Abstract
In consequence of the withdrawal of products that assisted animal production, such as antimicrobial growth promoters, once-controlled enteric diseases have returned and new multifactorial diseases causing gut disorders of unknown origin have emerged in broilers. One of these widespread syndromes causing intestinal health problems in broilers is in the field referred to as “dysbacteriosis”. During the present study, the histopathology of the intestinal tract of broilers affected with dysbacteriosis was analysed. Commercial broilers were given a macroscopic dysbacteriosis score by experienced veterinarians during necropsy. Samples from the duodenum and caecum were taken from each broiler for histopathological analysis. An increase in the macroscopic dysbacteriosis score coincided with increased villus atrophy, a decrease in the thickness of the tunica muscularis and an increase in T-lymphocyte infiltration in the gut mucosa. Also more and larger goblet cells were observed in the animals with high macroscopic dysbacteriosis scores. Although the exact aetiology still remains to be identified, dysbacteriosis in broiler chickens thus coincides with an inflammatory reaction in the gut mucosa.
Introduction
Since the ban of antimicrobial growth promoters in Europe, the broiler industry has been facing a rise in intestinal health problems, collectively referred to as “dysbacteriosis” among practitioners (Eshuis et al., Citation1998; Fabri, Citation2000; Rebel et al., Citation2006; De Gussem, Citation2007). Dysbacteriosis has been defined as the presence of a qualitatively and/or quantitatively abnormal microbiota in the proximal parts of the small intestine, inducing a cascade of reactions in the gastro-intestinal tract including reduced nutrient digestibility and impaired intestinal barrier function, increasing the risk of bacterial translocation and inflammatory responses (Fabri, Citation2000; Panneman, Citation2000; van der Klis & Lensing, Citation2007).
The syndrome is generally seen between 20 and 30 days of age (Fabri, Citation2000; Pattison, Citation2002; Wilson et al., Citation2005). Clinically, the main Signs are: pale, glistening or orange droppings with undigested food particles, wet and greasy droppings and hence dirty feathers, sometimes foamy caecal droppings, reduced physical activity, increased water intake, a decrease in feed intake with a check in weight or reduced gain rates and an increased feed conversion (Fabri, Citation2000; Pattison, Citation2002; Wilson et al., Citation2005; De Gussem, Citation2007). At necropsy, the main observations are thin, fragile, often translucent intestinal walls, watery or foamy intestinal contents and frequent orange mucus and undigested particles in the intestines, ballooning of the gut and intestinal inflammation (Pattison, Citation2002; De Gussem, Citation2007).
It is believed that both non-infectious and infectious factors can play a role in dysbacteriosis (Mortimer, Citation2002; De Gussem, Citation2007). Suspected non-infectious factors are different types of non-specific stressors, such as feed interruptions or dietary changes, nutritional imbalance, dietary stressors such as soluble ‘non-starch polysaccharides’, management disorders, genetic background, enzymatic dysfunction, and mycotoxins (Langhout et al., Citation1999; De Gussem, Citation2007; Teirlynck et al., Citation2009). Infectious agents that potentially play a role in dysbacteriosis are coccidia, Clostridium perfringens and other unidentified bacteria producing toxic metabolites (Morrow, Citation2001; De Gussem, Citation2007).
Despite the widespread nature and importance of the syndrome, there is a lack of scientific literature and still much controversy about the exact aetiology—and up until today the basis of the underlying pathophysiology is unknown. Hence, diagnosis in broilers faces a lot of challenges due to the incomplete characterization of the syndrome (Wilson et al., Citation2005).
The purpose of the present study was to gain insight into the histopathological changes at the level of the intestinal mucosa in field cases of dysbacteriosis.
Materials and Methods
Animals and sampling
Male Ross 308 broiler chickens were used in the study: 12 birds 18 days old (two flocks, one broiler farm), 38 birds 21 days old (four flocks, two broiler farms), 36 birds 28 days old (two flocks, one broiler farm) and 18 birds 42 days old (one flock, one broiler farm). In total, broilers of nine flocks from five different broiler farms were sampled. The flocks were selected adt random from an integrated poultry enterprise, thus all the birds originated from the same breeder flocks and were given similar feed including anticoccidials. The birds were receiving nicarbazin/narasin in the starter phase (day 1 until day 18) and salinomycin in the grower phase until 30 days of age at registered doses. This enterprise was reported by the field veterinarian to have above-average intestinal health problems, including dysbacteriosis, coccidiosis (mainly Eimeria maxima) and wet litter, although the (ventilation) management was evaluated as better than average. The birds that were selected were not clinically ill, and were considered as average, healthy birds by an experienced poultry veterinarian.
