three groups. At 2, 3 and 4 h after i.v. LPS administration at a dose of 1 500 000 u/kg BW, three chickens were euthanized. At 2, 3 and 4 h after pyrogen-free water administration, three chickens were euthanized. At the same time points, two control chickens were euthanized. Lungs were collected from LPS-treated, pyrogen-free-water-treated birds and control birds and stored in 10% formaldehyde for 24 h.
After embedding in paraffin, tissues were cut into sections of 3 µm and stained with a Giemsa stain according to standard procedures. Slides were evaluated microscopically, by counting the amount of heterophils in 10 microscopic fields using a 100x objective (Zeiss).
Statistical analysis
Students t test (α = 0.05) was used to demonstrate possible differences in the AUC0→5 h and AUC0→24 h values of the body temperature and blood pressure between LPS-treated chickens and control chickens. The results of the PCR, flow cytometric and histological analyses were also statistically evaluated, using the same test.
Statistical comparison of the IL-6 concentration between the different treatment groups was done 3 h after the administration of LPS or saline, the time point at which the secreted IL-6 concentration reached its highest value. A Mann − Whitney test was performed to demonstrate possible differences in cytokine concentration.
Results
Experiment 1
Body temperature
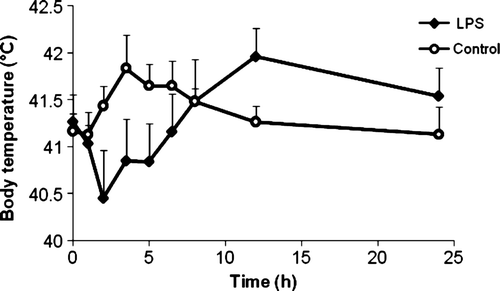
The course of the body temperature was characterized by a hypothermic phase followed by a fever phase in LPS-treated chickens, as shown in . There was a significant difference in AUC0→5 h value between LPS-treated chickens and control chickens, expressed as the mean ± standard deviation (LPS: 204.03 ± 1.52°C x h; control: 207.50 ± 0.96°C x h; P < 0.05). For the AUC0→24 h value, no significant difference could be found between LPS-treated chickens and control chickens, since the hypothermic phase cancels out the hyperthermic phase.
PCR analysis
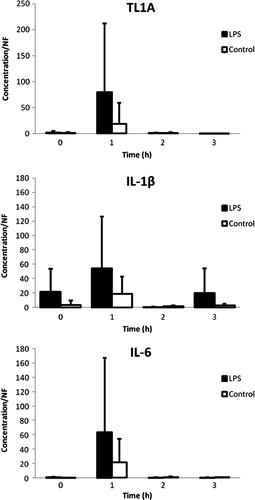
The mRNA levels of TL1A, IL-1β and IL-6 are depicted in . The levels were slightly increased in leukocytes and thrombocytes of LPS-treated chickens. However, no statistical significance could be demonstrated for any of the three cytokine levels.
Figure 2. Concentration/normalization factor of TL1A, IL-1β and IL-6 versus time graphs of LPS-treated chickens (n = 6) and control chickens (n = 6) expressed as the mean (+ standard deviation). A slight, although not significant, increase in mRNA levels in the leukocytes was seen 1 h after LPS administration.
Flow cytometry
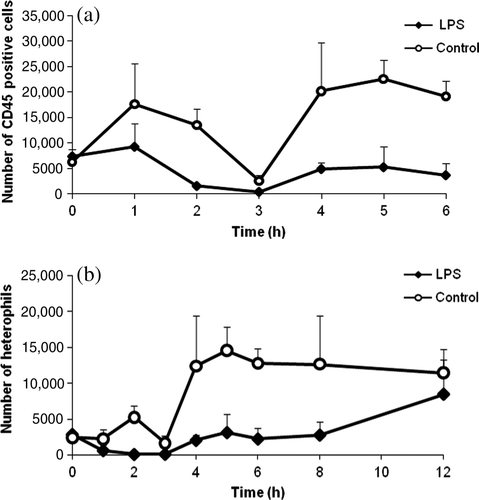
A decrease in circulating CD45-positive white blood cells was observed as shown in a. Specifically, in LPS-treated chickens there was a significant decrease in circulating heterophils as demonstrated in b. In control chickens, an increase in circulating heterophils could be found from 4 h after aqua ad injectabilia administration; this increase was attributed to stress due to handling of the animals.
