Abstract
In vitro antimicrobial susceptibility to tylosin, valnemulin, tiamulin, doxycycline, lincomycin and ampicillin was investigated by broth dilution in 48 Brachyspira spp. isolates from commercial laying hens (n=30) and free-living wild mallards (Anas platyrhynchos) (n=18). Presumed pathogens (Brachyspira alvinipulli, Brachyspira intermedia, Brachyspira pilosicoli), commensals (Brachyspira murdochii, Brachyspira innocens, “Brachyspira pulli”), and isolates of undetermined species affiliation were included. The laying hens had not been exposed to therapeutic levels of antimicrobials for at least 50 weeks before sampling, and low levels of environmental antimicrobial exposure were presumed in mallards. No isolates with decreased susceptibility to tylosin, valnemulin, tiamulin or doxycycline were found. Decreased susceptibility to lincomycin (minimum inhibitory concentration 16 µg/ml) was detected in two isolates (Brachyspira sp.) from laying hens. Five isolates showed decreased susceptibility to ampicillin (minimum inhibitory concentration 16 to >32 µg/ml), including two “B. pulli” and one B. alvinipulli from laying hens, and isolates of B. pilosicoli and “B. pulli” from mallards. Decreased susceptibility to ampicillin was associated with β-lactamase activity in four isolates. A new variant of a class D β-lactamase gene designated bla oxa-192 was identified in a B. pilosicoli isolate of mallard origin. This is the first time the genetic basis for antimicrobial resistance is described in Brachyspira spp. from a free-living wild bird. Isolates displaying decreased susceptibility to ampicillin were accompanied by fully susceptible isolates of the same species or other genotypes within three laying hen flocks. This underlines the need for performing antimicrobial susceptibility tests on single clones/genotypes, and to analyse multiple isolates from the same flock.
Introduction
Colonization with intestinal spirochaetes of genus Brachyspira commonly occurs in mammals (e.g. pigs, dogs, rodents and humans) and in domestic and free-living wild birds, especially gallinaceous and anseriform species. In chickens, a wide range of potentially pathogenic and commensal species may be found, including Brachyspira alvinipulli, Brachyspira intermedia and Brachyspira pilosicoli that have been linked to diarrhoea, wet litter, faecal staining of egg shells and impaired laying performance in adult chickens (Stanton et al., Citation1998; Hampson & McLaren, Citation1999; Stephens & Hampson, Citation2002). Among the remaining recognized and proposed species found in chickens, Brachyspira murdochii, Brachyspira innocens and “Brachyspira pulli” are presumed non-pathogenic species, and the swine dysentery agent Brachyspira hyodysenteriae, although an unusual finding in chickens (Feberwee et al., Citation2008), has not yet been linked to disease. Free-living wild mallards host a wide range of Brachyspira spp. including known pathogens of livestock—that is, B. hyodysenteriae (Jansson et al., Citation2004), “Brachyspira suanatina” (Råsbäck et al., Citation2007), B. pilosicoli, B. intermedia and B. alvinipulli, the presumed commensal “B. pulli”, and isolates that cannot presently be identified to species level (Jansson et al., 2011, unpublished observations). It is not known whether colonization of mallards is associated with enteric disease.
Antimicrobial agents, especially pleuromutilins, macrolides and lincosamides, are widely used to control swine dysentery in pig herds. In B. hyodysenteriae of porcine origin, widespread resistance has emerged to tylosin in the USA, Denmark, Australia, Finland, Belgium, Sweden and Spain (Kinyon & Harris, Citation1980; Rønne & Szancer, Citation1990; Smith et al., Citation1991; Hommez et al., Citation1998; Honkanen-Buzalski & Huovinen, Citation1999; Bengtsson et al., Citation2010; Hidalgo et al., Citation2010). Tiamulin resistance has been described in recent years from Hungary, the UK, the Czech Republic, Germany, the Netherlands, Spain and Japan (Molnár, Citation1996; Gresham et al., Citation1998; Lobova et al., Citation2004; Rohde et al., Citation2004; Duinhof et al., Citation2008; Hidalgo et al., Citation2010; Ohya & Sueyoshi, Citation2010). Compared with the situation in pigs, there is little information on antimicrobial susceptibility in Brachyspira spp. of avian origin. Attempts to treat chicken flocks colonized by intestinal spirochaetes have met with limitations mostly because of lack of approved products and application of long withdrawal times for eggs. Moreover, chickens are likely to get re-infected from environmental sources, especially when housed on litter, which may lead to recurrence of clinical signs (Smit et al., Citation1998; Stephens & Hampson, Citation1999). More recently, Burch et al. (Citation2006) reported improved egg production and reduced mortality in chickens colonized by B. pilosicoli that were treated with tiamulin.
