Abstract
Infectious bronchitis virus (IBV) is, in spite of vaccination, still a major cause of respiratory problems in broilers and of poor egg production in breeders and layers in many parts of the world. A possible cause of the insufficient protection induced by vaccination is an inadequate application of the vaccine. This paper reports the results of two field studies. In the first, the results of the α-IBV IgM enzyme-linked immunosorbent assay (ELISA) on post-vaccination sera were compared with the efficacy of the IBV vaccination against homologous challenge of the same broilers. The results showed that groups with at least 50% positive sera in the IgM ELISA at 10 days post vaccination had a high level of protection against challenge. Most groups of broilers with a low level of IgM ELISA positives had a low or moderate level of protection against challenge. In a second field study, the IgM response to IBV vaccination was compared with detailed information of the vaccination process of 360 spray-vaccinated flocks of about 2-week-old broilers, layer pullets, broiler breeders and broiler grandparents. The information included parameters such as flock size, type of chicken, housing, age of the chicken, application route, vaccine, dose, water quantity and temperature, ventilation and light management, combination with other vaccines and temperature of the house. The aim was to identify factors that might be associated positively or negatively with the IgM response and thereby with the expected level of protection against homologous challenge under field conditions. Significant associations were detected between the level of IgM response and factors regarding type of bird, flock size, housing type, ventilation management, light management, age/interval of vaccination, interval between vaccination and blood sampling, and temperature of the water that was used to reconstitute the vaccine. This knowledge can be useful to improve the average efficacy of IBV vaccination in the field.
Introduction
Infectious bronchitis virus (IBV) is, in spite of vaccination, a major cause of respiratory problems in broilers and of poor egg production in breeders and layers in many parts of the world. Possible reasons for partial or complete failure of the protection induced by vaccination are, for example, heterologous challenges, immunosuppression, very short or long intervals between vaccination and challenge and, last but not least, inadequate application of the vaccine. The mass application of IBV vaccines in the field is known for its many variations in: application technique (eye drop, course spray, drinking water, aerosol); quantity, quality and temperature of the water used to dilute the vaccine; dose; and the combination of different vaccines such as IBV with Newcastle disease vaccines (Jackwood et al., Citation2009). Many of these factors can have a negative effect on the efficacy of vaccination under field conditions. As many of these techniques and factors are developed or implemented for human comfort and are not necessarily favourable for the take of the vaccine virus, there is a need for a simple and cheap method to check the efficacy of IBV vaccination under field conditions. Routine determination of the level of protection by challenge is not practical or affordable. If a vaccination induces a humoral response, serology can be used for checking the vaccination efficacy by detection of a seroconversion using paired serum samples. This strategy is hampered for vaccination in the presence of maternally derived antibodies (De Wit, Citation2000), the need for two samplings and the usually low serological response to mild vaccines (De Wit et al., Citation1997). An alternative strategy for serodiagnosing the take of an IBV vaccination would be to look for the presence of an IBV-specific IgM response. In contrast to the other immunoglobulin classes, like IgG and IgA, that are produced in response to IBV vaccination and infection, IBV-specific IgM response is short-lived (Gillette, Citation1974; Mockett & Cook, Citation1986; Martins et al., Citation1990, Citation1991; Toro & Fernandez, Citation1994; De Wit et al., Citation1998). Detection of IgM is therefore indicative of a recent infection or vaccination.
This paper reports the results of two field studies. In the first study, the efficacy of IBV vaccination in protecting against homologous challenge was compared with the results of the α-IBV IgM enzyme-linked immunosorbent assay (ELISA) on sera that were collected at several intervals post vaccination of the same broilers. The predictive value of the IgM ELISA was determined for the efficacy of the vaccination.
In a second field study, the IgM response to IBV vaccination was compared with detailed information of the vaccination process of 360 spray-vaccinated flocks of about 2-week-old broilers, layer pullets, broiler breeders and broiler grandparents. The information included parameters such as flock size, type of chicken, housing, age of the chicken, vaccine application route, vaccine used, dosage, water quantity, ventilation and light management, combination with other vaccines and temperature of the house and quality of water used. The aim was to identify factors that might be associated positively or negatively with the IgM response, and thereby with the expected level of protection against homologous challenge under field conditions. This knowledge can be useful to improve the average efficacy of IBV vaccinations in the field.
