Abstract
Fledgling cliff swallows were cared for at a rehabilitation facility when clinical signs of ocular disease, characterized by conjunctivitis, epiphora, and hyperaemia of palpebrae and nictitans, were recognized. Treatment consisted of topical and oral antibiotic therapy and one topical steroid administration. However, one cliff swallow died and three were killed due to poor therapeutic response. Conjunctival swabs were obtained ante-mortem from the three cliff swallows and were submitted for mycoplasma culture and molecular diagnostics. Heads of the three birds were fixed in 10% neutral buffered formalin and submitted for histopathologic examination of oculonasal tissues. Mycoplasma cultures and molecular evaluation of isolates identified Mycoplasma sturni, but not Mycoplasma gallisepticum, from each specimen. Histopathologic examination revealed lymphoplasmacytic conjunctivitis, rhinitis and infraorbital sinusitis with follicular lymphoid hyperplasia, epithelial hyperplasia, and protozoal stages compatible with Cryptosporidium spp. arranged in and along the apical surfaces of epithelial cells. Identification of concurrent M. sturni and Cryptosporidium spp. infections in these cliff swallows demonstrates an alternative infectious condition that can produce gross and microscopic lesions comparable with those commonly observed in M. gallisepticum infections of house finches and other passerine species. Conjunctivitis associated with M. sturni and Cryptosporidium spp. in cliff swallows may represent an emerging disease risk to a naïve, high-density and colonial species such as colony-nesting cliff swallows.
Introduction
Mycoplasma gallisepticum conjunctivitis emerged in 1994 as a disease of free-ranging house finches (Carpodacus mexicanus) in the mid-Atlantic region of the USA and rapidly spread to house finches throughout their entire introduced eastern range (Ley et al., Citation1996; Luttrell et al., Citation1996; Dhondt et al., Citation1998; Hartup et al., Citation2001a, Citationb). The resulting epidemic of M. gallisepticum conjunctivitis produced an unprecedented decline in eastern house finch populations (Altizer et al., Citation2004; Hochachka & Dhondt, Citation2000). M. gallisepticum has also been isolated from other songbirds with conjunctivitis, notably American goldfinch (Carduelis tristis), purple finch (Carpodacus purpureus), evening grosbeak (Coccothraustes vespertinus) and pine grosbeak (Pinicola enucleator) (Fischer et al., Citation1997; Ley et al., Citation1997; Hartup et al., Citation2000; Mikaelian et al., Citation2001). A feature of both natural and experimental infections vital to the successful study of this disease has been the apparent very high correlation between M. gallisepticum infection and characteristic gross lesions of the eye and circumocular tissues. Mycoplasmas from all known susceptible host species had consistently been identified as M. gallisepticum, resulting in the assumption for both field and experimental observations that characteristic gross lesions in susceptible host species were evidence of M. gallisepticum infection. In 2006, Mycoplasma sturni was isolated from a California house finch with conjunctivitis, but experimental infection of house finches with the M. sturni isolate failed to reproduce the disease (Ley et al., Citation2010).
The original isolate and type strain (UCMF = ATCC 51945) of M. sturni was recovered from an adult European starling (Sturnus vulgaris) with bilateral conjunctivitis in Connecticut, USA (Forsyth et al., Citation1996; Frasca et al., Citation1997). Subsequently, M. sturni was isolated from northern mockingbirds (Mimus polyglottos) and blue jays (Cyanocitta cristata) in Florida, with clinical signs and gross lesions suggestive of M. gallisepticum conjunctivitis in house finches (Ley et al., Citation1998). In Minnesota, M. sturni was isolated from an American crow (Corvus brachyrhynchos) with conjunctivitis and in American crows without clinical signs caged with a nest-mate of the crow with conjunctivitis (Wellehan et al., Citation2001). M. sturni also was found in American robins (Turdus migratorius) and European starlings showing no clinical signs housed at the same facility as the crows. The authors concluded that M. sturni can infect American crows and American robins with or without the presence of clinical disease (Wellehan et al., Citation2001). Post-mortem sampling in Scotland of wild birds with a range of infectious and non-infectious conditions found M. sturni in 18 of 41 tested; all positive birds were immature, and included blackbird (Turdus merula), rook (Corvus frugilegus), carrion crow (Corvus corone), magpie (Pica pica), and European starling (Pennycott et al., Citation2005). However, the significance of isolating M. sturni from these birds with diverse diseases was unclear (Pennycott et al., Citation2005).