The objective of the present study was to investigate antimicrobial susceptibility of Brachyspira spp. isolates from laying hens with a history of low antimicrobial use and, for comparison, isolates from free-living wild mallards were included.
Materials and Methods
Spirochaete isolates
All analysed isolates () originated from commercial Swedish laying hens (n=30) or free-living wild mallards (Anas platyrhynchos) (n=18) that had been sampled in Sweden. The isolates have been investigated by phenotypic and molecular tests, and identified to species level when possible (Jansson et al., 2011, unpublished results). Information on sampling is available elsewhere (Jansson et al., Citation2004). The laying hens were approximately 65 weeks of age at sampling, and they had not been exposed to therapeutic levels of antimicrobials during the production period (i.e. approximately 50 weeks prior to sampling). No information was available on antimicrobial use during the rearing period. The environmental exposure to antimicrobials of the sampled mallards was assumed to be low. Following selective culture, the isolates had been subcultured once, and if necessary subcultured to pure genotypes by 10-fold serial dilution as described elsewhere (Jansson et al., Citation2008). The isolates were stored in liquid nitrogen (Fellström & Gunnarsson, Citation1995) in the strain collection of the National Veterinary Institute (SVA), Uppsala, Sweden until further use.
Table 1. In vitro activities (MICs) of six antimicrobial agents for isolates of Brachyspira spp. of avian origin tested by broth dilution.
Antimicrobial susceptibility tests
Thawed isolates were grown on fastidious anaerobe agar supplemented with 10% equine blood (FAA; SVA) and subcultured twice. The purity of all isolates was assessed by phase contrast microscopy. The minimum inhibitory concentrations (MICs) of tiamulin, valnemulin, doxycycline, lincomycin, tylosin, and ampicillin were determined by broth dilution in VetMIC™ Brachy panels (SVA) as described previously (Karlsson et al. Citation2003). The medium for the susceptibility tests was brain heart infusion broth (Difco, BD, Sparks, Maryland, USA) supplemented with 10% foetal calf serum (SVA). The MIC was read as the lowest concentration of the antimicrobial agent that prevented visible growth. Strains B78T (B. hyodysenteriae, ATCC 27164) and Wichita (Staphylococcus aureus subsp. aureus, ATCC 29213) were used as controls.
β-Lactamase detection
Nitrocefin disks (AB Biodisk, Solna, Sweden) were used to determine β-lactamase activity of isolates AN3382/1/03, AN304/04, AN1268/3/04, AN3635/3/02 and AN6052:2/1/00. The disks were moistened with phosphate-buffered saline and two 1 µl loops of bacterial cells from 3-day-old cultures from FAA plates were smeared on the disks and incubated for 30 min at room temperature. The β-lactamase activity was detected by observing the colour change from yellow to red after 5 and 30 min. Strain B78T (B. hyodysenteriae, ATCC 27164) was used as negative control and strain Rosenbach 1884 (S. aureus subsp. aureus, CCUG 15915) as positive control.
Amplification and sequencing of the β-lactamase and 23S rRNA genes
Polymerase chain reactions (PCRs) were performed to attempt to detect a β-lactamase gene of isolates AN3382/1/03, AN304/04, AN1268/3/04, AN3635/3/02 and AN6052:2/1/00. PCR amplification of the 23S rRNA gene (870 nucleotides, positions 1864 to 2733 Escherichia coli numbering, accession number J01695) of isolate AN1828/02 was performed to identify possible point mutations that could explain the decreased susceptibility to lincomycin displayed by this isolate. Chromosomal DNA was prepared from 3-day-old cultures on FAA plates as previously described (Råsbäck et al., 2006). Primers used for PCR amplification and sequencing of β-lactamase and 23S rRNA genes are listed in . Primers for the β-lactamase gene were designed based on sequences deposited in the GenBank database, accession numbers AY619003 (B. pilosicoli, strain BM4442; Meziane-Cherif et al., Citation2008), CP002025 (B. pilosicoli, strain 95/1000; Wanchanthuek et al., Citation2010), and AY227054 (Fusobacterium nucleatum subsp. polymorphum, strain N161; Voha et al., Citation2006). PCRs were performed under the following conditions: 3 min at 94°C, 30 cycles with 30 sec denaturation at 94°C, 40 sec annealing at 59°C and extension at 72 for 54 sec, followed by 10 min final extension at 72°C. Amplicons were analysed by electrophoresis in 1.5% agarose gels. Water was used as negative control and B. hyodysenteriae strain B78T (ATCC 27164) was used as positive control for amplification of the 23S rRNA gene. PCR amplicons were purified by the illustra GFX™ PCR DNA and Gel Band Purification Kit (GE Healthcare, Uppsala, Sweden). Sequencing reactions were performed using the BigDye® Terminator v3.1 kit (Applied Biosystems Carlsbad, CA, USA) in an ABI PRISM® 2700 Genetic Analyzer at Uppsala Genome Center, Uppsala University, Uppsala, Sweden (http://www.genpat.uu.se/node462). Sequences were edited and translated using the CLC bio Main Workbench (CLC bio, Åarhus N, Denmark). A neighbour-joining tree of class D β-lactamase genes of B. pilosicoli was constructed from a high-fidelity nucleotide alignment made by the algorithm included in the CLC Sequence Viewer free software, version 6.4 using default parameters.