Figure 3. (3a) Number of CD45-positive white blood cells versus time and (3b) number of circulating heterophils in LPS-treated chickens (n = 6) and control chickens (n = 6) expressed as the mean (+ standard deviation). A significant decrease in circulating leukocytes was seen in LPS-treated chickens due to sequestration and apoptosis.
In , significant differences in MFI 3 h after treatment between LPS-treated chickens and control chickens are shown for intracellular IL-1β and IL-6 in the heterophils.
Figure 4. Intracellular expression (MFI) of IL-1β and IL-6 3 h after treatment in the heterophils of LPS-treated chickens (n = 6) and control chickens (n = 6) expressed as the mean (+ standard deviation) *Means differ significantly from those of the control group (P < 0.05). A significant increase in intracellular IL-1β and IL-6 expression in the heterophils was seen 3 h after LPS administration.

Bio-assay
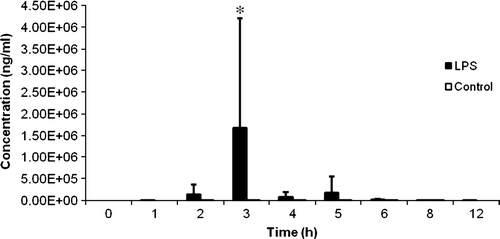
LPS-treated chickens had significantly higher levels of IL-6 in plasma at 3 h post-treatment compared with control chickens (P < 0.05), as shown in .
Figure 5. Concentration of secreted IL-6 versus time graph of LPS-treated chickens (n = 6) and control chickens (n = 6) expressed as the mean (+ standard deviation) *Mean differs significantly from that of the control group (P < 0.05). A significant increase in plasma IL-6 concentration was seen 3 h after LPS administration.
Light and fluorescence microscopy
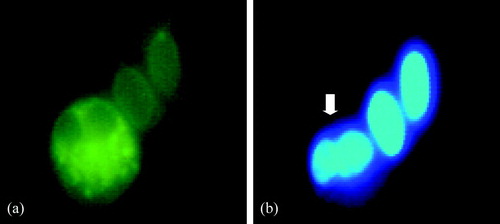
Microscopic examination of blood smears revealed a decrease in circulating heterophils in LPS-treated chickens. Moreover, 4 h after LPS administration, pregranulocytes appeared in circulation, characterized by the presence of blue granules in their cytoplasm and a less lobulated nucleus. In LPS-treated chickens, dark-coloured cells could be observed from 2 h post administration. These cells were identified as apoptotic cells using annexin V staining as illustrated in .
Figure 6. 6a: Fluorescence microscopic image of a fluorescein isothiocyanate − annexin-V-stained apoptotic heterophil; 6b: as identified by the shape of the nucleus (indicated with an arrow), two red blood cell nuclei can be noted on the right. Intravenous LPS administration to broiler chickens induced heterophil apoptosis.
In control chickens, an increase in circulating heterophils was seen. No marked difference in circulating monocytes and lymphocytes could be observed between LPS-treated chickens and control chickens.
Experiment 2
Blood pressure
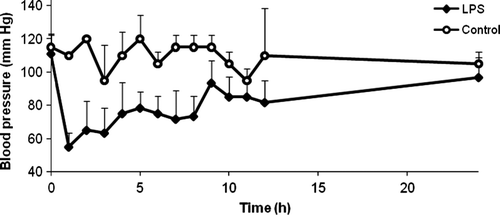
The administration of LPS resulted in a profound and sustained hypotension, as depicted in . Both the AUC0→5 h value (LPS: 352.92 ± 39.38 mmHg x h; control: 552.5 ± 38.89 mmHg x h) and the AUC0→24 h value (LPS: 1986.25 mmHg x h ± 212.47; control: 2607.5 mmHg x h ± 272.24) were significantly different between LPS-treated chickens and control chickens (P < 0.05).
Experiment 3
Histology
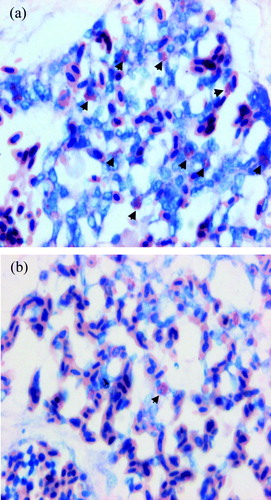
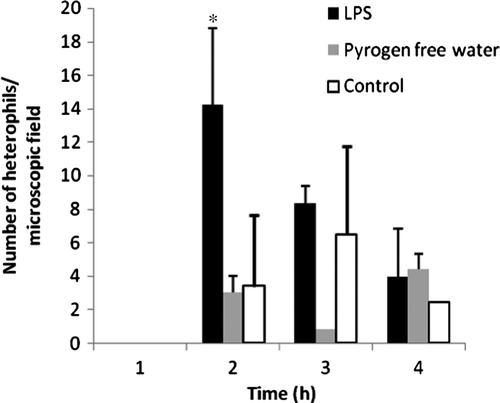
LPS administration significantly increased the number of heterophils in the lung (P < 0.05) 2 h after administration, as shown in . illustrates the difference in presence of heterophils in lung tissue after Giemsa staining of an LPS-treated chicken in comparison with a control chicken.
