Abstract
This is the first report of a primary, spontaneous and, most probably, congenital teratoma in a domestic turkey, localized in front of the left eyeball. The unique localization allowed surgical excision of the tumour. The histopathological examination revealed that the tumour included structures derived from all three germ cell layers: ectoderm, mesoderm and endoderm (e.g. cartilaginous, osseous, haematopoietic, fibrous, nervous, glandular, squamous epithelial and smooth muscle tissues). The presence of epithelial cells as well as smooth muscle cells was confirmed using anti-cytokeratin and anti-desmin antibodies, respectively. The proliferative activity of the tumour cells was confirmed using proliferating cell nuclear antigen immunostaining. The other cases of teratoma in wild and domestic birds are reviewed briefly.
Introduction
Teratoma arises from totipotential germ cells that have undergone somatic differentiation and have given rise to two or more of the embryonic layers (endoderm, mesoderm and ectoderm), with a variety of tissues present (Kennedy et al., Citation1998). Some researchers presumed that teratoma arises from non-germinal cells of the early conceptus, stem cells, extra-embryonic cells, “included” twins or various cell types, depending on the anatomic site (Schelling, Citation1994 after Gonzalez-Crussi, 1982). The mechanism of development of the teratoma is unclear; the “misinvolvement–misenfoldment” hypothesis assumed that the disturbances in cell migration during the early embryonic stage of development might lead to intracranial teratoma (Sano, Citation2001). Histologically, teratomas are classified as: mature teratomas, characterized by differentiated tissues; immature teratomas, composed of immature but non-malignant tissues; and malignant teratomas (Lo Curto et al., Citation2007). Spontaneous teratoma is only rarely reported in domestic fowl and sporadically reported in wild avian species. All available reports describe teratomas diagnosed post-mortem. This is the first report of teratoma in a domestic turkey (Meleagris gallopavo domestica), diagnosed in an excisional biopsy specimen from the area of the left eye.
Materials and Methods
Case history
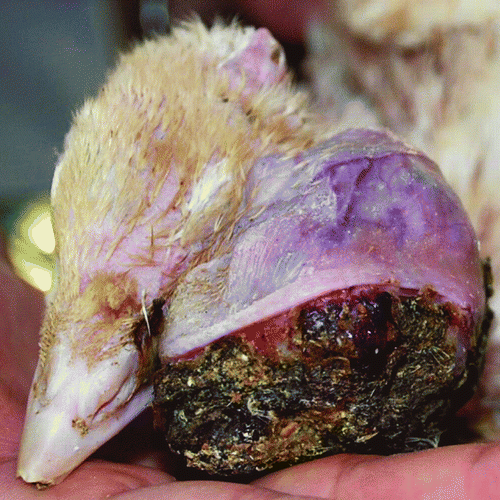
A 6-week-old turkey poult (male) was presented for clinical examination with a large excrescence in the area of the left eye (). The general condition and behaviour of the bird were unaffected. Despite moderate scratching, the excrescence seemed to have no influence on the bird's comfort. After 2 days of clinical observation, surgical excision (using ketamine/xylazine intravenous anaesthesia) of the excrescence was conducted. Intrasurgical observation revealed that the excrescence was a tumour situated rostral to and loosely attached to the eyeball. The tumour was poorly encapsulated and well vascularized. The left eyeball was excised as well. After excision, the skin was sutured with a gauze swab inside the empty orbit. The swab was changed every day during the period of convalescence, which lasted 24 days.
Macroscopically, the tumour was heterogeneous and partially covered with crusts (this area protruded outwards). The cut surface was pale and yellowish with visible aggregates of melanin. The consistency was firm, and the structure was granular and cystic.