The chickens were euthanized by means of cervical dislocation by an experienced veterinarian and immediately necropsied. Intestinal parameters were scored (see below). Samples of approximately 3 cm were taken from the second limb of the duodenum and the middle part of one caecum, rinsed in phosphate-buffered saline and fixed in 4% (v:v) buffered formalin.
Macroscopic scoring system
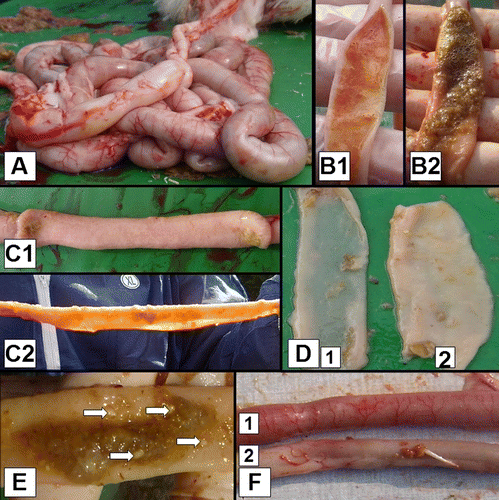
Each bird was given a score between 0 and 10 for intestinal dysbacteriosis parameters, 0 being a normal gastrointestinal tract and 10 being the most severe dysbacteriosis. In total 10 parameters were assessed and scored 0 when absent and 1 when present (). The parameters are: (1) ballooning of the gut; (2) significant redness of the serosal and/or mucosal side of the gut and/or presence of abnormally dilated blood vessels on the serosal side of the gut, cranial to Meckel's diverticulum; (3) a macroscopic visible and/or tangible reduction of the gut wall thickness and/or translucent guts in combination with increased fragility of the gut cranial to Meckel's diverticulum; (4) 3 sec after dissecting the gut, the edges of the gut cranial to Meckel's diverticulum are flaccid; (5) an abnormal appearance of the contents in the lumen of the gut (excessive slime, water, gas, greasy aspect or mixture of these) cranial to Meckel's diverticulum; (6) significant redness of the serosal and/or mucosal side of the gut and/or presence of abnormally dilated blood vessels on the serosal side of the gut, caudal to Meckel's diverticulum; (7) a macroscopic visible and/or tangible reduction of the gut wall thickness and/or translucent guts in combination with increased fragility of the gut caudal from Meckel's diverticulum; (8) 3 sec after dissecting the gut, the edges of the gut caudal to Meckel's diverticulum are flaccid; (9) abnormal appearance of the contents in the lumen of the gut (excessive slime, water, gas, greasy aspect or mixture of these) caudal to Meckel's diverticulum; and (10) undigested food particles caudal to ileo-caecal junction (De Gussem, Citation2010). During this lesion scoring, no intestinal sections were given scores of 0, 9 or 10.
Figure 1. Macroscopic dysbacteriosis score system parameters. 1A: Overall gut ballooning. 1B: Content of the intestinal tract: (B1) mucoid, orange intestinal content; and (B2) foamy intestinal content. 1C: Tonus of the intestinal tract: (C1) good tonus; and (C2) lack of tonus. 1D: Macroscopically visible thickness of the intestinal tract: (D1) macroscopically thin intestinal tract; and (D2) intestinal tract with normal thickness. 1E: Undigested particles in the colon (arrows). 1F: Inflammation of the gut: (F1) inflammation; and (F2) no inflammation.
