Abstract
Proliferative growth, consistent with poxvirus infection, encapsulated plastic beak-bits and covered the dorsal portion of the upper beak and nares of adult male and female captive-raised Hungarian partridges. Three representative birds were submitted to the Wisconsin Veterinary Diagnostic Laboratory for necropsy. Lesions in the necropsied birds extended through the nares, where the plastic bit ends are designed to rest. The lesions also variably extended caudally into the oropharynx and cranially within the beak epithelium, and included palate deformity and beak necrosis. Poxvirus was diagnosed in all of the birds examined based on histopathology, electron microscopy, and polymerase chain reaction amplification and sequencing. This report is the first to describe avian pox lesions associated with the application of beak-bits and the resulting beak and oral pathology.
Introduction
In poultry and game birds reared in captivity, plastic anti-pecking devices such as ring beak-bits can be used to prevent feather pecking, cannibalism, and egg eating (Savory & Hetherington, Citation1997). Beak-bits are widely used in the game bird industry because they have the advantage, over beak clipping or cautery, of preserving the normal appearance of the beak, and they are considered by some to be a better alternative in terms of animal welfare to the other techniques. To our knowledge there have been no significant adverse effects reported with the use of beak-bits. The bits are set in the nostrils and lie between the upper and lower beak to prevent complete closure. Manifestations of avian poxvirus infections are typically divided into clinically benign cutaneous or clinically severe diphtheritic forms. Cutaneous forms present on the non-feathered legs, feet, face, and base of the beak, and diphtheritic lesions occur in the mucous membranes of the oropharynx and respiratory tract; and outbreaks in farm-raised poultry (Tripathy, Citation2008) and free-living partridges (Gortá Zar et al., Citation2002; Buenestado et al., Citation2004) have been well described. Host specificity of poxviruses is known to play a role in clinical manifestation of these two forms, with some pox species presenting as diphtheritic lesions in one avian species and as cutaneous lesions in others (Kim et al., Citation2003), and both forms have been described in the same bird (Fallavena et al., Citation1993). In the present report, we describe the unusual extension of pox lesions from the skin into the upper respiratory and oral mucosa via eroded and necrotic nares, and possible promotion of this lesion by beak-bit mechanical abrasion.
Materials and Methods
The Hungarian partridges described in this report were commercially raised in a northern climate in an indoor facility. They were housed in groups of 120 to 150 in nine connected wire mesh indoor pens with shared side walls. Access to wild birds and mosquitoes was minimal. The birds were maintained on a 19% protein commercial pelleted game ration supplemented with Fenbendazole. Three adult male birds with beak lesions were humanely euthanized on the farm and submitted to the Wisconsin Veterinary Diagnostic Laboratory for necropsy.
Pathology preparations
Tissues from necropsied partridges were fixed in 10% buffered neutral formalin. Major organs (heart, lungs, trachea, spleen, kidney, pancreas, intestine, testis, crop, proventriculus, ventriculus, and liver) and sections of lesions that included the oropharynx, nares and beak-bits, maxillary beak and skin around the eyes were paraffin embedded, sectioned at 5 to 8 µm, and stained with haematoxylin and eosin. Crusts from the lesions were collected for electron microscopy. Negative staining of the crusts was done by pulverizing the crust with a wooden applicator stick and rinsing it in 50 µl of 2% phosphotungstic acid, then transferring it to a grid for viewing on a Hitachi H-7500 Transmission Electron Microscope. Lesions were also post-fixed in Karnofsky's and 2% buffered osmium tetroxide. In a series of increasing concentrations of ethanol, the samples were dehydrated and infiltrated in EPON resin, and then cut into 80 nm thick sections. All sections were post stained with uranyl acetate and lead citrate.
Extraction of nucleic acid from tissue
Samples obtained from 100 to 200 mg fresh tissue were collected into a vial containing ceramic beads (Roche, Denver, Colorado, USA) and 1 ml sterile phosphate-buffered saline, and were homogenized (Bertin Technologies, Montigny-le-Bretonneux, France) for 30 sec at 6500 rpm. DNA and RNA were co-eluted from 50 µl homogenate using the Mag Max 96 Viral RNA Isolation Kit (Ambion; ABI, Foster City, California, USA), as per the manufacturer's protocol.