Histopathology and immunohistochemistry
The tumour was surgically excised, and the samples were fixed in 10% buffered formalin, embedded in paraffin, cut at 5 µm and mounted. The sections were processed routinely and stained with Alcian blue-PAS (Bancroft & Gamble, 2008), as well as with haematoxylin and eosin, Mallory trichrome (staining kit; Bio-Optica, Milano, Italy) and cresyl violet, according to conventionally accepted procedures (Bagiński, Citation1969). Immunohistochemical examination was performed using anti-cytokeratin (1:50 dilution, clone AE1/AE3; DAKO, Carpinteria, California, USA), anti-proliferating cell nuclear antigen (1:200 dilution, clone PC10; DAKO, Glostrup, Denmark) and anti-desmin (1:50 dilution, clone D33; DAKO, Glostrup, Denmark) monoclonal mouse anti-human antibodies. The primary antibody was detected using a system based on a horseradish peroxidase-labelled polymer conjugated with secondary antibodies (Impress Universal Reagent Anti Mouse/Rabbit Ig, VECTOR, Burlingame, California, USA). The antigen–antibody complexes were visualized using 3,3-diaminobenzidine (DAB) and a chromogen substrate (DAB Substrate Kit for Peroxidase; VECTOR, Burlingame, California, USA), which resulted in a brown precipitate at the antigen site. The specimens were counterstained with Mayer's haematoxylin (3 min), dehydrated and mounted using Canadian balm. For the negative control, primary antibody was replaced with Mouse IgG2a (1:200; DAKO, Glostrup, Denmark). The appropriate normal canine tissue sections were used as positive control. Each section was investigated using a Panoramic Scanner MIDI (3DHISTECH, Budapest, Hungary). Photographs were prepared using Panoramic Viewer software (3DHISTECH, Budapest, Hungary).
Results
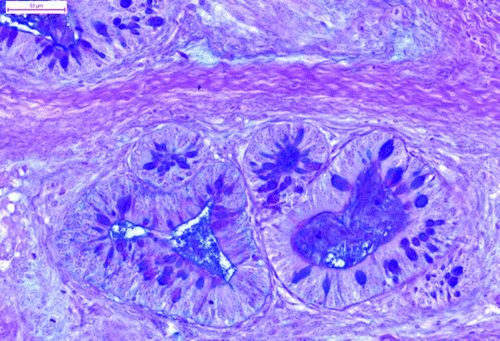
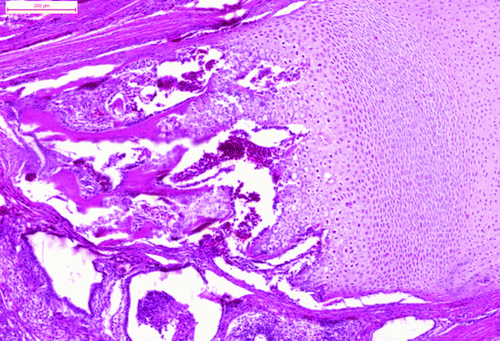
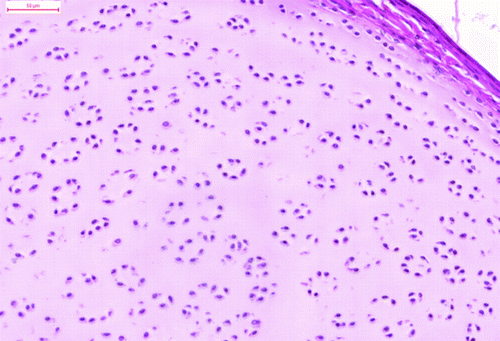
The tumour comprised many different tissue components. The most apparent feature was the presence of the multiform cystic spaces lined by simple columnar epithelium with numerous goblet cells. The goblet cells contained acidic or neutral mucus, depending on their localization (). These glandular structures formed crypts and simulated the intestine. The pseudostratified ciliated epithelium resembled the epithelium of the respiratory system. The hyaline cartilage formed islands of various sizes, scattered throughout the tumour mass. Some of these islands contained embryonic hyaline cartilage with an irregular chondrocyte arrangement. The chondrocytes of the embryonic hyaline cartilage did not form chondrones and concentrated in wreath-like structures in some areas (). There were also areas of endochondral ossification (). The trabecular bone cavities contained bone marrow, which consisted of heterophils and lymphoblasts. The glandular tissue was surrounded by smooth muscle cells in the form of thick strands. The melanocytes were organized in bands that resembled those of the ciliary body. The stratified squamous epithelium occasionally formed keratin pearls (). Primitive nervous tissue formed rosettes with cystic spaces; these areas mimicked the neural tube. The areas of well-developed neurons (dispersed, with pale nuclei and distinct nucleoli) were well circumscribed and showed distinct angiogenesis. There were also small areas of ganglion cells surrounded by satellite glial cells.