Morphological examination
Formalin-fixed intestinal segments were dehydrated in xylene, and embedded in paraffin. Sections of 4 µm were cut using a microtome (Microm; Prosan, Merelbeke, Belgium). Deparaffinization was done in xylene (2×5 min). The sections were then rehydrated in isopropylene (5 min), 95% alcohol (5 min) and 50% alcohol (5 min) and were stained with haematoxylin and eosin. Histological lesions were studied using standard light microscopy. Villus length in the duodenum was measured by random measurement of nine villi per section using an Olympus BX61 Digital Camera DP50 (Olympus NV, Aartselaar, Belgium) and a computer-based image analysis system (Analysis® J-2; P4 Technologies, Inc., Waldorf, Maryland, USA). Only intact villi were measured, meaning villi for which the tip as well as the base of the villus were in the plane of the section. The thickness of the tunica muscularis in duodenum and caeca was also measured using the Analysis® J-2 software. For each section, eight measurements were performed on different locations. Measurements were done on cross-sections of ring-shaped intestinal segments that allow unbiased perpendicular measurements. Detection of goblet cells was done using periodic acid Schiff staining as described by Forder et al. (Citation2007)
Immunohistochemical examination
Deparaffinization of paraffin-embedded tissue sections (4 µm) was performed as described above. The pressure cooker antigen retrieval method (Tender Cooker, Nordic Ware, Minneapolis, USA) was applied to the samples. Immunohistochemical labelling of leukocytes was performed as described by Mast et al. (Citation1998). Briefly, endogenous peroxidase in the tissue sections was blocked with H2O2 (3%) in methanol for 30 min (21°C). After rinsing with phosphate-buffered saline sections were incubated for 1 h (21°C) with monoclonal antibodies directed against T lymphocytes (KUL05). After rinsing thoroughly, a goat anti-mouse IgG1 conjugate, labelled with peroxidase (Dako, Heverlee, Belgium), was added for 30 min (21°C). After rinsing again, tissue sections were incubated with ABC horseradish peroxidase complex (Dako, Heverlee, Belgium) for 30 min (21°C). After another rinse, positive cells were stained brown after conversion of the substrate (3,3′-diaminobenzidine tetrahydrochloride; Sigma, St Louis, Missouri, USA) in the presence of H2O2. The number of T lymphocytes in the propria mucosae was scored with an automatic image analysis system (Optimas 6.5; Media Cybernetics, Silver Spring, USA), measuring the area percentage occupied by the labelled cells. For each section, eight randomly selected sites were analysed by the image analysis program.
Statistical analysis
The relation between the macroscopic lesion score and the outcome variables villus length, thickness of the tunica muscularis and T-lymphocyte infiltration was evaluated by means of a linear mixed model taking into account the chicken as a random factor since multiple observations were made on one animal. In addition, other co-variables such as age of the bird, the person scoring the bird and the intestinal segment were also taken into account. First, all independent variables were tested univariably, and subsequently all significant variables were included in a multivariable model. In this multivariable model, all two-way interactions were also tested. In the multivariable model, all main effects and interactions with P<0.05 were retained. For the outcome variables that were not normally distributed, a log transformation was performed. Model fit was evaluated by means of evaluation of the residuals. The number of chickens belonging to a certain macroscopic dysbacteriosis score group per age group is presented in .
Table 1. Number of chickens analysed per age per macroscopic dysbacteriosis group.
Results
Intestinal morphology
Villus length
Duodenal villus length generally decreased with an increase in the macroscopic “dysbacteriosis” score within one age group (). Statistical analysis showed that, starting from macroscopic dysbacteriosis score 5, the villus length was significantly lower compared with macroscopic dysbacteriosis score 1 (P=0.01). Score 8 differed significantly from scores 5, 6 and 7 (P=0.03).
Table 2. Length of villi (µm) in duodenal sections in relation to the macroscopic score.
Cystic crypts
In the duodenum, a mild to severe dilatation of the crypts of Lieberkühn was observed. Although not quantitatively analysed, the number of cystic crypts appeared to increase with age and with the macroscopic dysbacteriosis score. The age effect seemed to have the highest impact.
Thickness of the tunica muscularis
For each age interval, the thickness of the tunica muscularis generally decreased with an increasing macroscopic dysbacteriosis score (). Statistical analysis showed that the thickness of the tunica muscularis of the gut of the animals having macroscopic dysbacteriosis score 5 and higher was significantly lower as compared with score 1 (P=0.01).
Table 3. Thickness of the tunica muscularis (µm) in duodenal and caecal sections in relation to the macroscopic score.