Table 2. Primers used for PCR and DNA sequencing.
Results
Antimicrobial susceptibility and β-lactamase activity
The MICs of tested antimicrobial agents are presented in . No isolates with decreased susceptibility to tylosin, valnemulin, tiamulin or doxycycline were found. Two isolates of Brachyspira sp. (AN1828/03 and AN1831/03) from the same organic laying hen flock (Flock A, ) showed decreased susceptibility to lincomycin (MIC 16 µg/ml), and five isolates showed decreased susceptibility to ampicillin (MIC 16 to >32 µg/ml). The latter isolates included two “B. pulli” (AN3382/1/03 and AN304/04) and one B. alvinipulli (AN1268/3/04) from three separate laying hen flocks (Flocks C, Q and R, ), and a B. pilosicoli (AN3635/3/02) and a “B. pulli” (AN6052:2/1/00) isolate from free-living wild mallards. Four of these isolates (AN3382/1/03, AN304/04 AN1268/3/04 and AN3635/3/02) and the positive control strain Rosenbach 1884 produced β-lactamase that hydrolysed the chromogenic cephalosporin nitrocefin, in less than 5 min, and the fifth isolate (AN6052:2/1/00) showed a weak colour change after 30 min. For B. hyodysenteriae B78T, no colour change was observed after 30 min. Susceptible isolates as well as isolates with decreased susceptibility to ampicillin were found when several isolates from the same laying hen flock (Flocks C, Q and R, ) were analysed. Based on previously published phenotypic and molecular analyses (Jansson et al., Citation2008), isolates originating from the same chicken flocks represented different species (Flocks C, Q and R, ) or different genotypes of the same species (Flock R). These isolates originated either from a single faecal sample (Flocks C, and R) or from different faecal samples from the same flock (Flocks C, Q and R). Similarly, susceptible Brachyspira isolates (AN3635/2/02 and AN3635/5/02) were found together with an isolate with decreased susceptibility (AN3635/3/02) in a sample from a mallard.
Amplification and sequencing of β-lactamase and 23S rRNA genes
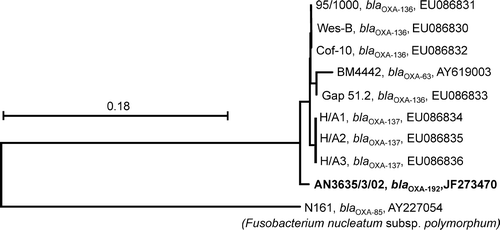
For isolates AN3382/1/03, AN304/04, AN1268/3/04, and AN6052:2/1/00 no amplicons of predicted sizes were detected by the β-lactamase gene PCRs, but for isolate AN3635/3/02 amplicons of predicted sizes were obtained with primer combinations BlaF1/BlaR3, BlaF2/R3, BlaF3/R3 and BlaF3/BlaR4 (). A complete β-lactamase gene sequence was obtained from the sequenced and assembled amplicons of primer combinations BlaF1/BlaR3 and BlaF3/BlaR4, and sequence information is available in the GenBank database (accession number JF273470). Comparison of the predicted amino acid sequence in the GenBank database revealed closest similarity to class D β-lactamase genes from B. pilosicoli human isolate H/A1 (OXA-137 gene, accession number ABW76138) (260/269 amino acid identities, E value 3×10−147), B. pilosicoli porcine strain 95/1000 (OXA-136 gene, accession number YP_003785827, 262/269 amino acid identities, E value 2×10−140), B. pilosicoli human isolate Gap 51.2 (OXA-136 gene, accession number ABW76137, 261/269 amino acid identities, E value 4×10−140), and B. pilosicoli human isolate BM4442 (OXA-63 gene, accession number AAU88145, 248/269 amino acid identities, E value 6×10−130). The new gene variant described in this paper was designated bla oxa-192. A tree showing the genetic relationship of this gene to previously published class D β-lactamase genes of B. pilosicoli and to the gene bla OXA-85 of F. nucleatum subsp. polymorphum, strain N161 is shown in . No mutations were identified within the 23S rRNA gene fragment of isolate AN1828/02, compared with the wild-type sequence of B. hyodysenteriae.