Figure 3. The chondrocytes of the embryonic cartilage formed wreath-like structures (haematoxylin and eosin). Bar = 50 μm.
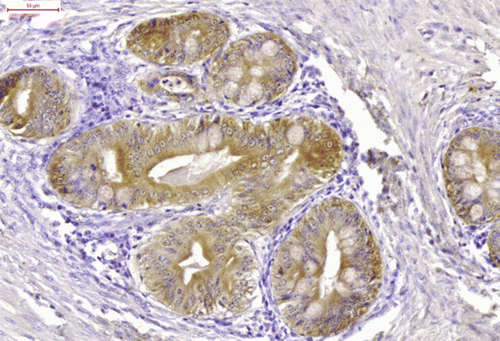
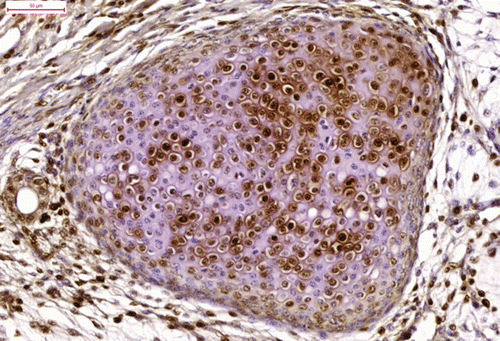
Cytokeratin immunoreactivity was positive in the cytoplasm of the epithelial cells (cuboidal cylindrical, pseudostratified, squamous) () and strongly positive in the horny pearls. The bundles of myocytes and the myoepithelial cells surrounding the glandular tissue were strongly immunoreactive for desmin (). Proliferating cell nuclear antigen immunoreactivity was strong in the epithelial cells, myocytes, many of the chondrocytes (), satellite glial cells and proliferating endothelial cells.
These components represent all three germ layers (). All tissues represented in the tumour were mature, except for poorly differentiated cells in the area resembling a neural tube.
Figure 5. The stratified squamous epithelium formed a horny pearl (haematoxylin and eosin). Bar =50 μm.
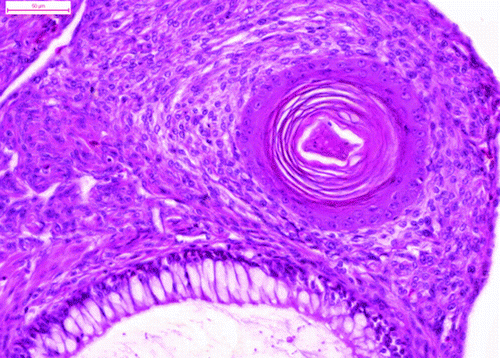
Table 1. Derivation of tissue components present in the teratoma.
The eye was approximately 15 to 18 mm in diameter, spherical, and loosely attached to the tumour mass. The surface was irregular and nodular. The tumour mass attached to the sclera did not penetrate the eyeball and displayed a structure similar to that of the main tumour mass. The retina displayed rod and cone layer dysplasia. Rods and cones were degenerated and arranged irregularly. The cornea displayed cutaneous metaplasia, with nodular protrusions filled with adipose tissue and covered with squamous epithelium that formed at the border of the cornea and sclera (corneal limbus). The squamous epithelium was dysplastic (displayed dysplastic cornification) and formed cystic spaces. The outer, cornified layer displayed massive exfoliation and was focally strongly infiltrated with heterophils. The ossicles normally present in the avian sclera were focally hyperplastic, and intramembranous ossification was observed focally. The Harderian gland consisted of columnar epithelial cells of foamy appearance, with strong secretory activity; individual cells demonstrated secretory retention with balloon enlargement. The gland was infiltrated by large numbers of heterophils.