Figure 8. Number of heterophils/microscopic lung field (100x) versus time curve for LPS-treated chickens (n = 3), pyrogen-free-water-treated chickens (n = 3) and control chickens (n = 2) expressed as the mean (+ standard deviation) *Mean differs significantly from those of the control group and the pyrogen-free-water-treated group (P < 0.05). Two hours after intravenous LPS administration to broiler chickens a significant sequestration of heterophils in the lungs was noted.
Discussion
Recognition of microbial components by Toll-like receptors triggers a cascade of cellular signals that culminates in the activation of NF-κB, leading to the expression of genes encoding pro-inflammatory cytokines like TNF-α, IL-1β and IL-6, inducible NO synthase, cyclooxygenase 2 and phospholipase A2, and clearance of the infectious agent (Witkamp & Monshouwer, Citation2000; Verstrepen et al., Citation2008).
IL-1β, IL-6 and TNF-α are considered the most important pyrogenic cytokines or endogenous pyrogens in the circulation (Dinarello, Citation2000; Witkamp & Monshouwer, Citation2000). In mammals, TNF-α is considered one of the first mediators released during inflammation, which in turn promotes the production of IL-1β and IL-6 (Witkamp & Monshouwer, Citation2000). To date, TNF-α has not been identified in the chicken. However, chicken TL1A belongs to the TNF ligand superfamily, a family of cytokines regulating cell growth/survival/apoptosis, and shows a similar mode of action as TNF-α (Gaur & Aggarwal, Citation2003; Takimoto et al., Citation2008).
In the current experiment, mRNA levels of IL-1β, IL-6 and TL1A in white blood cells were slightly elevated 1 h after i.v. injection of the Toll-like receptor 4 ligand, LPS. It should be mentioned that mRNA levels do not necessarily equate to protein levels and that cytokines can be produced by other cell types than leukocytes (Kogut et al., Citation2005). In the current study, the IL-1β and IL-6 intracellular protein levels in white blood cells were found to be significantly elevated in heterophils, the avian counterpart of mammalian neutrophils, 3 h after LPS administration. These results from in vivo administration of LPS confirm the in vitro results from heterophils, macrophages and thrombocytes (Dil & Qureshi, Citation2002; Kogut et al., Citation2005; Ferdous et al., Citation2008). The concentration of secreted bioactive IL-6, which is produced by white blood cells as well as by other cell types such as endothelial cells, was also significantly elevated 3 h after LPS administration. This could be expected since there is generally a good correlation between intracellular detection of cytokines and the secreted amounts of cytokines (Schuerwegh et al., Citation2003).
As a result of the production of these cytokines, a change in body temperature occurs. In the first experiment of this study, a biphasic response in body temperature (i.e. a fever phase preceded by a hypothermic phase) was seen. The rise in body temperature of the control chickens could be attributed to several factors, including circadian rhythm and handling of the chickens (Dawson & Whittow, Citation2000; Cabanac & Aizawa, Citation2000). It is proposed that hypothermic and pyrogenic mechanisms are independently activated during an LPS-induced acute phase response and that both behaviours are adaptive (Romanovsky et al., Citation1996; Almeida et al., Citation2006b; Akarsu & Mamuk, Citation2007). Hypothermia can potentially lower tissue hypoxia and thus plays a defensive role in septic shock and is probably aimed at preserving the host's vital systems (Romanovsky et al., Citation1997; Almeida et al., Citation2006a). The similarities in time course of the hypothermia and the reduction in oxygen consumption strongly suggest that hypothermia is a reflection of reduced thermogenesis (Derijk et al., Citation1994), but the time course of the LPS-induced hypothermia coincides with the time course of arterial hypotension. Intravenous LPS administration triggers widespread inducible NO synthase expression, resulting in copious production of NO (Bowen et al., Citation2007; Saia et al., Citation2008). NO seems to be involved in LPS-induced hypothermia, since it may participate in vasodilatation, increasing heat loss and intensifying the magnitude of hypothermia (Vayssettes-Courchay et al., Citation2003). As time passes after the administration of a high dose of LPS, the level of endotoxemia decreases, the arterial