Materials and Methods
Field study 1: IgM IBV response and level of protection against IBV challenge
Study design and housing
At 13 days of age, six groups of 10 broilers were collected from six non-vaccinated broiler flocks and housed in separate negative-pressure isolators at the Animal Health Service (AHS), the Netherlands. These birds were vaccinated (eye–nose drop) at 14 days of age with one dose of the same vaccine (combination of H120 and D274) as the remaining birds of these flocks housed in the field (). At 15 days of age, 10 birds from each of the flocks that had been vaccinated at the farm (spray or water application), were transported to the AHS and also housed in separate isolators. Blood samples were taken 1 day before vaccination and 10 days post vaccination (d.p.v.) and were stored at −20°C until testing. Non-vaccinated specific pathogen free broilers were used as control birds.
Table 1. Field study 1: IgM response and level of protection after infectious bronchitis vaccination at 14 days of age with a combined Mass and D274 vaccine followed by a homologous Mass M41 challenge.
IgM IBV ELISA
The ELISA was performed as described by De Wit et al. (1998). Briefly, a monoclonal antibody specific to chicken IgM was used as the catching antibody and coated onto microtitre plates. Subsequent steps included the addition of test serum in duplicate (diluted 1:50 in phosphate-buffered saline, pH 7.3, containing 0.65 M NaCl, 1 mM ethylenediamine tetraacetic acid [Merck], 0.05% [v/v] Tween 80 and 0.5% [v/v] casein [Sigma]), IBV-M41 antigen or control antigen (negative allantoic fluid), enzyme-labelled anti-IBV monoclonal antibody (CVI-IBVNp-48.4), and enzyme substrate. Results were expressed as the percentage positivity (PP%) compared with the results of the positive control (PC) sample. The PP% of a test sample (ts) was calculated as follows:
Challenge
All birds were challenged by eye drop with 104 median embryo infectious doses (EID50) of the M41 strain of IBV in 0.1 ml diluent per bird between 16 and 21 d.p.v. Non-vaccinated specific pathogen free broilers (AHS) were also challenged with M41 or with negative allantoic fluid at 49 days of age.
Ciliostasis test
The level of protection was determined using the ciliostasis test on five tracheal rings per bird. The tracheas were placed in Hank's minimum essential medium immediately after killing the chickens by an intravenous injection of 0.1 to 0.2 ml T61 (Intervet Nederland, Boxmeer, the Netherlands) and subsequent bleeding. Then, five rings (equally divided over the total length of the trachea) were cut and placed in medium at 37°C. The level of ciliostasis was determined independently by two technicians between 1 and 4 h after the birds were killed. The level of beating of the cilia for each ring was expressed as 0 (0% beating of cilia, total lack of protection), 1 (>0 to 25% beating), 2 (>25 to 50% beating), 3 (>50 to 75% beating) or 4 (>75 to 100% beating). One bird could score between 0 and 20 (five rings from each trachea: maximum score 4 per ring). An individual chick was recorded as protected against challenge if the ciliostasis score was 10 or more (Cook et al., Citation1999). For each group, a protection score (0 to 100%) was calculated by the formula:
Field study 2: IgM response and associations with vaccination parameters
Flocks
In the second field study, 360 flocks of broilers, pullets, rearing broiler breeders and broiler grandparents were vaccinated at about 14 days of age with an IBV vaccine by spray. All layer pullet, rearing broiler breeder, and broiler grandparent flocks had been vaccinated in the hatchery with a Massachusetts (Mass) strain vaccine or a Mass/D274 vaccine. In these flocks, the vaccine used at around 14 days of life was of the heterologous 793B (4/91,CR88) serotype (). The vaccination schedule was the same in 78% of the broiler flocks. The other 22% of the flocks had not been vaccinated in the hatchery and were vaccinated for the first time against IBV at approximately 14 days of age with a Mass strain vaccine or a combination of a Mass and another IBV strain (D274 or Arkansas). Detailed information of the vaccination procedure and conditions in the house were recorded on an enquiry sheet. These data included, for example, the type of bird, breed, age, flock size, vaccine used, dose, use of stabilizer, application method, amount of water allowed per bird, vaccination reaction, temperature of house, temperature of water to reconstitute and spray the vaccine, ventilation system, ventilation on/off, light on/off, other vaccinations (which, when), and season. At about 10 days after the vaccination, blood was collected from 10 birds per house and tested for the presence of IgM against IBV by ELISA as described above.