Figure 1. Neighbour-joining tree showing the genetic relationship among class D β-lactamase genes (793 nucleotide positions) of B. pilosicoli strains originating from humans (BM4442, Wes-B, H/A1, H/A2, H/A3 and Gap 51.2), pigs (Cof-10 and 95/1000) and an isolate from the present study (free-living wild mallard, isolate AN3625/3/02, shown in bold). Strain and β-lactamase gene designations and GenBank database accession numbers are shown. The bla OXA-85 class D β-lactamase gene from F. nucleatum subsp. polymorphum strain N161 was used as outgroup. Scale bar represents the distance equivalent to 18 substitutions per 100 nucleotide positions.
Discussion
Reports on antimicrobial susceptibility of Brachyspira spp. isolates from avian hosts are few and have focused on the enteropathogenic species B. alvinipulli, B. intermedia and B. pilosicoli of chickens and B. hyodysenteriae from farmed common rheas (Trampel et al., Citation1999; Hampson et al., 2006b). Trampel et al. (1999) investigated MICs of 11 antimicrobial agents by agar dilution test for four isolates each from chickens (B. pilosicoli, B. alvinipulli) and common rheas (B. hyodysenteriae) of US origin. All isolates were susceptible to lincomycin, carbadox and tiamulin, whereas the MICs of chlortetracycline, oxytetracycline, bacitracin, erythromycin, tylosin, neomycin, penicillin and streptomycin varied between isolates. Notably, the MICs of penicillin ranged from 17.0 to 170 IU/ml in chickens but were higher in isolates from common rheas; 1700 IU/ml. In another study, Hampson et al. (Citation2006b) investigated MICs of tiamulin, lincomycin, tylosin, metronidazole and tetracycline by agar dilution tests for 42 isolates of B. intermedia and B. pilosicoli from chickens, of which the majority were of Australian origin. In total, 22 isolates, represented by both B. intermedia and B. pilosicoli, showed reduced susceptibility to tylosin, and the MICs of ampicillin for two isolates of B. pilosicoli from one farm were >32 mg/l. Neither of these studies supplied information on prior exposure to antimicrobials. The isolates investigated in the present study came from chickens that had not been exposed to antimicrobials at least 50 weeks prior to sampling (Jansson et al., Citation2008) and also the overall use of antimicrobials in Swedish poultry is low (Bengtsson et al., Citation2010). For many years, only one product has been approved for laying hens (oxytetracycline) and its long withdrawal period for eggs (7 days) poses an important obstacle to its use. Further, it can be assumed that the environmental exposure to antimicrobials in Brachyspira spp. originating from free-living wild birds is low.
Brachyspira colonization is common among commercial Swedish laying hens, particularly in non-caged systems indoors and in free-range birds (Jansson et al., Citation2008), but only a few farmers report health problems or production losses in Brachyspira colonized flocks. However, severe production losses, diarrhoea and faecal smears on egg shells in association with Brachyspira spp. colonization have been encountered (Jansson, 2008, unpublished observations). To the best of the authors’ knowledge, no laying hen flocks have so far been treated for intestinal spirochaete colonization in Sweden. In the present study it was therefore assumed that the investigated Brachyspira isolates represent a more or less naive spirochaetal population in terms of selective pressure on resistance determinants, which explains the fewer findings of decreased susceptibility among the isolates from chickens in this study compared to previous reports.