pressure returns to its normal values and, along with the alleviation of tissue hypoperfusion, cold-seeking behaviour changes into warmth-seeking behaviour and fever emerges (Romanovsky et al., Citation1996; Almeida et al., Citation2006a). We agree with the concept proposed by Almeida et al. (Citation2006a) that hypotension evokes hypoperfusion and less oxygen consumption, whereby there is less oxygen for heat production, resulting in hypothermia. In this way, hypothermia can be seen as a result of the hypotension, another hallmark of LPS administration in various species such as chickens, rats, dogs, rabbits, primates and humans (Jordan & Hinshaw, Citation1964; Wakabayashi et al., Citation1991; Leturcq et al., Citation1996; Miyamoto et al., Citation1996; Romanovsky et al., Citation1997; Mayr et al., Citation2008). NO is also believed to be responsible for the hypotension after endotoxin administration (Doursout et al., Citation2008), so either way NO seems to be the causative agent of both hypotension and hypothermia. However, in this experiment a sustained hypotension was seen over a longer period than the hypothermic phase. Probably in the initial phase of the inflammatory response, hypotension has a profound effect on the body temperature—while later on the mechanisms evoking fever overrule the effect of hypotension.
In dogs and pigs, hypothermia by cooling of the animals induced neutropaenia (Fedor et al., Citation1958; Biggar et al., Citation1983, Citation1984). Also in our experiments, hypothermia was accompanied by a decrease in circulating granulocytes. More in general, there was a decrease of all CD45-positive cells. In sheep and dwarf goats, LPS administration was shown to induce a decrease in peripheral white blood cell counts, which was caused by margination and accumulation of leukocytes in the lungs (Meyrick & Brigham, Citation1983; Van Miert et al., Citation1997). Normal lungs contain a large pool of marginated neutrophils since they are less deformable than erythrocytes, which have similar dimensions but pass the lung capillary segments faster (Hogg & Doerschuk, Citation1995; Kitagawa et al., Citation1997; Klut et al., Citation1998; Kuebler et al., Citation2000; Walther et al., Citation2000; Suwa et al. Citation2001; Jones et al., Citation2002). Another key feature in a localized response to LPS or inflammation is sequestration of leukocytes from the circulation to the endothelium, resulting in emigration into the surrounding, inflamed tissue. In the case of sepsis or endotoxemia, the leukocytes become trapped in the lungs and are not able to recirculate, as seen in rabbits, rats and mice (Haslett et al., Citation1987; Cardozo et al., Citation1991; Yipp et al., Citation2002). Endotoxin decreases neutrophil deformability by stiffening of the cytoskeleton by formation of microfilaments and increases the density of neutrophils in pulmonary capillaries (Hogg & Doerschuk, Citation1995; Kitagawa et al., Citation1997; Klut et al., Citation1998; Kuebler et al., Citation2000; Walther et al., Citation2000; Suwa et al. Citation2001). Interestingly, in rabbits, injection of recombinant human IL-6 induces a decrease in deformability of neutrophils (Suwa et al., Citation2001). Also L-selectin-mediated leukocyte/endothelial interaction, endothelium-derived Toll-like receptor 4 and the adhesion molecules ICAM1 and ELAM1, which bind neutrophils on endothelial cells, are reported to participate in the process of sequestra tion (Osborn, Citation1990; Hogg & Doerschuk, Citation1995; Kuebler et al., Citation2000; Andonegui et al., Citation2003).
The decrease in circulating white blood cells, seen in our experiments, is at least partly caused by the sequestration of these cells, as shown by the abundant presence of especially granulocytes in the lungs 2 h after LPS administration. A second reason could be apoptosis of these cells, which is reported in vivo in pigs and chickens and in vitro for fish lymphocytes (Norimatsu et al., Citation1995; Shini et al., Citation2008a; Xiang et al., Citation2008). In the current study, an annexin-V staining of isolated white blood cells of LPS-treated chickens showed more apoptotic cells compared with the samples from control chickens. In mice, it was suggested that these apoptotic cells have beneficial effects against LPS-induced endotoxic shock and even have therapeutic potential by reducing the release of pro-inflammatory cytokines (Ren et al., Citation2008).