Table 2. General information about the 360 flocks and the IBV spray vaccinations used for field study 2.
Statistical analysis
Analysis of variance (UNIANOVA in SPSS 17.0) was used to analyse the data. This method has the prerequisites that the variance should be homogeneous within groups and that the residuals should be normally distributed. Because of lack of homogeneity in the variance, the results of the broilers, pullets, broilers' grandparents and broiler breeders had to be analysed separately. The analyses of variance were applied stepwise backwards, starting with all potentially influential variables. At each step the least significant variable was removed and the analyses were repeated until all remaining variables had a P value of 0.05 or less. For the final model the normality of the residuals was tested.
Results
Field study 1: IgM IBV response and level of protection
The six groups of broilers that had been vaccinated by eye drop at the laboratory showed at least 50% positive sera in the IgM ELISA at 10 d.p.v. and a protection level against challenge between 89% and 100% (). The six groups of broilers that had been vaccinated at the farm showed, on average, 7% positives in the IgM ELISA at 10 d.p.v. and an average level of protection of 43% with a range from 0% to 86%. Surprisingly, the differences in levels of protection between different houses on the three different farms were high. The differences in average protection of the birds of the different houses of the same farm were 51%, 76% and 36% for Farms A, B and C, respectively. According to the vaccinator, the vaccination procedures had been the same on the farm level—except for Farm C where there was a difference in dosage of the vaccine used, since House 1 received one-half of a recommended dose of vaccine/bird.
Field study 2: IgM response and associations with vaccination parameters
Detailed information about the vaccination process was obtained from 360 flocks that had been vaccinated by the spray method (). The average percentage of responders was 46%. The results of the statistical analyses that were significant are summarized in . The average percentages of responders of the broilers (38%, 88 flocks) and of the layer pullets (46%, 178 flocks) were significantly lower than that of the grandparents (61%, 36 flocks). The average percentage of responders of the broiler breeders (52%, 58 flocks) was not significantly different from that of the other groups. Sixteen per cent (95% confidence interval: 8.3 to 23.5%) of the broiler flocks had no detectable IgM response (all samples negative) compared with 0% of the grandparent flocks, 3% (95% confidence interval: 0 to 8.1%) of the broiler breeders flocks and 7% (95% confidence interval: 3.5 to 11.1%) for the pullet flocks (). The percentage of the pullet flocks without a detectable IgM response was significantly higher (P=0.029) than the percentages of the other groups.
Figure 1. Overview of the infectious bronchitis IgM responses in each flock after IBV vaccination at approximately 2 weeks of age in 360 broiler, pullet, broiler breeder and broiler grandparent flocks. x axis: percentage of sera from a flock that was positive in the α-IBV IgM ELISA. y axis: percentage of flocks with a certain percentage of positive sera.
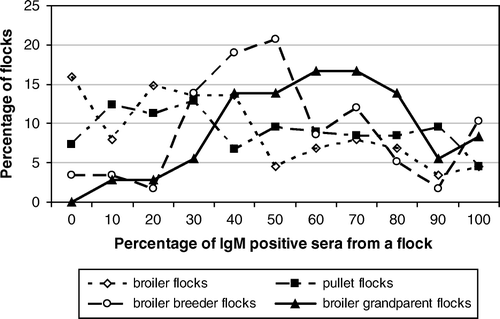
Table 3. Statistically significant correlations between parameters of the IBV spray vaccination procedure and the average IgM response in each flock in 360 flocks of approximately 2-week-old broilers, layer pullets, broiler breeders and broiler grandparents.