Materials and Methods
Clinical history
Three fledgling cliff swallows (CLSWs; Petrochelidon pyrrhonota) were admitted to the veterinary hospital at Lindsay Wildlife Museum (Walnut Creek, California, USA) in 2010 on June 28 (day 0, CLSW-1), June 29 (day 1, CLSW-2) and July 2 (day 5, CLSW-3) because they had fallen from the nest. All came from the same colony, had minor injuries, and were housed as a group. On day 25, an additional fledgling cliff swallow (CLSW-4) was transferred for overwintering from another facility that rehabilitates a range of wildlife including non-natives such as European starlings (S. vulgaris). Since single-housed swallows do not thrive in captivity and all four swallows were free of clinical signs of infectious disease after 7 days, they were combined. All four birds remained free of clinical signs until day 76 when CLSW-4 developed mild increased respiratory effort, right unilateral conjunctivitis, epiphora, and hyperaemia of palpebrae and nictitans with no corneal ulcers detected. The bird was treated with topical antibiotics (bacitracin/neomycin/polymyxin ointment in the right eye every 12 h for 7 days) for possible trauma. Over the course of days 85 through 116, clinical signs resolved and recurred twice, the bird was treated with systemic antibiotics—enrofloxacin (compounded by BCP Veterinary Pharmacy, Houston, Texas, USA) and erythromycin (Abbott, Abbott Park, Illinois, USA)—as well as ophthalmic ofloxacin (Falcon Pharmaceuticals, Fort Worth, Texas, USA). On day 162, CLSW-3 developed similar ocular signs that consisted of right unilateral mild conjunctivitis and clear ocular discharge. As a consequence of the discharge, the peri-ocular feathers became stiffened and added a physical trauma by curving and scratching the cornea, and so were regularly cleaned. Although CLSW-1 and CLSW-2 did not have ocular signs, they did exhibit mildly increased respiratory effort. At this time all four birds were treated with erythromycin (per orally, 20 mg/kg every 24 h for 14 days; Abbot Laboratories, Abbot Park, Illinois, USA). Clinical signs abated but did not resolve, and it was considered that persistence of ocular signs could be due to a physical irritation or to an infectious agent that was not susceptible to the antimicrobials used. Financial limitations precluded extensive diagnostics; it was therefore decided to administer a single topical dose of prednisolone (OmniPred®; Alcon Laboratories Inc., Fort Worth, Texas, USA). Resolution of the condition would support physical irritation as a cause, whereas a rapid worsening of clinical signs would suggest an infectious agent and the animals would be killed owing to the non-responsiveness of the clinical signs and the potential of infecting others. Clinical signs improved briefly but then worsened. On day 189, CLSW-1 was dead in its enclosure. No gross lesions were found on necropsy. Conjunctival swabs of CLSW-2, CLSW-3 and CLSW-4 were taken on day 198. Left and right conjunctival swabs (Pur-Wraps® polyester tipped mini swabs; Puritan Medical Products, Co. Gullford, Maine, USA) were pooled (one tube/bird) in 1 ml Universal Transport Medium (Becton Dickenson/Copan, Sparks, Maryland, USA) and shipped overnight on cold packs to North Carolina State University College of Veterinary Medicine (Raleigh, North Carolina, USA) for mycoplasma testing. Owing to a lack of response to various treatments, a worsening of the ocular clinical signs, and a continued deterioration of feather condition, the decision was made to kill the swallows. A second set of conjunctival swabs was taken ante-mortem from each bird and sent to North Carolina State University College of Veterinary Medicine. Intact heads from these three cliff swallows were placed in 10% neutral buffered formalin and later sent to the Connecticut Veterinary Medical Diagnostic Laboratory (Department of Pathobiology and Veterinary Science, University of Connecticut, Storrs, Connecticut, USA) for histopathologic evaluation. Gross necropsy findings were similar for all three birds and revealed thin birds but no other gross lesions.