Goblet cells
Although not quantitatively analysed, periodic acid Schiff staining generally showed more and larger goblet cells both on the villi and in the crypts of the gut of animals with higher macroscopic dysbacteriosis scores.
T-lymphocyte infiltration
Generally T-lymphocyte infiltration in the duodenum and caecum increased within one age group, with increased macroscopic dysbacteriosis scores (). Statistical analysis showed that T-lymphocyte infiltration in the gut mucosa of animals having a macroscopic dysbacteriosis score of 6 and higher was significantly higher as compared with score 1 (P=0.02).
Table 4. T-lymphocyte infiltration (area percentage) for duodenal and caecal sections in relation to the macroscopic score.
Discussion
Definitive diagnosis of dysbacteriosis has been challenging due to the non-specific nature of the clinical signs and lesions and because the aetiology is still unknown (Wilson et al., Citation2005). The present study has identified several more or less characteristic changes, which may aid in confirming the diagnosis of “dysbacteriosis”, and which help in differentiating this entity from other intestinal disorders, such as malabsorption syndrome (MAS) and runting–stunting syndrome (RSS).
MAS has been described as a gastro-intestinal disease affecting broilers during the first 2 weeks post-hatch (Zekarias et al., Citation2002; van Hemert et al., Citation2004). The main clinical signs of MAS are weight gain depression with non-uniform growth, stunting, diarrhoea with undigested food particles resulting in wet litter, retarded and defective feathering, pigment loss, distended abdomens, depression and early mortality. At necropsy, lesions are found in the digestive organs, especially the small intestine, which is grossly pale and distended, with mucoid contents, and there is lower mineralization of the thigh bones (Kouwenhoven et al., Citation1978a, Citationb; Bracewell & Randall, Citation1984; Reece et al., Citation1984; Szabo et al., Citation1989; Reece & Frazier, Citation1990; McNulty & McFerran, Citation1993; Sályi & Glávitis, Citation1999; Songserm et al., Citation2002a, Citationb; Van Hemert et al., Citation2004; Rebel et al., Citation2006). Histologically, intestinal lesions such as cystic crypts of Lieberkühn and villus atrophy are observed (Rebel et al., Citation2004).
RSS typically affects birds within the first 2 weeks of life and can be defined as a syndrome in which a number of individuals in a flock appear considerably small due to delayed growth (Dufour-Zavala, Citation2005; Nili et al., Citation2007). Clinically, the main signs of RSS are immobility, increased feed conversion and poor performance, uneven growth and stunting, inappetance and excessive water consumption, diarrhoea, retarded and defective feathering, distended abdomens and bone defects (Vertommen et al., Citation1980a, Citationb; Ruff, Citation1982; Calnek et al., Citation1997; Shapiro et al., Citation1997, Citation1998; Sályi and Glávits, Citation1999; Dufour-Zavala, Citation2005; Nili et al., Citation2007). At necropsy, lesions are found in the small intestine, which is pale and thin, almost translucent, containing undigested food particles.
A main difference between dysbacteriosis and MAS/RSS, of which the latter could be identical entities with different names, is that in field cases of dysbacteriosis the growth retardation due to poorer absorption of nutrients is probable but very often average growth will still be in line with breed standards, and also the homogeneity of the flock will typically not be dramatically affected as with RSS/MAS. Also the age of disease induction is lower (below 2 weeks of age) in MAS/RSS as compared with dysbacteriosis (3 to 4 weeks of age). Dysbacteriosis and MAS/RSS are clearly distinct from subclinical necrotic enteritis because no ulcerations in the gut are found.