For the broilers, 68% of the variation was explained by the variations in the parameters: flock size, ventilation, light and interval between vaccination and sampling time:
| • | Increasing flock sizes was correlated with decreasing IgM responses (P=0.04) (). Every extra 1000 birds resulted, on average, in a 1% lower IgM response (). | ||||
| • | Flocks that had been vaccinated with the ventilation turned off during spray vaccination showed a 15.5% higher IgM response (P=0.037) than the flocks that had been vaccinated with the ventilation system running (). | ||||
| • | Flocks that had been vaccinated with the lights turned on during spray vaccination showed a 41% higher IgM response (P=0.009) than the flocks that had been vaccinated with the lights turned off (). | ||||
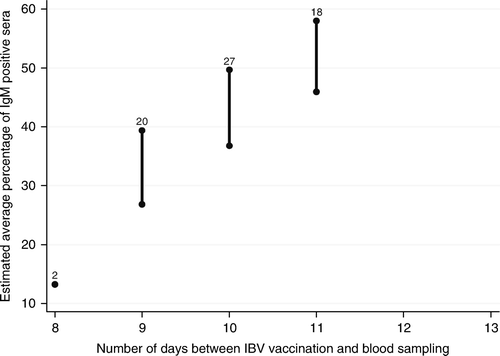
| • | A longer interval between vaccination and sampling resulted in an increased IgM response (P=< 0.001) (). | ||||
Figure 2. Association between the size of the broiler flocks and the average IgM response in each flock after IBV spray vaccination at approximately 2 weeks of age. Bar: 95% confidence interval of the estimated average percentage of IgM-positive sera for each flock size. Number on top of bar: number of flocks with that flock size.
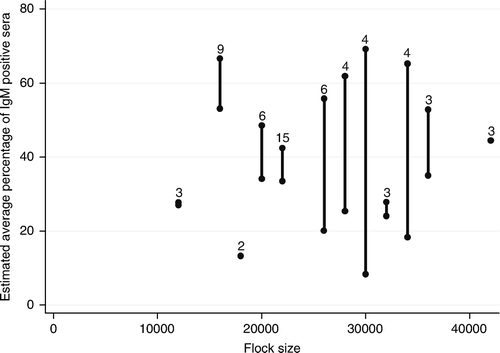
Figure 3. Association between the number of days between vaccination and sampling and the average IgM response for broiler flocks after the IBV spray vaccination at approximately 2 weeks of age. Bar: 95% confidence interval of the estimated average percentage of IgM-positive sera for each number of days between vaccination and blood sampling. Number on top of bar: number of flocks with that number of days between vaccination and blood sampling.
For the layer pullets, 44% of the variation was explained by the housing type and number of days between the previous IBV vaccination at day 1 and the vaccination around 14 days of age:
| • | Housing type. The average response for the birds housed in cages, an aviary system and on the floor covered with wood shavings was 31%, 48% and 53%, respectively (difference cage – ground, P=0.010; and cage – aviary, P=0.052; ). There were considerable differences in average flock sizes for the housing types. The average flock size housed in the cages was 41,873 compared with 30,474 birds for the aviaries and 17,642 birds for the floor housing (). The parameters housing type and flock size were not independent from each other. | ||||
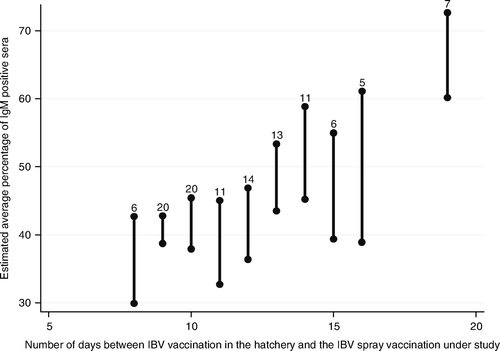
| • | A longer interval between the previous vaccination (Mass or Mass/D274) at day of hatch and the second heterologous 793B-type vaccination resulted in a higher IgM response (P=0.005, ). Every extra day between the vaccination at day 1 and the second vaccination (ranging from 8 to 19 days) resulted in a 2.5% higher IgM response (). This was especially the case for the period of 8 to 13 days. Vaccinations from 13 days onwards resulted in comparable IgM responses. | ||||
Figure 4. Association between the interval between the heterologous IBV vaccination in the hatchery and the spray vaccination under study and the average IgM response per layer pullet flock after IBV spray vaccination at approximately 2 weeks of age. Bar: 95% confidence interval of the estimated average percentage of IgM-positive sera for each number of days between the vaccination in the hatchery and the spray vaccination under study. Number on top of bar: number of flocks with that number of days between vaccination in the hatchery and the spray vaccination under study.