From 4 h after LPS administration, granulocytosis was seen in LPS-treated chickens. Uchiyama et al. (Citation2005) reported granulocytosis in quails 3 h after LPS administration and Shini et al. (2008b) demonstrated an increased heterophil:lymphocyte ratio at 1, 3 and 24 h after LPS treatment, which is earlier than in our experiment. LPS accelerates the release of neutrophils from bone marrow pools and stimulates an increase in immature heterophils or band cells, in the bone marrow and peripheral blood as confirmed in chicken blood in the present experiment (Biggar et al., Citation1983; Klut et al., Citation1996, Citation1998; Shini et al., Citation2008b). In rabbits it was demonstrated that administration of recombinant human IL-6 accelerates the release of plymorphonuclear leukocyte from the bone marrow between 9 and 24 h after treatment (Suwa et al., Citation2001).
It should be noted that in the control group an increase in circulating heterophils was observed 4 h after the administration of pyrogen-free water. This can be attributed to stress due to the frequent handling of the chickens. An increase in the blood heterophil:lymphocyte ratio is used as a general stress indicator and is related to corticosterone production in chickens, which can rise due to simple experimental procedures (e.g. confinement, isolation and multiple blood sampling) in birds such as pigeons and geese (Machin et al., Citation2001; Takahashi et al., Citation2002).
In conclusion, the parameters characterizing this i.v. E. coli LPS-induced inflammation model in chickens are profound hypotension accompanying the hypothermic phase of the body temperature curve, slight elevation of mRNA levels of TL1A, IL-1β and IL-6 at 1 h after LPS administration, peak of expression of intracellular IL-1β and IL-6 in heterophils by 3 h after LPS administration, and also peak levels of secreted IL-6 by 3 h post LPS treatment. Furthermore, LPS induced a decrease in circulating CD45-positive cells, partly caused by sequestration of the heterophils in the lungs on the one hand and apoptosis of the circulating leukocytes on the other. In future experiments, the influence of non-steroidal anti-inflammatory drug administration on these parameters will be evaluated.
Acknowledgements
The authors would like to thank W. Burms for the quantification of IL-6 in plasma by bio-assay, S. Stuyvaert for the help with the PCR analysis and K. Demeyere for performing the flow cytometric analysis. C. Puttevils and D. Ameye are acknowledged for the help with the Giemsa staining of the tissues. The help of E. Neirinckx, D. De Clercq and K. Jonckheere with the animal experiments is greatly appreciated.
References
- Akarsu , E. and Mamuk , S. 2007 . Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats . American Journal of Physiology–Regulatory, Integrative and Comparative Physiology , 292 : R1846 – R1850 .
- Almeida , M. , Steiner , A. , Branco , L. and Romanovsky , A. 2006a . Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation . European Journal of Neuroscience , 23 : 3359 – 3367 .
- Almeida , M. , Steiner , A. , Branco , L. and Romanovsky , A. 2006b . Neural substrate of cold-seeking behavior in endotoxin shock . PLoS ONE , 1 : 1 – 10 .
- Andonegui , G. , Bonder , C. , Green , F. , Mullaly , S. , Zbytnuik , L. Rahario , E. 2003 . Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs . The Journal of Clinical Investigation , 111 : 1011 – 1020 .
- Anonymous 2004 Draft Appendix A of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS No. 123) . Guidelines for Accommodation and Care of Animals (Article 5 of the Convention), EMEA .
- Biggar , W. , Bohn , D. and Kent , G. 1983 . Neutrophil circulation and release from bone marrow during hypothermia . Infection and Immunity , 40 : 708 – 712 .
- Biggar , W. , Bohn , D. , Kent , G. , Barker , C. and Hamilton , G. 1984 . Neutrophil migration in vitro and in vivo during hypothernia . Infection and Immunity , 46 : 857 – 859 .
- Bowen , O. , Erf , G. , Chapman , M. and Wideman , R. 2007 . Plasma nitric oxide concentrations in broilers after intravenous injections of lipopolysaccharide or microparticles . Poultry Science , 86 : 2550 – 2554 .
- Cabanac , M. and Aizawa , S. 2000 . Fever and tachycardia in a bird (Gallus domesticus) after simple handling . Physiology & Behaviour , 69 : 541 – 545 .
- Cardozo , C. , Edelman , J. , Jagirdar , J. and Lesser , M. 1991 . Lipopolysaccharide-induced pulmonary vascular sequestration of polymorphonuclear leukocytes is complement independent . The American Review of Respiratory Disease , 144 : 173 – 178 .
- Christensen , J. , Fosse , R. , Halvorsen , O. and Morild , I. 1987 . Comparison of various anesthetic regimens in the domestic fowl . American Journal of Veterinary Research , 48 : 1649 – 1657 .