Although most investigated isolates displayed high susceptibility to all tested antimicrobials, a few isolates showed decreased susceptibility to ampicillin and lincomycin. β-Lactam antibiotics are among the most widely used antibiotics in both humans and livestock. Decreased susceptibility to β-lactam antibiotics has been reported in Brachyspira isolates from pigs (Dassanayake et al., Citation2005; Mortimer-Jones et al., Citation2008), humans (Tompkins et al., Citation1987; Brooke et al., Citation2003; Dassanayake et al., Citation2005; Meziane-Cherif et al., Citation2008; Mortimer-Jones et al., Citation2008), and chickens (Hampson et al., Citation2006b). Except for the early paper by Tompkins et al. (Citation1987), in which isolates were not identified to species level, all the isolates displaying decreased susceptibility to β-lactam antibiotics were identified as B. pilosicoli. Chromosomally located class D β-lactamase genes (i.e. bla OXA-63, bla OXA-136 and bla OXA-137) have been identified in B. pilosicoli isolated from humans and pigs (Meziane-Cherif et al., Citation2008; Mortimer-Jones et al., Citation2008). The extensive use of β-lactam antibiotics in both humans and pigs to treat a wide range of bacterial infections may have selected for resistance in B. pilosicoli as a side effect. However, the finding in this study of a closely related β-lactamase gene in an isolate of B. pilosicoli from a free-living wild mallard () with presumed low level of exposure to β-lactam antibiotics suggests alternative explanations. A β-lactamase gene could have been acquired by horizontal gene transfer within the genus Brachyspira or from an unrelated species as suggested by Mortimer-Jones et al. (Citation2008). Alternatively, the isolate may have been exposed to β-lactam antibiotics when present in another niche. B. pilosicoli has a broad host range that includes humans (Trivett-Moore et al., Citation1998), a variety of domestic animals (e.g. pigs, dogs, and poultry; Trott et al., Citation1996; McLaren et al., Citation1997; Duhamel et al., Citation1998) and free-living feral and wild birds (Oxberry et al., Citation1998; Jansson et al., 2011, unpublished observations), and the fact that it may be found in faecally contaminated surface waters (Oxberry et al., Citation1998) lends some support to this hypothesis. Further, isolates of B. pilosicoli may cross species barriers by experimental inoculation (Sacco et al., Citation1997; Trott & Hampson, Citation1998) and zoonotic spread of this species has been suggested (Hampson et al., Citation2006a). In the present study we also found β-lactamase activity in the chicken enteropathogen B. alvinipulli and in presumed non-pathogenic “B. pulli” isolates from chickens and a mallard, but we failed to amplify any β-lactamase gene(s). Thus, our results suggest that β-lactamase genes may be more broadly distributed among members of the genus Brachyspira than currently recognized.
Several mutations that cause decreased susceptibility to lincosamides and macrolides in other bacteria are located within domain V of the 23S ribosomal RNA gene (Vester & Douthwaite, Citation2001). In B. hyodysenteriae, an A2058T 23S rRNA mutation is associated with both macrolide and lincosamide resistance (Karlsson et al., Citation1999). However, no mutations were found when a DNA fragment covering domain V was sequenced for one of the isolates with decreased lincomycin susceptibility (AN1828/02) in this study. Additionally this isolate and AN1831/03 were both macrolide susceptible, which makes this antimicrobial susceptibility phenotype unusual.
Despite selective culture and repeated subculture, a common problem when studying avian Brachyspira spp. is the occurrence of isolates that contain more than a single species or genotype (Jansson et al., Citation2008). This is caused by the intrinsic motility and diffuse growth pattern of Brachyspira spp. on solid agar media. Definitive separation of species and genotypes from such isolates often requires 10-fold serial dilution and subculture. In the present paper we show that chicken flocks as well as individual chickens and mallards can be simultaneously colonized by fully susceptible and Brachyspira spp. isolates with decreased susceptibility. If antimicrobial susceptibility tests are performed on isolates that consist of several species or genotypes with different susceptibility patterns, the result of the test will reflect the number of cells in the sample and their relative growth rate, which may lead to incorrect MICs.
In conclusion, the results obtained in the present study showed that a majority of Brachyspira spp. isolates from commercial laying hens were susceptible to the antimicrobials that may be of therapeutic value in cases of intestinal spirochaete colonization, given a low level of antimicrobial exposure of the target population. We also showed that antimicrobial susceptibility tests should only be performed on isolates consisting of a single species and genotype, and that more than one isolate from a chicken flock should be investigated before treatment is initiated. Further, decreased susceptibility to amoxicillin was found in an isolate of B. pilosicoli from a mallard. To the best of our knowledge this is the first report of a β-lactamase gene of Brachyspira spp. found in an avian host, and also in a free-living wild animal species. The β-lactamase gene described here (bla OXA-192) was similar but not identical to previously described β-lactamase genes in B. pilosicoli of human and porcine origin. The finding of β-lactamase activity in “B. pulli” and “B. alvinipulli” points to a broader occurrence of β-lactamase genes within the genus Brachyspira than previously known. Further work is required to characterize these genes and to determine their occurrence.