For the young replacement broiler breeders, the variation in the parameters was relatively small due to the number of vaccinators that were involved in the vaccinations of these flocks. Forty-six per cent of the variation could be explained by:
| • | More days between previous vaccination (Mass or Mass/D274) at day of hatch and second heterologous 793B vaccination resulted in a lower IgM response (P=0.018) (). Every extra day between vaccination at day 1 and the second vaccination (ranging from 8 to 13 days) resulted in a 6.6% lower IgM response (). | ||||
| • | Higher temperatures of the water that was used to reconstitute and spray the vaccine (P=0.021) resulted in lower IgM responses (). Every degree centigrade higher temperature (ranging from 6 to 18°C) resulted in an average 3.2% lower IgM response after vaccination (). | ||||
Figure 5. Association between the interval between the heterologous IBV vaccination in the hatchery and the spray vaccination under study and the average IgM response per broiler breeder flock after IBV spray vaccination at approximately 2 weeks of age. Bar: 95% confidence interval of the estimated average percentage of IgM-positive sera for each number of days between the vaccination in the hatchery and the spray vaccination under study. Number on top of bar: number of flocks with that number of days between vaccination in the hatchery and the spray vaccination under study.
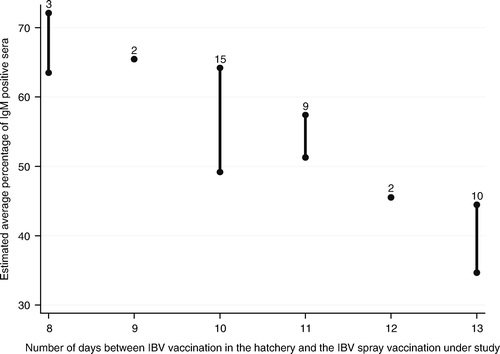
Figure 6. Association between the temperature of the water that was used to reconstitute and spray the vaccine and the average IgM response per broiler breeder flock after IBV spray vaccination at approximately 2 weeks of age. Bar: 95% confidence interval of the estimated average percentage of IgM-positive sera for each temperature of the water. Number on top of bar: number of flocks with that temperature of the water that was used to reconstitute and spray the vaccine.
For the young broiler grandparents, the variation in parameters was small. There were no significant correlations between the parameters investigated and the IgM results.
Discussion
This paper reports the results of two field studies. In the first, the efficacy of IBV vaccination against homologous challenge was compared with the results of the α-IBV IgM ELISA on sera that were collected at 10 d.p.v. The results showed that groups with at least 50% positive sera in the IgM ELISA at 10 d.p.v. had a protection of at least 89% against challenge. Groups of broilers with a low level of IgM positives showed an average protection rate of 43%, ranging from 0 to 86%. These results show that a higher percentage of positives in the IgM ELISA is an indication of a high efficacy of the vaccination. A low percentage of positives in the IgM ELISA should be interpreted as a warning, since the efficacy of the vaccination might be moderate, low or even non-existent. It was concluded that the results of the IgM ELISA could be used as an indicator for the efficacy of the IBV vaccination in the second field study, which aimed to identify possible factors that might be associated positively or negatively with the IgM response and thereby with the expected level of protection against homologous challenge. This second study was performed to detect statistically significant associations between certain factors related to the vaccination procedure and management circumstances and the IgM response. However, it is important to realize that a significantly positive or negative association of a factor with a seroresponse does not necessarily mean that it is a causal relationship. For example, if factor A causes a disease that results in manifestations B and C, there will be a statistical causal association between A and B, and between A and C. However, there will also be a statistical association between the two response variables, B and C, arising from their separate associations with A, but this is a non-causal association (Martin et al., Citation1987).