In the dysbacteriosis field cases, the duodenal villus length, thickness of the tunica muscularis and T-lymphocyte infiltration in the mucosa of the duodenum and caecum were altered. No necrotic lesions were observed. This combination of morphological and inflammatory changes combined with the different clinical appearance compared with other syndromes (such as MAS/RSS) is in our opinion sufficiently unique and characteristic to classify this syndrome as a separate entity, although microscopic measurements of MAS/RSS samples are necessary for confirmation. The microscopic findings of dysbacteriosis may explain the clinical signs, being depressed growth, wet litter and clinical depression (Toskes et al., Citation1975; Kaldhusdal & Hofshagen, Citation1992; Hoerr, Citation2001). Based on the measurements, variations in intestinal distension between healthy gut and severe dysbacteriosis can explain the variations in thickness of the tunica muscularis. Thus ballooning of the gut and the flaccid aspect of the gut wall may be directly associated with reduced tonus/rigour of the tunica muscularis. Variations in intestinal distension, however, can only partly explain the reduction in villus length and increase in villus thickness as observed in the severe dysbacteriosis cases. Thus severe dysbacteriosis is associated with an absolute reduction in the available absorptive surface area. The change in the size and number of goblet cells could explain the mucous content of the intestinal tract of affected birds. Cystic crypt formation could be due to a vitamin deficiency as a secondary effect of the malabsorption caused by villus atrophy and increased T-lymphocyte infiltration (Klasing & Austic, Citation2003).
Dysbacteriosis is defined in human medicine as a condition characterized by a shift in the microbiota favouring abnormal populations of bacteria to predominate within the intestinal tract with minimal intestinal pathology (Dumitrasco et al., Citation1980; Schoorel et al., Citation1980; Sidorchuk & Bondarenko, Citation1984; Klemparskaya et al., Citation1987). Despite the definition of dysbacteriosis, the microbiota composition and possible microbiota shifts have not been studied and the name dysbacteriosis is not really substantiated. However, there are indications suggesting that the intestinal microbiota composition may play a role in this syndrome. These indications include the response to certain antibiotic treatments, the consistency of the droppings and the inflammatory nature of the intestinal lesions.
In conclusion, dysbacteriosis in broilers is characterized by villus atrophy, a decrease in the thickness of the tunica muscularis and an increase in T-lymphocyte infiltration in the gut mucosa. These histological observations can explain performance problems and macroscopic observations of the gut at necropsy, in broilers with dysbacteriosis.
Acknowledgements
The authors would like to thank Sarah Loomans, Christian Puttevils and Delphine Ameye for their skilful technical assistance. Dieter Vancraeynest and Maja Mariën (Alpharma) are acknowledged for their help with macroscopic lesion scoring. The present work was funded by grant S6169 of the Federal Public Service Health, Food Chain Safety and Environment.
References
- Bracewell , C.D. and Randall , C.J. 1984 . The infectious stunting syndrome . World's Poultry Science Journal , 40 : 31 – 37 .
- Calnek , B.G. , Barnes , H.J. , Beard , C.W. , McDougald , L.R. & Saif , Y.M. 1997 . Diseases of Poultry , 10th edn Ames : Iowa State Press .
- De Gussem , M. 2007 . Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health . In Proceedings of the XVI European Symposium on Poultry Nutrition (pp. 160 169 . Strasbourg , France .
- De Gussem , M. 2010 . Macroscopic scoring system for bacterial enteritis in broiler chickens and turkeys In WVPA Meeting . Merelbeke Belgium
- Dufour-Zavala , L. 2005 . Cystic enteritis: reproduction of the disease and attempted control measures . In Proceedings of the 40th National Meeting on Poultry Health and Processing (pp. 20 21 . Ocean City , Maryland, , USA .
- Dumitrasco , D. , Grigoresco , M. , Parau , N. , Suciu , A. and Erdosy , S. 1980 . Dysbacteriosis in enteropathies . Medicine Interne , 18 : 239 – 245 .
- Eshuis , J.W. , Van Dobbenburgh , O.A. , Delhaes , L.M. , Van Der Meij , D. & Nijland , J.T. 1998 . Bacterie-regelaar Tylan helpt tegen dysbacteriose . Pluimveehouderij , 13 .
- Fabri T.H.F. 2000 . Necrotic enteritis, Clostridial enteritis or dysbacteriosis? In Proceedings of the Elanco Symposium . Cork, Ireland .
- Forder , R.E. , Howarthe , G.S. , Tivey , D.R. and Hughes , R.J. 2007 . Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry . Poultry Science , 86 : 2396 – 2403 .
- Hoerr , F.J. 2001 . Intestinal integrity and the impact of losing it . World Poultry–Elsevier , 17 : 16 – 18 .