Acknowledgements
The authors wish to thank Dr George A. Jacoby, Lahey Clinic, Burlington, Massachusetts, USA, for generous assistance with the designation of the new β-lactamase gene bla OXA-192. Funding was kindly provided by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) and the Swedish Farmers’ Foundation for Agricultural Research (SLF).
References
- Bengtsson , B. , Eriksson Unnerstad , H. , Greko , C. , Grönlund Andersson , U. & Landén , A. 2010 . In SVARM 2009, Swedish Veterinary Antimicrobial Resistance Monitoring 2009 ( pp. 8–13 and 35–36 ). Uppsala, , Sweden : National Veterinary Institute (SVA) .
- Brooke , C.J. , Hampson , D.J. and Riley , T.V. 2003 . In vitro antimicrobial susceptibility of Brachyspira pilosicoli isolates from humans . Antimicrobial Agents and Chemotherapy , 47 : 2354 – 2357 .
- Burch , D.G. , Harding , C. , Alvarez , R. and Valks , M. 2006 . Treatment of a field case of avian intestinal spirochaetosis caused by Brachyspira pilosicoli with tiamulin . Avian Pathology , 35 : 211 – 216 .
- Dassanayake , R.P. , Sarath , G. and Duhamel , G. 2005 . Penicillin-binding proteins in the pathogenic intestinal spirochaete Brachyspira pilosicoli . Antimicrobial Agents and Chemotherapy , 49 : 1561 – 1563 .
- Duhamel , G.E. , Trott , D.J. , Muniappa , N. , Mathiesen , M.R. , Tarasiuk , K. , Lee , J.I. and Hampson , D.J. 1998 . Canine intestinal spirochetes consist of Serpulina pilosicoli and a newly identified group provisionally designated “Serpulina canis” sp. nov . Journal of Clinical Microbiology , 36 : 2264 – 2270 .
- Duinhof , T.F. , Dierikx , C.M. , Koene , M.G. , van Bergen , M.A. , Mevius , D.J. Veldman , K.T. 2008 . Multiresistant Brachyspira hyodysenteriae in a Dutch sow herd . Tijdschrift voor Diergeneeskunde , 133 : 604 – 608 .
- Feberwee , A. , Hampson , D.J. , Phillips , N.D. , La , T. , van der Heijden , H.M.J.F. Wellenberg , G.J. 2008 . Identification of Brachyspira hyodysenteriae and other pathogenic Brachyspira species in chickens from laying flocks with diarrhea and/or reduced production . Journal of Clinical Microbiology , 46 : 593 – 600 .
- Fellström , C. and Gunnarsson , A. 1995 . Phenotypical characterisation of intestinal spirochaetes isolated from pigs . Research in Veterinary Science , 59 : 1 – 4 .
- Gresham , A.C. , Hunt , B.W. and Dalziel , R.W. 1998 . Treatment of swine dysentery—problems of antibiotic resistance and concurrent salmonellosis . The Veterinary Record , 143 : 619
- Hampson , D.J. and McLaren , A.J. 1999 . Experimental infection of laying hens with Serpulina intermedia causes reduced egg production and increased faecal water content . Avian Pathology , 28 : 113 – 117 .
- Hampson , D.J. , Oxberry , S.L. and La , T. 2006a . Potential for zoonotic transmission of Brachyspira pilosicoli . Emerging Infectious Diseases , 12 : 869 – 870 .
- Hampson , D.J. , Stephens , C.P. and Oxberry , S.L. 2006b . Antimicrobial susceptibility testing of Brachyspira intermedia and Brachyspira pilosicoli isolates from Australian chickens . Avian Pathology , 35 : 12 – 16 .
- Hidalgo , A. , Carvajal , A. , Pringle , M. , Rubio , P. and Fellström , C. 2010 . Characterization and epidemiological relationships of Spanish Brachyspira hyodysenteriae field isolates . Epidemiology and Infection , 138 : 76 – 85 .
- Hommez , J. , Castryck , F. , Miry , C. , Lein , A. , Devriese , L.A. and Haesebrouck , F. 1998 . Susceptibility of different Serpulina species in pigs to antimicrobial agents . Vlaams Diergeneeskundig Tijdschrift , 67 : 32 – 35 .
- Honkanen-Buzalski , T. and Huovinen , P. 1999 . Bacterial Resistance to Antimicrobial Agents in Finland: FINRES 1999 , 32 – 33 . Helsinki, , Finland : Ministry of Agriculture and Forestry and Ministry of Social Affairs and Health .