Polymerase chain reaction
A segment of the gene encoding the fowlpox 4b core protein was amplified using previously published primers (Lee & Lee, Citation1997): P1 forward, CAGCAGGTGCTAAACAACAA; and P2 reverse, CGGTAGCTTAACGCCGAATA. The 50 µl polymerase chain reaction (PCR) mix contained 1x PCR Buffer (GeneAmp®; ABI), an additional 2.5 mM MgCl2, 0.25 mM each dNTP, 1 µM each primer, 2.5 u AmpliTaq® polymerase (ABI), and 5 µl sample or control. A GeneAmp PCR System 9700 (ABI) was used with the following parameters: initial denaturation at 95°C for 5 min; 40 cycles of 95°C for 30 sec, 50°C for 30 sec, and 72°C for 1 minute; and a final extension at 72°C for 7 min. Amplicons were excised from a 1.5% agarose gel, purified with the QIAquick Gel Extraction Kit (Qiagen, Valencia, California, USA) and sequenced at the University of Wisconsin Biotechnology Center.
Results
Necropsy and histopathology
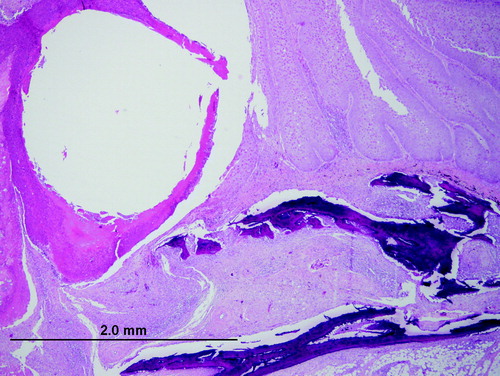
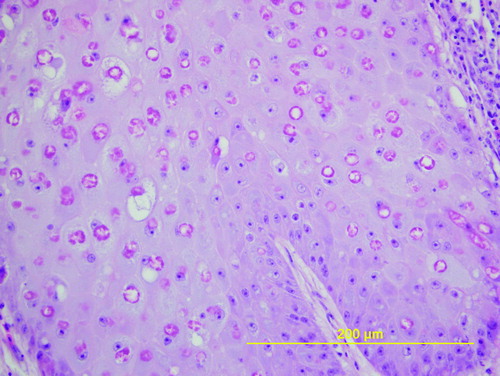
Examination of the upper beaks of the birds revealed firm tan nodules that completely obscured the nares and plastic beak-bit ends. The lesions extended to the skin around the eye of one bird, and completely replaced the entire upper beak of another bird (). None of the birds had lesions on other non-feathered parts of the body and all three had adequate muscle mass and were in good body condition (weight 278.0 to 383.0 g). The plastic bits formed the centre of the lesions, with thick circumferential crusts of necrotic tissue encasing them (). On histopathologic section, sheets of necrotic cells surrounded the space left by the removed plastic bit. This tissue was surrounded by hyperplastic and papillary outgrowths of epithelium that variably extended ventrally and caudally into the palate and oropharynx or cranially within the beak, undermining the keratin layer of the distal beak epithelium. The cells of the stratum spinosum were markedly swollen and vacuolated and contained eosinophilic cytoplasmic inclusions (). Bacteria were also noted in the crusts and superficial stratum spinosum of some sections.
Figure 1. Hungarian partridge with proliferative growths on the upper beaks that surround plastic beak-bits and associated beak necrosis.

Confirmatory testing
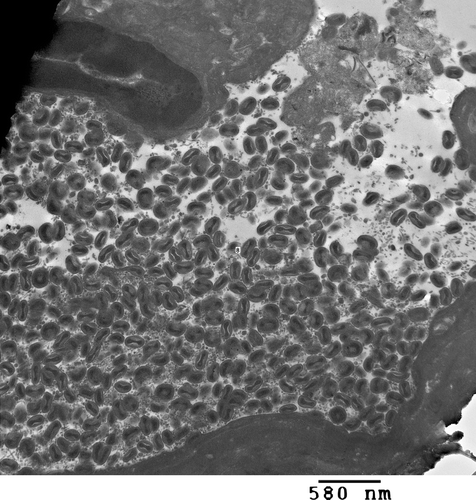
Negative staining of crusts (not shown) and electron microscopy of embedded tissue () revealed sheets of brick-shaped virus particles falling in the range of 250 to 300 nm x 180 to 200 nm and demonstrating inner dumbbell-shaped cores. Polymerase chain amplification for fowl poxvirus resulted in the predicted 578 base pair band and was confirmed by sequence analysis (data not shown).
Figure 4. Epithelium from a proliferative mass at the base of the beak surrounded by sheets of poxvirus particles. Electron microscopy magnification 22,000 x at 80 kV.