Mycoplasma culture and polymerase chain reaction
Upon overnight delivery on cold packs, 200 µl of each sample in Universal Transport Medium was passed to 2 ml Frey's mycoplasma broth (Kleven, Citation2008) with 15% (v/v) swine serum and 1% (v/v) Fungizone (250 µg/ml amphotericin B, GIBCO catalogue number 15290–018), and incubated in humidified air at 37°C with periodic transfer of aliquots to Frey's mycoplasma agar. First-passage cultures showing signs of contaminant overgrowth (early colour change, turbidity) were filtered (0.45 µm) and passed to Frey's mycoplasma broth and agar media. Mycoplasmal colonies on agar were tested for M. gallisepticum by direct immunofluorescence (Kleven, Citation2008) using fluorescein-conjugated rabbit antiserum (Leiting & Kleven, Citation2000) provided by S. H. Kleven (College of Veterinary Medicine, University of Georgia, Athens, Georgia, USA), and also tested for M. sturni by indirect immunofluorescence (Ley et al., Citation1998) using M. sturni rabbit antiserum (Forsyth et al., Citation1996) provided by S. Geary (Center of Excellence for Vaccine Research, Department of Pathobiology & Veterinary Science, University of Connecticut).
Samples collected on day 210 were tested directly by polymerase chain reaction (PCR) using 200 µl of each sample in Universal Transport Medium for DNA extraction and purification with the QIamp® DNA Mini Kit (QIAGEN Sciences, Germantown, Maryland, USA) according to the manufacturer's instructions. The same procedure was used for DNA extraction of mycoplasma isolates in broth cultures made from both sample submissions. Conventional PCR for M. gallisepticum was performed using primers to the mgc2 surface protein gene (Hong et al., Citation2005), and for mycoplasmas using primers (GPF/MGSO) to the 16S rRNA gene, reported to detect Mycoplasma species, Acholeplasma species (Lierz et al., Citation2007) and Ureaplasma species (D. H. Ley, data not shown). Amplified PCR products were separated in 2% agarose gels and visualized by ethidium bromide staining and ultraviolet transillumination.
DNA sequencing
Selected partial 16S rRNA gene amplicons were sequenced using the same GPF/MGSO primers by a commercial service (GENEWIZ, South Plainfield, New Jersey, USA). Forward and reverse sequences were scanned visually using FinchTV (http://www.geospiza.com/Products/finchtv.shtml) to evaluate chromatograms for quality and to truncate sequences. Forward and reverse sequences were assembled to a contiguous sequence without further editing using the ContigExpress tool of Vector NTI Advance 11.0 (Invitrogen Corp., Carlsbad, California, USA), and these were compared with 16S microbial rDNA sequences in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) using BLASTn (Altschul et al., Citation1990).
Histopathology
Heads of CLSW-2, CLSW-3 and CLSW-4 fixed in 10% neutral buffered formalin were received by the Connecticut Veterinary Medical Diagnostic Laboratory. Each head was dissected to remove the lower jaw and tongue and to examine the choanal cleft. The brain was removed and was serially and transversely sliced. The remaining tissues, which included both eyes and the nasal cavity, were decalcified by immersion into 0.5 M ethylenediamine tetraacetic acid for 24 h, and then transferred to 70% ethanol for an additional 24 h, after which time each eye, together with its circumocular tissues (e.g. palpebrae and conjunctivae), was dissected from the head. Each ocular globe together with its circumocular tissues was sliced in the mid-sagittal plane. The nasal cavity was sliced transversely at the base of the beak. Samples of the brain, each eye and its associated circumocular tissues, and the nasal cavity were placed individually into separate plastic cassettes, processed routinely, embedded in paraffin and sectioned at 4 µm. Histological sections were mounted on glass slides, stained with haematoxylin and eosin and examined by light microscopy to evaluate the microscopic features of lesions.
Results
Mycoplasma culture and PCR
Conjunctival swab samples collected on day 198 from CLSW-2, CLSW-3 and CLSW-4 yielded mycoplasma isolates from cultures. All were negative for M. gallisepticum and positive for M. sturni by immunofluorescence (data not shown). Furthermore, all were negative with M. gallisepticum PCR using mgc2 primers but all were positive with PCR using GPF/MGSO primers (data not shown), indicating that mycoplasma DNA was detected but it was not that of M. gallisepticum. Conjunctival swab samples collected on day 210 from CLSW-2, CLSW-3 and CLSW-4 also yielded mycoplasma isolates from cultures, and again all were negative for M. gallisepticum and positive for M. sturni by immunofluorescence (data not shown). Whether testing samples directly or using culture isolates, all M. gallisepticum PCR results using mgc2 primers were negative but all mycoplasmal PCR results using GPF/MGSO primers were positive (data not shown). PCR results were as expected with both primer sets using positive control reference strains of M. gallisepticum and M. sturni, and qualitatively identical results were obtained when samples were tested directly or as culture isolates. Based on these results, the following GPF/MGSO amplicons were chosen for sequencing to validate the method for M. sturni and to confirm the immunofluorescence results: M. sturni reference strain, CLSW-4 direct sample, and CLSW-3 culture isolate.