The average IgM responses of the broiler and layer pullet flocks were significantly lower than that of the broiler grandparents replacement flocks. It cannot be excluded that genetic aspects may play a role in this difference (Cook et al., Citation1990; Otsuki et al., Citation1990; Nakamura et al., Citation1991). However, the difference in average results was highest between the broilers and the grandparents, which are most probably genetically more related to the broilers than the pullets (which were not significantly different). This makes it unlikely that genetic aspects play a major role. A major difference in the results between the type of chicken was the percentage of flocks with no detectable IgM response. Sixteen per cent of the broiler flocks had no detectable IgM response compared with 0% of the broiler grandparent flocks, 3% of the broiler breeder flocks and 7% for the pullet flocks. Remarkably, this order is also the order of economical value of the birds, and this possibly had an influence on the attention and time that is invested in the vaccination procedure. Another part of the explanation might be the effect of the difference in average flock sizes, a significant factor with the broilers, as the average flock sizes of broilers and pullets is higher than the relatively small flock size of broiler breeders and broiler grandparents. Another factor that might play a role, especially for the broilers, is the tendency to perform the vaccination of broilers in such a way that the vaccination reaction is not noticeable or is as mild as possible (Smith et al., Citation1985; Di Matteo et al., Citation2000; Matthijs et al., Citation2003; Bijlenga et al., Citation2004). Small disturbances in the performance of the broilers due to vaccination reactions in the short fattening period can already have a relevant economic impact, whereas this is hardly the case in the long-living pullets, broiler breeders and grandparents. This tendency to vaccinate mildly might logically result in a higher risk of inadequate take of the vaccine, resulting in low or no protection.
The statistical associations between the seroresponse and the ventilation and light management could be explained well with existing knowledge about IBV. Leaving the ventilation running during spray vaccination will reduce the vaccine virus concentration in the air because it is blown out of the house before it reaches the bird. The reason for the better response when the lights are on during the vaccination might be due to the fact that the eyes are open when the lights are turned on and closed when the light is turned off. It is well known that IBV vaccines applied onto the conjunctiva can be very effective (Davelaar & Kouwenhoven, Citation1976; Survashe et al., Citation1979; Toro et al., Citation1996) and eye-drop application is generally considered to be the gold standard for application of live IBV vaccines.
The significant positive association between the interval between vaccination and sampling and the higher IgM response in the broiler flocks might well be caused by spreading of the vaccine virus after replication to some of the housemates. When only a part of the flock is effectively vaccinated, the vaccine may start to spread from bird to bird (Matthijs et al., Citation2008; Jackwood et al., Citation2009). This will result in a delayed IgM response at flock level.
The significantly lower IgM response detected in the layer pullets housed in cages compared with the birds on the floor and in aviary housing was not expected. The general idea was that birds in cages can more easily be vaccinated as they cannot run away, although they can hide behind other birds in large cages. A potential reason for the lower response in the cages might be the usually big flock size (see negative correlation between flock size and IgM response of the broilers), but also the potentially fewer possibilities of spread of the vaccine from bird to bird compared with birds that are in contact with faeces in the case of suboptimal application of the vaccine.