- Coceani , F. and Akarsu , E. 1998 . Prostaglandin E2 in the pathogenesis of fever. An update . Annals of the New York Academy of ScienceS , 856 : 76 – 82 .
- Dawson , W. and Whittow , G. 2000 . Regulation of body temperature. In “Sturkie's Avian Physiology , 5th edn , 343 – 390 . London : Academic Press .
- De Boever , S. , Beyaert , R. , Vandemaele , F. , Baert , K. , Duchateau , L. Goddeeris , B. 2008a . The influence of age and repeated lipopolysaccharide administration on body temperature and the concentration of interleukin-6 and IgM antibodies against lipopolysaccharide in broiler chickens . Avian Pathology , 31 : 39 – 44 .
- De Boever , S. , Vangestel , C. , De Backer , P. , Croubels , S. and Sys , S.U. 2008b . Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens . Veterinary Immunology and Immunopathology , 122 : 312 – 317 .
- Derijk , R. , Van Kampen , M. , Van Rooijen , N. and Berkenbosch , F. 1994 . Hypothermia to endotoxin involves reduced thermogenesis, macrophage-dependent mechanisms, and prostaglandins . American Journal of Physiology–Regulatory, Integrative and Comparative Physiology , 266 : R1 – R8 .
- Dil , N. and Qureshi , M. 2002 . Involvement of lipopolysaccharide related receptors and nuclear factor κB in differential expression of inducible nitric oxide synthase in chicken macrophages from different genetic backgrounds . Veterinary Immunology and Immunopathology , 88 : 149 – 161 .
- Dinarello , C. 2000 . Proinflammatory cytokines . Chest , 118 : 503 – 508 .
- Doursout , M. , Oguchi , T. , Fischer , U. , Liang , Y. , Chelly , B. , Hartley , C. and Chelly , J. 2008 . Distribution of NOS isoforms in a porcine endotoxin shock model . Shock , 29 : 692 – 702 .
- Dwars , R. , Matthijs , M. , Daemen , A. , van Eck , J. , Vervelde , L. and Landman , W. 2009 . Progression of lesions in the respiratory tract of broilers after single infection with Escherichia coli compared to superinfection with E. coli after infection with infectious bronchitis virus . Veterinary Immunology and Immunopathology , 127 : 65 – 76 .
- Fedor , E.J. , Fisher , B. and Fisher , E.R. 1958 . Observations concerning bacterial defense mechanisms during hypothermia . Surgery , 43 : 807 – 814 .
- Ferdous , F. , Maurice , D. and Scott , T. 2008 . Broiler chick thrombocyte response to lipopolysaccharide . Poultry Science , 87 : 61 – 63 .
- Gaur , U. and Aggarwal , B. 2003 . Regulation of proliferation, survival and apoptosis by members of the TNF superfamily . Biochemical Pharmacology , 66 : 1403 – 1408 .
- Haslett , C. , Worthen , G. , Giclas , P. , Morrison , D. , Henson , J. and Henson , P. 1987 . The pulmonary vascular sequestration of neutrophils in endotoxemia is initiated by an effect on the neutrophil in the rabbit . The American Review of Respiratory Disease , 136 : 9 – 18 .
- Hogg , J. and Doerschuk , C. 1995 . Leukocyte traffic in the lung . Annual Review of Physiology , 57 : 97 – 114 .
- Jones , H. , Paul , W. and Page , C. 2002 . A new model for the continuous monitoring of polymorphonuclear leukocyte trapping in the pulmonary vasculature of the rabbit . Journal of Pharmacological and Toxicological Methods , 48 : 21 – 29 .
- Jordan , M. and Hinshaw , L. 1964 . Species resistance to histamine and endotoxin: the response of the chicken . Proceedings of the Society for Experimental Biology and Medicine , 115 : 455 – 458 .
- Kitagawa , Y. , Van Eeden , S. , Redenbach , D. , Blair , M. , Klut , M. , Wiggs , B. and Hogg , J. 1997 . Effect of mechanical deformation on structure and function of polymorphonuclear leukocytes . Journal of Applied Physiology , 82 : 1397 – 1405 .
- Klut , M. , van Eeden , S. , Whalen , B. , Verburght , L. , English , D. and Hogg , J. 1996 . Neutrophil activation and lung injury associated with chronic endotoxemia in rabbits . Experimental Lung Research , 22 : 449 – 465 .
- Klut , M. , van Eeden , S. and Hogg , J. 1998 . Neutrophil structural changes associated with chronic endotoxemia and lung injury . Journal of Inflammation , 48 : 1 – 12 .