Discussion
The most common location of avian teratoma is the ovary, testicle or coelom; there are few reports of avian teratomas situated retrobulbarly or intracranially () and only one report of an avian teratoma on the head surface (Homer, 1991). No previous reports of extragonadal teratoma in turkeys were found. The origin of the teratoma described is uncertain, as it involved a large part of the cutis and subcutis of the head and was extending to the eyeball. The tumour was probably derived from misplaced embryonic cells. The teratomas in birds are in most cases benign, but due to their large size and location can often lead to death (). In the case reported here, the mature character of the tumour and its external location allowed surgical removal of the tumour and the affected eyeball.
Figure 7. The bundles of myocytes showed strong desmin immunoreactivity (desmin immunostaining). Bar = 200 μm.
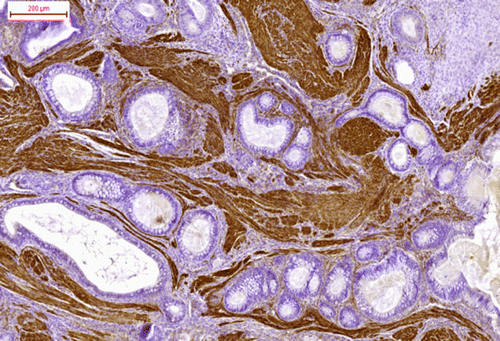
Table 2. Reports of teratoma in wild and domestic avian species.
Immunohistochemistry examination was previously used in diagnosing avian teratoma, using vimentin, smooth muscle actin, pancytokeratin and neuron-specific enolase (Hooper, Citation2008). In this study, the combination of histochemical and immunohistochemical stainings allowed identification and description of all of the tissue components. The proliferating cell nuclear antigen immunoreactivity of most of the tissue components of the teratoma suggests that the tumour had the capacity for growth.
References
- Bagiński , S. 1969 . Technika mikroskopowa. Praktyczny poradnik mikroskopowy (Microscopic technique. Practical guide). , 2nd edn , Warsaw : PWN .
- Baker , J.R. & Chandler , D.J. 1981 . Suspected teratoma in a black headed gull (Larus ridibundus) . The Veterinary Record , 109 , 60.r
- Bancroft , J.D. and Gamble , M. 2008 . Theory and practice of histological techniques. , 6th edn , Philadelphia : Churchill Livingstone Elsevier .
- Bolte , A.L. and Burkhardt , E. 2000 . A teratoma in a Muscovy duck (Cairina moschata) . Avian Pathology , 29 : 237 – 239 .
- Campbell , J.G. and Appleby , E.C. 1966 . Tumours in young chickens bred for rapid body growth (broiler chickens): a study of 351 cases . The Journal of Pathology & Bacteriology , 92 : 77 – 90 .
- Campbell , J.G. 1962 . A retro-ocular teratoma containing pinealomatous tissue in a young chicken . British Journal of Cancer , 16 : 258 – 266 .
- Campbell , J.G. 1951 . Some unusual gonadal tumours of the fowl . British Journal of Cancer , 5 : 69 – 82 .
- Cooper , J.M. , Watson , J. and Payne , L.N. 1978 . A mixed cell tumour in a Seychelles kestrel (Falco araea) . Avian Pathology , 7 : 651 – 658 .
- Cullen , J.M. , Newbold , J.E. and Sherman , G.J. 1991 . A teratoma in a duck infected congenitally with duck hepatitis B virus . Avian Diseases , 35 : 638 – 641 .
- Feldman , W.H. and Olson , C. Jr. 1959 . “ Neoplastic diseases of the chicken ” . In Diseases of Poultry , 4th edn , Edited by: Biester , H.E. and Schwarte , L.E. 688 – 690 . Ames : Iowa State University Press .
- Forbes , N.A. , Cooper , J.E. and Higgins , R.J. 2000 . “ Neoplasms of birds of prey ” . In Raptor Biomedicine III , Edited by: Lumeij , J.T. , Remple , J.D. , Redig , P.T. , Lierz , M. and Cooper , J.E. 127 – 146 . Lake Worth , FL : Zoological Education Network Inc .