DNA sequence analysis
Amplicons from the M. sturni ATCC reference strain, CLSW-4 direct sample, and CLSW-3 culture isolate resulted in contiguous sequences of 861, 865, and 777 nucleotides, respectively. BLASTn analysis showed that each had 99% identity with M. sturni (strain UC/MF 16S ribosomal RNA, partial sequence; GenBank NR_025968.1).
Pathological findings
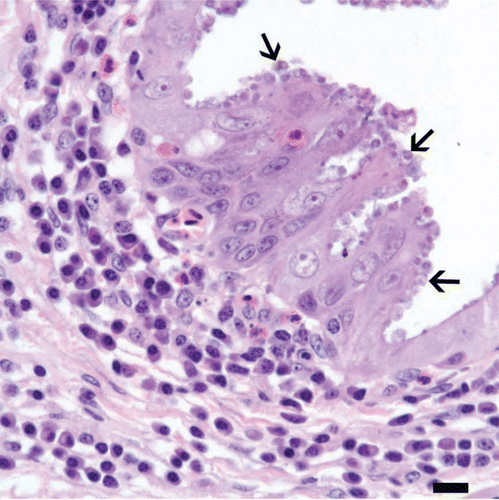
Grossly, there was bilateral mild to moderate eyelid swelling in each specimen with loss of feathers from the palpebral and peri-ocular skin (). Microscopically, there were varying degrees, including severe, of conjunctivitis, rhinitis and infraorbital sinusitis in each of the three specimens. Inflammatory infiltrates involved bulbar and palpebral conjunctivae and were lymphoplasmacytic in each specimen, with varying degrees of follicular lymphoid hyperplasia (). There was mild to marked hyperplasia of conjunctival epithelium, with intra-epithelial infiltrates of low numbers of heterophils (). Inflammatory infiltrates in the respiratory mucosae of the nasal cavities and infraorbital sinuses were also lymphoplasmacytic, with mild to marked follicular lymphoid hyperplasia, and were accompanied by hyperplasia of lining epithelium and intra-epithelial heterophils, as with the conjunctivae ( and ). Distributed along segments of the conjunctival, nasal and sinus epithelia in all three swallows were round protozoa 2 to 5 µm in diameter, which were associated with the apical surfaces of epithelial cells () and were histomorphologically compatible with species of Cryptosporidium.
Figure 1. Lateral view of a cliff swallow head. There is mild swelling of the eyelids and nictitans, with loss of feathers from the skin of the upper and, to a lesser extent, the lower eyelid and from the skin at the medial canthus.

Figure 2. Conjunctiva from a cliff swallow with conjunctivitis. Lymphocytes and plasma cells (inset: bar = 10 µm), along with a focus of follicular lymphoid hyperplasia (*), infiltrate and expand the lamina propria. Bar = 20 µm. Haematoxylin and eosin.
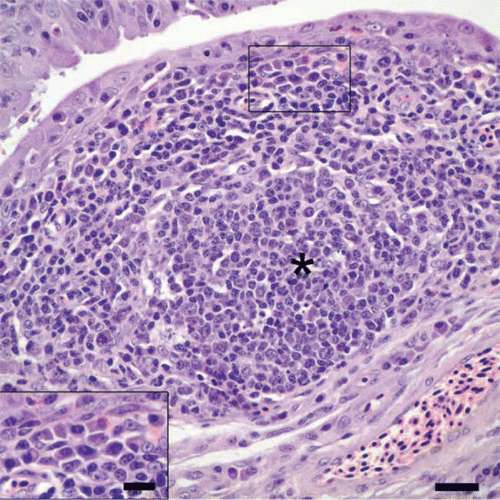
Figure 3. Fornix of the conjunctiva from a cliff swallow with conjunctivitis. There is hyperplasia of conjunctival epithelium, evidenced by tufts and pilings of epithelial cells, and heterophils in low numbers are scattered in the hyperplastic epithelium. Note that the lamina propria is infiltrated by lymphocytes and plasma cells, and few cryptosporidial stages are present along the surfaces of epithelial cells. Bar = 20 µm. Haematoxylin and eosin.