- Kogut , M. , He , H. and Kaiser , P. 2005 . Lipopolysaccharide binding protein/CD14/TLR4-dependent recognition of salmonella LPS induces the functional activation of chicken heterophils and up-regulation of pro-inflammatory cytokine and chemokine gene expression in these cells . Animal Biotechnology , 16 : 165 – 181 .
- Kuebler , W. , Borges , J. , Sckell , A. , Kuhnle , G. , Bergh , K. , Messmer , K. and Goetz , A. 2000 . Role of L-selectin in leukocyte sequestration in lung capillaries in a rabbit model of endotoxemia . American Journal of Respiratory Critical Care Medicine , 161 : 36 – 43 .
- Landegren , U. 1984 . Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications of detection of lymphokines and cell surface antigens . Journal of Immunological Methods , 67 : 379 – 388 .
- Leshchinsky , T. and Klasing , K. 2003 . Profile of chicken cytokines induced by lipopolysaccharide is modulated by dietary alpha-tocopheryl acetate . Poultry Science , 82 : 1266 – 1273 .
- Leturcq , D. , Moriaty , A. , Talbott , G. , Winn , R. , Martin , T. and Ulevitch , R. 1996 . Antibodies against CD14 protect primates from endotoxin-induced shock . The Journal of Clinical Investigation , 98 : 1533 – 1538 .
- Li , Y.P. , Handberg , K.J. , Juul-Madsen , H.R. , Zhang , M.F. and Jørgensen , P.H. 2007 . Transcriptional profiles of chicken embryo cell cultures following infection with infectious bursal disease virus . Archives of Virology , 152 : 463 – 478 .
- Lorenzoni , A. and Wideman , R. 2008 . Intratracheal administration of bacterial lipopolysaccharide elicits pulmonary hypertension in broilers with primed airways . Poultry Science , 87 : 645 – 654 .
- Machin , K. , Tellier , L. , Lair , S. and Livingston , A. 2001 . Pharmacodynamics of flunixin and ketoprofen in mallard ducks (Anas platyrhynchos) . Journal of Zoo and Wildlife Medicine , 32 : 222 – 229 .
- Mayr , F. , Firbas , C. , Leitner , J. , Spiel , A. , Reiter , R. , Beyer , D. , Meyer , M. , Wolff , G. and Jilma , B. 2008 . Effects of the pan-selectin antagonist bimosiamose (TBC1369) in experimental human endotoxemia . Shock , 29 : 475 – 482 .
- Meyrick , B. and Brigham , K.L. 1983 . Acute effects of Escherichia coli endotoxin on the pulmonary microcirculation of anesthetized sheep. Structure: function relationship . Laboratory Investigation , 48 : 458 – 470 .
- Miyamoto , T. , Fujinaga , T. , Yamashita , K. and Hagio , M. 1996 . Changes of serum cytokine activities and other parameters in dogs with experimentally induced endotoxic shock . Journal of Veterinary Research , 44 : 107 – 118 .
- Netea , M. , Kullberg , B. and Van der Meer , J. 2000 . Circulating cytokines as mediators of fever . Clinical Infectious Diseases , 31 : S178 – S184 .
- Norimatsu , M. , Ono , T. , Aoki , A. , Ohishi , K. , Takahashi , T. Watanabe , G. 1995 . Lipopolysaccharide-induced apoptosis in swine lymphocytes in vivo . Infection and Immunity , 63 : 1122 – 1126 .
- Osborn , L. 1990 . Leukocyte adhesion to endothelium in inflammation . Cell , 13 : 3 – 6 .
- Ren , Y. , Xie , Y. , Jiang , G. , Fan , J. , Yeung , J. Li , W. 2008 . Apoptotic cells protect mice against lipopolysaccharide-induced shock . The Journal of Immunology , 180 : 4978 – 4985 .
- Romanovsky , A. , Shido , O. , Sakurada , S. , Sugimoto , N. and Nagasaka , T. 1996 . Endotoxin shock: thermoregulatory mechanisms . American Journal of Physiology–Regulatory, Integrative and Comparative Physiology , 270 : R693 – R703 .
- Romanovsky , A.A. , Shido , O. , Sakurada , S. , Sugimoto , N. and Nagasaka , T. 1997 . Endotoxin shock-associated hypothermia. How and why does it occur? . Annals of the New York Academy of ScienceS , 813 : 733 – 737 .
- Saia , R. , Anselmo-Franci , J. and Carnio , E. 2008 . Hypothermia during endotoxemic shock in female mice lacking nitric oxide synthase . Shock , 29 : 119 – 126 .