A potential cause for the lower response in pullets when the vaccine was applied within 13 days of the first vaccination might be the recovery time needed by the tracheal epithelium after the first vaccination at day of hatch using a Mass strain or a combination of a Mass and a D274 strain (Winterfield & Fadly, Citation1972; Cook et al., Citation1990; Di Matteo et al., Citation2000; Bijlenga et al., Citation2004). Another explanation could be the findings of Davelaar & Kouwenhoven (Citation1977), who reported a decreased efficacy of H120 vaccination at 6 and 10 days of age, compared with vaccination at 1, 15 and 20 days of age. This decreased efficacy of a vaccination at 6 or 10 days was not confirmed in the work of Darbyshire & Peters (Citation1985), where they reported full protection against a M41 challenge at about 4 weeks post vaccination, using H120 at days 1, 7 and 14 in commercial layer pullets. However, these results were based on only three to four birds per group. The significant negative correlation between the length of the interval between the first, at hatch, and the second vaccinations and the IgM response in the broiler breeders is in contrast with the results in the layers. A biologically plausible cause is hard to find. These results show that other, at present unknown, factors are most probably interfering.
The extent of the influence of the temperature of the water used to reconstitute the vaccine (P=0.021) was remarkable, but in line with the known susceptibility of IBV to higher temperatures (Cavanagh & Gelb, Citation2008). Every degree centigrade higher temperature (ranging from 6 to 20°C) resulted in a 3.2% lower IgM response after the second vaccination.
As can be expected in these types of field study, not all results can easily be explained. Both experimental and field studies have advantages and disadvantages. In experimental studies, the variables can often be well controlled, which makes it easier to draw conclusions from the results. Whether the results are relevant for the field is not always clear. Field trials are performed in the “real world”, but the translation of the results can be very complicated due to the presence of many uncontrolled and unknown variables. However, the results of these field studies provided information and knowledge that can be used for improving the average efficacy of IBV spray vaccination in the field. For this, the IgM IBV ELISA is a helpful tool.
Acknowledgements
This research was supported by a grant from the National Board for Poultry and Eggs (PPE) of the Netherlands. The authors thank the contributing poultry farmers, veterinarians and integrations for their technical assistance to this work.
References
- Bijlenga , G. , Cook , J.K.A. , Gelb , J. Jr and De Wit , J.J. 2004 . Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review . Avian Pathology , 33 : 550 – 557 .
- Cavanagh D. Gelb J.J. 2008 Infectious Bronchitis In Y.M. Saif A.M. Fadly J.R. Glisson L.R. McDougald L.K. Nolan D.E. Swayne Diseases of Poultry , 12th edn 117 135 Blackwell Publishing Professional, Ames Iowa , , USA
- Cook , J.K.A. , Orbell , S.J. , Woods , M.A. and Huggins , M.B. 1999 . Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes . Avian Pathology , 28 : 477 – 485 .
- Cook , J.K.A. , Otsuki , K. , Huggins , M.B. and Bumstead , N. 1990 . Investigations into resistance of chicken lines to infection with infectious bronchitis virus . Advances in Experimental Medicine and Biology , 276 : 491 – 496 .
- Darbyshire , J.H. and Peters , R.W. 1985 . Humoral antibody response and assessment of protection following primary vaccination of chicks with maternally derived antibody against avian infectious bronchitis virus . Research in Veterinary Science , 38 : 14 – 21 .
- Davelaar , F.G. and Kouwenhoven , B. 1976 . Changes in the Harderian gland of the chicken following conjunctival and intranasal infection with infectious bronchitis virus in one- and 20-day-old chickens . Avian Pathology , 5 : 39 – 50 .
- Davelaar , F.G. and Kouwenhoven , B. 1977 . Influence of maternal antibodies on vaccination of chicks of different ages against infectious bronchitis . Avian Pathology , 6 : 41 – 50 .
- De Wit , J.J. 2000 . Detection of infectious bronchitis virus . Avian Pathology , 29 : 71 – 93 .
- De Wit , J.J. , Mekkes , D.R. , Koch , G. and Westenbrink , F. 1998 . Detection of specific IgM antibodies to infectious bronchitis virus by an antibody-capture ELISA . Avian Pathology , 27 : 155 – 160 .
- De Wit , J.J. , Mekkes , D.R. , Kouwenhoven , B. and Verheijden , J.H. 1997 . Sensitivity and specificity of serological tests for infectious bronchitis virus antibodies in broilers . Avian Pathology , 26 : 105 – 118 .