- Jansson , D.S. , Fellström , C. , Råsbäck , T. , Vågsholm , I. , Gunnarsson , A. , Ingermaa , F. and Johansson , K.-E. 2008 . Phenotypic and molecular characterization of Brachyspira spp. isolated from laying hens in different housing systems . Veterinary Microbiology , 130 : 348 – 362 .
- Jansson , D.S. , Johansson , K.-E. , Olofsson , T. , Råsbäck , T. , Vågsholm , I. , Pettersson , B. , Gunnarsson , A. and Fellström , C. 2004 . Brachyspira hyodysenteriae and other strongly β-haemolytic and indole‐positive spirochaetes isolated mallards (Anas platyrhynchos) . Journal of Medical Microbiology , 53 : 293 – 300 .
- Karlsson , M. , Fellström , C. , Gunnarsson , A. , Landén , A. and Franklin , A. 2003 . Antimicrobial susceptibility testing of porcine Brachyspira (Serpulina) species isolates . Journal of Clinical Microbiology , 41 : 2596 – 2604 .
- Karlsson , M. , Fellström , C. , Heldtander , M.U. , Johansson , K.-E. and Franklin , A. 1999 . Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae . FEMS Microbiology Letters , 172 : 255 – 260 .
- Kinyon , J.M. & Harris , D.L. 1980 . In vitro susceptibility of Treponema hyodysenteriae and Treponema innocens by the agar dilution method . In Proceedings of the Second International Symposium of Veterinary Laboratory Diagnosticians (pp. 1125 – 1128 ). Lucerne, Switzerland .
- Lobova , D. , Smola , J. and Cizek , A. 2004 . Decreased susceptibility to tiamulin and valnemulin among Czech isolates of Brachyspira hyodysenteriae . Journal of Medical Microbiology , 53 : 287 – 291 .
- McLaren , A.J. , Trott , D.J. , Swayne , D.E. , Oxberry , S.L. and Hampson , D.J. 1997 . Genetic and phenotypic characterization of intestinal spirochetes colonizing chickens and allocation of known pathogenic isolates to three distinct genetic groups . Journal of Clinical Microbiology , 35 : 412 – 417 .
- Meziane-Cherif , D. , Lambert , T. , Dupêchez , M. , Courvalin , P. and Galimand , M. 2008 . Genetic and biochemical characterization of OXA-63, a new class D β-lactamase from Brachyspira pilosicoli BM4442 . Antimicrobial Agents and Chemotherapy , 52 : 1264 – 1268 .
- Molnár , L. 1996 . Sensitivity of strains of Serpulina hyodysenteriae isolated in Hungary to chemotherapeutic drugs . The Veterinary Record , 138 : 158 – 160 .
- Mortimer-Jones , S.M. , Phillips , N.D. , La , T. , Naresh , R. and Hampson , D.J. 2008 . Penicillin resistance in the intestinal spirochaete Brachyspira pilosicoli associated with OXA-136 and OXA-137, two new variants of the class D β-lactamase OXA-63 . Journal of Medical Microbiology , 57 : 1122 – 1128 .
- Ohya , T. & Sueyoshi , M. 2010 . In vitro antimicrobial susceptibility of Brachyspira hyodysenteriae strains isolated in Japan from 1985 to 2009 . The Journal of Veterinary Medical Science , 72, 1651–1653 .
- Oxberry , S.L. , Trott , D.J. and Hampson , D.J. 1998 . Serpulina pilosicoli, waterbirds and water: potential sources of infection for humans and other animals . Epidemiology and Infection , 121 : 219 – 225 .
- Pringle , M. , Poehlsgaard , J. , Vester , B. and Long , K.S. 2004 . Mutations in ribosomal protein L3 and 23S ribosomal RNA at the peptidyl transferase centre are associated with reduced susceptibility to tiamulin in Brachyspira spp. isolates . Molecular Microbiology , 54 : 1295 – 1306 .
- Råsbäck , T. , Fellström , C. , Gunnarsson , A. and Aspán , A. 2006 . Comparison of culture and biochemical tests with PCR for detection of Brachyspira hyodysenteriae and Brachyspira pilosicoli . Journal of Microbiological Methods , 66 : 347 – 353 .
- Råsbäck , T. , Jansson , D.S. , Johansson , K.-E. and Fellström , C. 2007 . A novel enteropathogenic, strongly haemolytic spirochaete isolated from pig and mallard, provisionally designated ‘Brachyspira suanatina’ sp. nov . Environmental Microbiology , 9 : 983 – 991 .