- Schuerwegh , A. , De Clerck , L. , Bridts , C. and Stevens , W. 2003 . Comparison of intracellular cytokine production with extracellular cytokine levels using two flow cytometric techniques . Cytometry Part B , 55B : 52 – 58 .
- Shini , S. , Kaiser , P. , Shini , A. and Bryden , W. 2008a . Differential alterations in ultrastructural morphology of chicken heterophils and lymphocytes by corticosterone and lipopolysaccharide . Veterinary Immunology and Immunopathology , 122 : 83 – 93 .
- Shini , S. , Kaiser , P. , Shini , A. and Bryden , W. 2008b . Biological response of chickens (Gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin . Comparative Biochemistry and Physiology , 149 : 324 – 333 .
- Suwa , T. , Hogg , J. , Klut , M. , Hards , J. and van Eeden , S. 2001 . Interleukin-6 changes deformability of neutrophils and induces their sequestration in the lung . American Journal of Respiratory Critical Care Medicine , 163 : 970 – 976 .
- Takahashi , K. , Kawamata , K. , Akiba , Y. , Iwata , T. and Kasai , M. 2002 . Influence of dietary conjugated linoleic acid isomers on early inflammatory responses in male broiler chickens . British Poultry Science , 43 : 47 – 53 .
- Takimoto , T. , Sato , K. , Akiba , Y. and Takahashi , K. 2008 . Role of chicken TL1A on inflammatory responses and partial characterization of its receptor . The Journal of Immunology , 180 : 8327 – 8332 .
- Thiemermann , C. 1997 . Nitric oxide and septic shock . General Pharmacology , 2 : 159 – 166 .
- Uchiyama , R. , Morimoto , T. , Kai , O. , Uwatoko , K. , Inoue , Y. and Nakanishi , T. 2005 . Counting absolute number of lymphocytes in quail whole blood by flow cytometry . Journal of Veterinary Medical Science , 67 : 441 – 444 .
- Van Miert , A. , Van Duin , C. and Wensing , T. 1997 . Effects of pentoxifylline and polymyxin B on the acute-phase-response to Escherichia coli endotoxin in dwarf goats . Journal of Veterinary Pharmacology and Therapeutics , 20 : 61 – 68 .
- Van Snick , J. , Cayphas , S. , Vink , A. , Uyttenhove , C. , Coulie , P.G. , Rubira , M.R. and Simpson , R.J. 1986 . Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas . Proceedings of the National Academy of Sciences of the United States of America , 83 : 9679
- Vayssettes-Courchay , C. , Chataigneau , M. , Protin , C. , Ragonnet , C. and Verbeuren , T. 2003 . Cutaneous venous dysfunction studied in vivo in the LPS-treated rabbit: implication of NO in saphenous vein hyporeactivity. Naunyn-Schmiedeberg's . Archives of Pharmacology , 367 : 516 – 523 .
- Verstrepen , L. , Bekaert , T. , Chau , T. , Tavernier , J. , Chariot , A. and Beyaert , R. 2008 . TLR-4, IL-1R, TNF-R signalling to NF-kappaB: variations on a common theme . Cellular and Molecular Life Science , 65 : 2965 – 2978 .
- Wakabayashi , G. , Gelfand , J. , Burke , J. , Thompson , R. and Dinarello , C. 1991 . A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits . The FASEB Journal , 5 : 338 – 343 .
- Walther , A. , Weihrauch , M. , Schmidt , W. , Gebhard , M. , Martin , E. and Schmidt , H. 2000 . Leukocyte-independent plasma extravasation during endotoxemia . Critical Care Medicine , 8 : 2943 – 2948 .
- Witkamp , R. and Monshouwer , M. 2000 . Signal transduction in inflammatory processes, current and future therapeutic targets: a mini review . Veterinary Quarterly , 22 : 11 – 16 .
- Xiang , L. , Peng , B. , Dong , W. , Yang , Z. and Shao , J. 2008 . Lipopolysaccharide induces apoptosis in Carassius auratus lymphocytes, a possible role in pathogenesis of bacterial infection in fish . Developmental and Comparative Immunology , 32 : 992 – 1001 .
- Yipp , B. , Andonegui , G. , Howlett , C. , Robbins , S. , Hartung , T. , Ho , M. and Kubes , P. 2002 . Profound differences in leukocyte–endothelial cell responses to lipopolysaccharide versus lipoteichoic acid . The Journal of Immunology , 168 : 4650 – 4658 .