While the large poxvirus lesions that developed around beak bits were associated with subsequent beak necrosis and oral invasion, there were no deaths directly attributable to the lesions and only mild weight loss was reported.
Discussion
In the present report, Hungarian partridges developed cutaneous lesions centred on the nares of the upper beak. Initially 20 birds in a pen of 120 to 150 birds were identified with lesions, with subsequent spread to approximately 30% of the birds in the pen over the following month. The birds from the infected pen were shipped out once the diagnosis was made, and no further spread was reported. Low morbidity and no mortality are typical of cutaneous poxvirus outbreaks in poultry and game birds, and the infiltration of the oral mucosa in the birds we describe, did not result in the more severe disease associated with the diphtheritic presentation of avian pox. The bit application tool was considered a potential source of spread of the virus, acting as a fomite capable of transferring virus particles from one beak to the next. However, the time between application of the bits and the first observed lesion was 2 to 3 months, thus not supporting this mechanism of spread. Mode of entry of the virus into the flock also remains unclear, but the presence of the beak bits within the nares appears to have facilitated extension of the cutaneous lesions into the oropharynx and beak by mechanical abrasion of the nasal mucosa. Beak necrosis, and in some cases complete loss of the upper beak, followed deep extension of the pox lesion. Pox infection was associated with upper and lower beak necrosis in the context of a large outbreak in a commercial layer farm (Moayyedian et al., Citation2008). The lesions in that report involved the entire beak, with beak loss following circumferential development of cutaneous lesions. In this report only the upper beaks were affected and the lesions were centred on the nares and the bit implants. Histopathologic examination of the beak lesions showed a wave of hyperplastic and atypical epithelium replacing normal beak epithelium, as well as extension through nasal chonchae. Thus avascular necrosis following loss of structural integrity of the beak was considered a possible pathogenesis, with bacterial infection and inflammation acting as contributing factors (Cheng et al., Citation1976). This case highlights the importance of dermal abrasion in the development of poxvirus lesions, and the potential of external devices such as beak-bits that are seated in the nares to cause such abrasions, and facilitate deep mucosal infection. Early observation of the development of poxvirus lesions and the removal of beak bits during an outbreak may reduce the severity of lesions and prevent beak necrosis.
References
- Buenestado , F. , Gortá Zar , C. , Millá , J. , Hö Fle , U. and Villafuerte , R. 2004 . Descriptive study of an avian pox outbreak in wild red-legged partridges (Alectoris rufa) in Spain . Epidemiology and Infection , 132 : 369 – 374 .
- Cheng , K.J. , Gardiner , E.E. and Costerton , J.W. 1976 . Bacteria associated with beak necrosis in broiler breeder hens . The Veterinary Record , 99 : 503 – 505 .
- Fallavena , L.C.B. , Rodrigues , N.C. , Scheufler , W. , Martins , N.R.S. , Braga , A.C. , Salle , C.T.P. and Moraes , H.L.S. 1993 . Atypical fowlpox in broiler chickens in Southern Brazil . The Veterinary Record , 132 : 635
- Gortá Zar , C. , Millá , N.J. , Hö Fle , U. , Buenestado , F. , Villafuerte , R. and Kaleta , E.F. 2002 . Pathology of avian pox in wild red-legged partridges (Alectoris rufa) in Spain . Annals of the New York Academy of Sciences , 969 : 354 – 357 .
- Kim , T.-J. , Schnitzlein , W.M. , McAloose , D. , Pessier , A.P. and Tripathy , D.N. 2003 . Characterization of an avianpox virus isolated from an Andean Condor (Vulture gryphus) . Veterinary Microbiology , 96 : 237 – 246 .
- Lee , L.H. and Lee , K.H. 1997 . Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection . Journal of Virological Methods , 63 : 113 – 119 .
- Moayyedian , H. , Mirmohammad-Sadeghi , A. and Hasanshahi , R. 2008 . Clinical and histopathological survey of lesions similar to pox skin lesions in three flocks of a large commercial layer farm . Journal of Applied Poultry Research , 17 : 556 – 558 .
- Savory , C.J. and Hetherington , J.D. 1997 . Effects of plastic anti-pecking devices on food intake and behaviour of laying hens fed on pellets or mash . British Poultry Science , 38 : 125 – 131 .
- Tripathy , D.N. and Reed , W.M. 2008 . “ Pox ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 291 – 308 . Ames , IA : ISU Press .