- Rohde , J. , Kessler , M. , Baums , C.G. and Amtsberg , G. 2004 . Comparison of methods for antimicrobial susceptibility testing and MIC values for pleuromutilin drugs for Brachyspira hyodysenteriae isolated in Germany . Veterinary Microbiology , 102 : 25 – 32 .
- Rønne , H. & Szancer , J. 1990 . In vitro susceptibility of Danish field isolates of Treponema hyodysenteriae to chemotherapeutics in swine dysentery (SD) therapy. Interpretation of MIC results based on the pharmacokinetic properties of the antibacterial agents . In Proceedings of the 11th International Pig Veterinary Society Congress (p. 1126 ). Lausanne, Switzerland .
- Sacco , R.E. , Trampel , D.W. and Wannemuehler , M.J. 1997 . Experimental infection of C3H mice with avian, porcine, or human isolates of Serpulina pilosicoli . Infection and Immunity , 65 : 5349 – 5353 .
- Smit , H.F. , Dwars , R.M. , Davelaar , F.G. and Wijtten , G.A. 1998 . Observations on the influence of intestinal spirochaetosis in broiler breeders on the performance of their progeny and on egg production . Avian Pathology , 27 : 133 – 141 .
- Smith , S.C. , Muir , T. , Holmes , M. and Coloe , P.J. 1991 . In vitro antimicrobial susceptibility of Australian isolates of Treponema hyodysenteriae . Australian Veterinary Journal , 68 : 408 – 409 .
- Stanton , T.B. , Postic , D. and Jensen , N.S. 1998 . Serpulina alvinipulli sp. nov., a new Serpulina species that is enteropathogenic for chickens . International Journal of Systematic Bacteriology , 48 : 669 – 676 .
- Stephens , C.P. and Hampson , D.J. 1999 . Prevalence and disease association of intestinal spirochaetes in chickens in eastern Australia . Avian Pathology , 28 : 447 – 454 .
- Stephens , C.P. and Hampson , D.J. 2002 . Experimental infection of broiler breeder hens with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli causes reduced egg production . Avian Pathology , 31 : 169 – 175 .
- Tompkins , D.S. , Millar , M.R. , Heritage , J. and West , A.P. 1987 . β-Lactamase production by intestinal spirochaetes . Journal of General Microbiology , 133 : 761 – 765 .
- Trampel , D.W. , Kinyon , J.M. and Jensen , N.S. 1999 . Minimum inhibitory concentration of selected antimicrobial agents for Serpulina isolated from chickens and rheas . Journal of Veterinary Diagnostic Investigation , 11 : 379 – 382 .
- Trivett-Moore , N.L. , Gilbert , G.L. , Law , C.L.H. , Trott , D.J. and Hampson , D.J. 1998 . Isolation of Serpulina pilosicoli from rectal biopsy specimens showing evidence of intestinal spirochetosis . Journal of Clinical Microbiology , 36 : 261 – 265 .
- Trott , D.J. and Hampson , D.J. 1998 . Evaluation of day-old specific pathogen-free chicks as an experimental model for pathogenicity testing of intestinal spirochaete species . Journal of Comparative Pathology , 118 : 365 – 381 .
- Trott , D.J. , Stanton , T.B. , Jensen , N.S. , Duhamel , G.E. , Johnson , J.L. and Hampson , D.J. 1996 . Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis . International Journal of Systematic Bacteriology , 46 : 206 – 215 .
- Vester , B. and Douthwaite , S. 2001 . Macrolide resistance conferred by base substitutions in 23S rRNA . Antimicrobial Agents and Chemotherapy , 45 : 1 – 12 .
- Voha , C. , Docquier , J.D. , Rossolini , G.M. and Fosse , T. 2006 . Genetic and biochemical characterization of FUS-1 (OXA-85), a narrow-spectrum class D beta-lactamase from Fusobacterium nucleatum subsp. polymorphum . Antimicrobial Agents and Chemotherapy , 50 : 2673 – 2679 .
- Wanchanthuek , P. , Bellgard , M.I. , La , T. , Ryan , K. , Moolhuijzen , P. , Chapman , B. , Black , M. , Schibeci , D. , Hunter , A. , Barrero , R. , Phillips , N.D. and Hampson , D.J. 2010 . The complete genome sequence of the pathogenic intestinal spirochete Brachyspira pilosicoli and comparison with other Brachyspira genomes . PLoS One , 5 ( 7 ) : e11455