- Ford , S. L. , Wentz , S. and Garner , M. 2006 . Intracelomic teratoma in a juvenile bald eagle (Halieateus leucocephalus) . Journal of Avian Medicine and Surgery , 20 : 175 – 179 .
- Gupta , B.N. 1976 . Teratoma in a chicken (Gallus domesticus) . Avian Diseases , 20 : 761 – 768 .
- Hamir , A.N. 1985 . Teratoma in a duck . The Veterinary Record , 117 : 314
- Helmboldt , C.F. , Migaki , G. , Langheinrich , K.A. and Jakowski , R.M. 1974 . Teratoma in domestic fowl (Gallus gallus) . Avian Diseases , 18 : 142 – 148 .
- Homer , B.L. and Riggs , M.W. 1991 . Cranial teratomas in two domestic ducks (Anas platyrhynchos domesticus) . Avian Diseases , 35 : 994 – 998 .
- Hooper , C.C. 2008 . Teratoma in the cerebrum of a fantail pigeon . Avian Pathology , 37 : 141 – 143 .
- Jones , L.D. 1964 . Avian cerebellar teratoma . Avian Diseases , 8 : 581 – 584 .
- Kennedy , P.C. , Cullen , J.M. , Edwards , J.F. , Goldschmidt , M.H. , Larsen , S. , Munson , L. & Nielsen , S. 1998 . Histological Classification of Tumors of the Genital System of Domestic Animals . World Health Organization International Histological Classification of Tumors of Domestic Animals 2nd edn (p. 26 ). Washington : Armed Forces Institute of Pathology .
- Lo Curto , M. , D'Angelo , P. , Cecchetto , G. , Klersy , C. , Dall'Igna , P. , Federico , A. , Siracusa , F. , Alaggio , R. , Bernini , G. , Conte , M. , De Laurentis , T. , Di Cataldo , A. , Inserra , A. , Santoro , N. , Tamaro , P. and Indolfi , P. 2007 . Mature and immature teratomas: results of the first paediatric Italian study . Pediatric Surgery International , 23 : 315 – 322 .
- López , R.M. and Múrcia , D.B. 2008 . First description of malignant retrobulbar and intracranial teratoma in a lesser kestrel (Falco naumanni) . Avian Pathology , 37 : 413 – 414 .
- Mashar , U. 1932 . Über teratoide Geschwülste in der Leibeshöhle beim Haushuhn . Virchows Archiv , 285 : 155 – 168 .
- Mutinelli , F. and Vascellari , M. 2002 . Thoracic teratoma in a white Pekin duck (Anas platyrhinchos domestica) . Avian Diseases , 46 : 493 – 496 .
- Neumann , U. , Kummerfeld , N. and Uhde , S. 1986 . Tumorous lesions in budgerigars: diagnostic aspects in view of clinical and morphological observations . Israeli Journal of Veterinary Medicine , 42 : 98 – 102 .
- Reece , R.L. and Lister , S.A. 1993 . An abdominal teratoma in a domestic goose (Anseriformes, Anser anser domesticus) . Avian Pathology , 22 : 193 – 196 .
- Rigdon , R.H. and Leibovitz , L. 1970 . Spontaneous-occurring tumors in the duck: review of the literature and report of three cases . Avian Diseases , 14 : 431 – 444 .
- Rigdon , R.H. 1967 . Neoplasms in sterile hybrid ducks. A melanoma and two teratomas . Avian Diseases , 11 : 79 – 89 .
- Sano , K. 2001 . Intracranial dysembryogenetic tumors: pathogenesis and their order of malignancy . Neurosurgical Review , 24 : 162 – 167 .
- Schelling , S.H. 1994 . Retrobulbar teratoma in a great blue heron (Ardea herodias) . Journal of Veterinary Diagnostic Investigation , 6 : 514 – 516 .
- Sugiyama , M. , Isoda , M. and Tomizawa , A. 1983 . Polycystic immature teratoma observed at the head of Meckel's diverticulum of a chick . Journal of Veteterinary Science , 45 : 383 – 385 .