- Kaldhusdal , M. and Hofshagen , M. 1992 . Barley inclusion and Avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis . Poultry Science , 71 : 1145 – 1153 .
- Klasing , K. and Austic , R.E. 2003 . “ Nutritional diseases ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 1027 – 1053 . Ames : Iowa State Press .
- Klemparskaya , N.N. , Pinegin , B.V. , Shal'nova , G.A. , Korshunov , V.M. , Maltsev , V.N. Glad'ko , I.A. 1987 . Normalizing effect of immunoglobulins in the treatment of endogenous infection and intestinal dysbacteriosis in irradiated mice . Journal of Hygiene, Epidemiology, Microbiology and Immunology , 31 : 91 – 98 .
- Kouwenhoven , B. , Vertommen , M. and Vaneck , J.H.H. 1978a . Runting and leg weakness in broilers—involvement of infectious factors . Veterinary Science Communications , 2 : 253 – 259 .
- Kouwenhoven , B. , Davelaar , F.G. and Vanwalsum , J. 1978b . Infectious proventriculitis causing runting in broilers . Avian Pathology , 7 : 183 – 187 .
- Langhout , D.J. , Schutte , J.B. , Van Leeuwen , P. , Wiebenga , J. and Tamminga , S. 1999 . Effect of dietary high- and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks . British Poultry Science , 40 : 340 – 347 .
- Mast , J. , Goddeeris , B.M. , Peeters , K. , Vandesande , F. and Berghman , L.R. 1998 . Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01 . Veterinary Immunology and Immunopathology , 61 : 343 – 357 .
- McNulty , M.S. and McFerran , J.B. 1993 . “ The runting stunting syndrome general assessment ” . In Virus Infections of Birds , Edited by: McFerran , J.B. and McNulty , M.S. 519 – 528 . Amsterdam : Elsevier .
- Morrow , C. 2001 . Solving the problems of necrotic enteritis . British Poultry Science , 42 : 64 – 68 .
- Mortimer , I. 2002 . The detection of dysbacteriosis . In Proceedings of the Elanco Global Enteritis Symposium . Cambridge UK
- Nili , H. , Jahantigh , M. and Nazifi , S. 2007 . Clinical observation, pathology, and serum biochemical changes in infectious stunting syndrome of broiler chickens . Comparative Clinical Pathology , 16 : 161 – 167 .
- Panneman , H. 2000 . Clostridial enteritis/dysbacteriosis, fast diagnosis by T-RFLP, a novel diagnostic tool . In Proceedings of the Elanco Global Enteritis Symposium . Cork Ireland .
- Pattison , M. 2002 . Some clinical and pathological features of enteritis in broilers—observations on treatment in the UK . In Proceedings of the Elanco Global Enteritis Symposium (pp. 1 7 . Cambridge, , UK .
- Rebel , J.M.J. , van Dam , J.T.P. , Zekarias , B. , Balk , F.R.M. , Post , J. , Minambres , A.F. and ter Huurne , A.A.H.M. 2004 . Vitamin and trace mineral content in feed of breeders and their progeny: effects of growth, feed conversion and severity of malabsorption syndrome of broilers . British Poultry Science , 45 : 201 – 209 .
- Rebel , J.M.J. , Balk , F.R.M. , Post , J. , Van Hemert , S. , Zekarias , B. and Stockhofe , N. 2006 . Malabsorption syndrome in broilers . World's Poultry Science Journal , 62 : 17 – 29 .
- Reece , R.L. and Frazier , J.A. 1990 . Infectious stunting syndrome of chickens in Great Britain—field and experimental studies . Avian Pathology , 19 : 723 – 758 .
- Reece , R.L. , Hooper , P.T. , Tate , S.H. , Beddome , V.D. , Forsyth , W.M. , Scott , P.C. and Barr , D.A. 1984 . Field, clinical and pathological observations of a runting and stunting syndrome in broilers . Veterinary Record , 115 : 483 – 485 .
- Ruff , M.D. 1982 . Nutrient absorption and changes in blood-plasma of stunted broilers . Avian Diseases , 26 : 252 – 259 .
- Sályi , G. and Glávits , R. 1999 . Infectious stunting syndrome associated with disturbances of mineral metabolism and bone development in broiler chickens . Acta Veterinaria Hungarica , 47 : 361 – 378 .