- Di Matteo , A.M. , Sonez , M.C. , Plano , C.M. and Von Lawzewitsch , I. 2000 . Morphologic observations on respiratory tracts of chickens after hatchery infectious bronchitis vaccination and formaldehyde fumigation . Avian Diseases , 44 : 507 – 518 .
- Gillette , K.G. 1974 . Avian infectious bronchitis: Demonstration of serum IgG- and IgM-neutralizing antibody by sucrose density-gradient centrifugation and mercaptoethanol reduction . Avian Diseases , 18 : 515 – 525 .
- Jackwood , M.W. , Hilt , D.A. , McCall , A.W. , Polizzi , C.N. , McKinley , E.T. and Williams , S.M. 2009 . Infectious bronchitis virus field vaccination coverage and persistence of Arkansas-type viruses in commercial broilers . Avian Diseases , 53 : 175 – 183 .
- Martin , S.W. , Meek , A.H. and Willeberg , P. 1987 . Veterinary Epidemiology, Principles and Methods , Ames : Iowa State University Press .
- Martins , N.R. , Mockett , A.P.A. , Barrett , A.D. and Cook , J.K.A. 1991 . IgM responses in chicken serum to live and inactivated infectious bronchitis virus vaccines . Avian Diseases , 35 : 470 – 475 .
- Martins , N.R. , Mockett , A.P.A. and Cook , J.K.A. 1990 . A method for the rapid purification of serum IgM for the diagnosis of recent viral infections of chickens . Journal of Virological Methods , 29 : 117 – 125 .
- Matthijs , M.G. , Bouma , A. , Velkers , F.C. , van Eck , J.H. and Stegeman , J.A. 2008 . Transmissibility of infectious bronchitis virus H120 vaccine strain among broilers under experimental conditions . Avian Diseases , 52 : 461 – 466 .
- Matthijs , M.G. , van Eck , J.H. , Landman , W.J. and Stegeman , J.A. 2003 . Ability of Massachusetts-type infectious bronchitis virus to increase colibacillosis susceptibility in commercial broilers: a comparison between vaccine and virulent field virus . Avian Pathology , 32 : 473 – 481 .
- Mockett , A.P.A. and Cook , J.K.A. 1986 . The detection of specific IgM to infectious bronchitis virus in chicken serum using an ELISA . Avian Pathology , 15 : 437 – 446 .
- Nakamura , K. , Cook , J.K.A. , Otsuki , K. , Huggins , M.B. and Frazier , J.A. 1991 . Comparative study of respiratory lesions in two chicken lines of different susceptibility infected with infectious bronchitis virus: histology, ultrastructure and immunohistochemistry . Avian Pathology , 20 : 241 – 257 .
- Otsuki , K. , Huggins , M.B. and Cook , J.K.A. 1990 . Comparison of the susceptibility to avian infectious bronchitis virus infection of two inbred lines of white leghorn chickens . Avian Pathology , 19 : 467 – 475 .
- Smith , H.W. , Cook , J.K.A. and Parsell , Z.E. 1985 . The experimental infection of chickens with mixtures of infectious bronchitis virus and Escherichia coli . Journal of General Virology , 66 : 777 – 786 .
- Survashe , B.D. , Aitken , I.D. and Powell , J.R. 1979 . The response of the Harderian gland of the fowl to antigen given by the ocular route. I. Histological changes . Avian Pathology , 8 : 77 – 93 .
- Toro , H. and Fernandez , I. 1994 . Avian infectious bronchitis: specific lachrymal IgA level and resistance against challenge . Zentralblatt fur Veterinarmedizin, B , 41 : 467 – 472 .
- Toro , H. , Reyes , E. , Redmann , T. and Kaleta , E.F. 1996 . Local and systemic specific antibody response of different chicken lines after ocular vaccination against infectious bronchitis . Zentralblatt fur Veterinarmedizin, B , 43 : 449 – 454 .
- Winterfield , R.W. and Fadly , A.M. 1972 . Some characteristics of isolates of infectious bronchitis virus from commercial vaccines . Avian Diseases , 16 : 746 – 755 .