- Schoorel , E.P. , Giesberts , M.A. , Blom , W. and Van Gelderen , H.H. 1980 . D-Lactic acidosis in a boy with short bowel syndrome . Archives of Disease in Childhood , 55 : 810 – 812 .
- Shapiro , F. , Mahagna , M. and Nir , I. 1997 . Stunting syndrome in broilers: effect of glucose or maltose supplementation on digestive organs, intestinal disaccharidases, and some blood metabolites . Poultry Science , 76 : 369 – 380 .
- Shapiro , F. , Nir , I. and Heller , D. 1998 . Stunting syndrome in broilers: effect of stunting syndrome inoculum obtained from stunting syndrome affected broilers, on broilers, leghorns and turkey poults . Poultry Science , 77 : 230 – 236 .
- Sidorchuk , I.I. and Bondarenko , V.M. 1984 . Selection of a biologically active mutant of Propionibacterium shermanii and the possibility of its use in complex therapy of enteral dysbacteriosis . Journal of Hygiene, Epidemiology, Microbiology and Immunology , 28 : 331 – 338 .
- Songserm , T. , Engel , B. , van Roozelaar , D.J. , Kok , G.L. , Pijpers , A. , Pol , J.M.A. and ter Huurne , A.A.H.M. 2002a . Cellular immune response in the small intestine of two broiler chicken lines orally inoculated with malabsorption syndrome homogenates . Veterinary Immunology and Immunopathology , 85 : 51 – 62 .
- Songserm , T. , Zekarias , B. , van Roozelaar , D.J. , Kok , R.S. , Pol , J.M.A. , Pijpers , A. and ter Huurne , A.A.H.M. 2002b . Experimental reproduction of malabsorption syndrome with different combinations of reovirus, Escherichia coli, and treated homogenates obtained from broilers . Avian Diseases , 46 : 87 – 94 .
- Szabo , J. , Salyi , G. and Rudas , P. 1989 . Effect of malabsorption-syndrome on pancreatic function in broilers . Poultry Science , 68 : 1553 – 1560 .
- Teirlynck , E. , Bjerrum , L. , Eeckhaut , V. , Huygebaert , G. , Pasmans , F. Haesebrouck , F. 2009 . The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens . British Journal of Nutrition , 102 : 1453 – 1461 .
- Toskes , P.P. , Giannella , R.A. , Jervis , H.R. , Rout , W.R. and Takeuchi , A. 1975 . Small intestinal mucosal injury in the experimental blind loop syndrome; light and electron-microscopic and histochemical studies . Gastroenterology , 68 : 1193 – 1203 .
- van der Klis , J.D. and Lensing , M. 2007 . Wet litter problems relate to host–microbiota interactions . World Poultry , 23 : 20 – 22 .
- Van Hemert , S. , Hoekman , A.J. , Smits , M.A. and Rebel , J.M.J. 2004 . Differences in intestinal gene expression profiles in broiler lines varying in susceptibility to malabsorption syndrome . Poultry Science , 83 : 1675 – 1682 .
- Vertommen , M. , Vaneck , J.H.H. , Kouwenhoven , B. and Vankol , N. 1980a . Infectious stunting and leg weakness in broilers. Pathology and biochemical-changes in blood-plasma . Avian Pathology , 9 : 133 – 142 .
- Vertommen , M. , Van-Der-Laan , A. and Veenendaa-Lhesselman , H.M. 1980b . Infectious stunting and leg weakness in broilers. II. Studies on alkaline phosphatase iso enzymes in blood plasma . Avian Pathology , 9 : 143 – 152 .
- Wilson , J. , Tice , G. , Brash , M.L. and St Hilaire , S. 2005 . Manifestations of Clostridium perfringens and related bacterial enteritides in broiler chickens . Worlds Poultry Science Journal , 61 : 435 – 449 .
- Zekarias , B. , Songserm , T. , Post , J. , Kok , G.L. , Pol , J.M.A. , Engel , B. and ter Huurne , A.A.H.M. 2002 . Development of organs and intestinal mucosa leukocytes in four broiler lines that differ in susceptibility to malabsorption syndrome . Poultry Science , 81 : 1283 – 1288 .