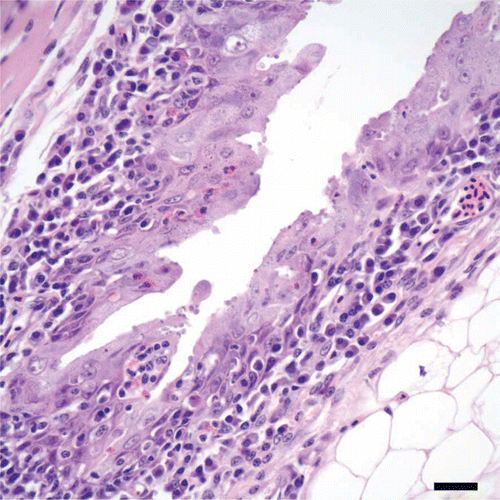
Figure 4. Nasal concha of a cliff swallow with rhinitis. The lamina propria of the nasal mucosa overlying the turbinate (t) is expanded by a lymphoplasmacytic infiltrate with follicular lymphoid hyperplasia. Note the cluster of cyrptosporidial stages along the surface of the epithelium. Bar = 20 µm. Haematoxylin and eosin.
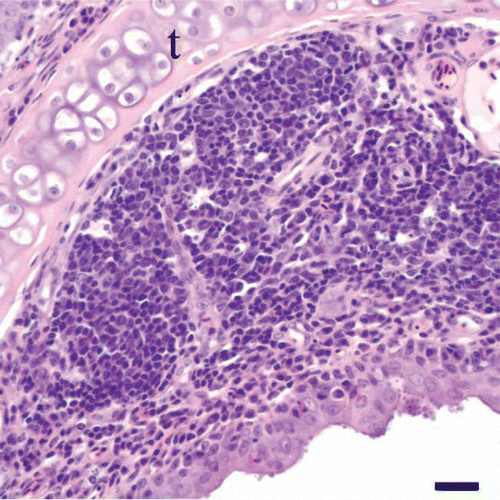
Figure 5. Nasal septum of a cliff swallow with rhinitis. There is hyperplasia of the nasal mucosal epithelium along the nasal septum (s), as evidenced by piling and layering of epithelial cells, together with scattered intra-epithelial heterophils. Note the infiltrate of lymphocytes and plasma cells in the lamina propria and the cryptosporidial stages along the surfaces of epithelial cells. Bar = 20 µm. Haematoxylin and eosin.
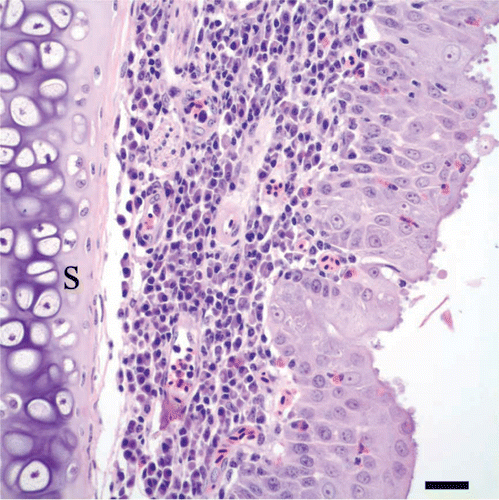
Figure 6. Conjunctiva from a cliff swallow with conjunctivitis. Numerous, round protozoa 2 to 5 µm in diameter, consistent with cryptosporidial developmental stages (arrows), are located in or at the apical margins of epithelial cells along this segment of hyperplastic conjunctival epithelium. An infiltrate of plasma cells with occasional heterophils is present in the underlying connective tissue of the lamina propria. Bar = 10 µm. Haematoxylin and eosin.
Discussion
In the course of monitoring birds at selected wildlife care and rehabilitation facilities for eye lesions compatible with those of M. gallisepticum conjunctivitis and to collect isolates from house finches to further document the range of hosts affected by this disease, we encountered the cases in cliff swallows described here. Cliff swallows are small migratory birds that nest in colonies throughout much of western North America and are highly social, participating in all of their activities as large groups (Brown & Brown, Citation1986), which could facilitate the impact of transmissible infectious diseases such as mycoplasmosis and cryptosporidiosis. Identification of both in these cliff swallows, which had gross lesions comparable with those of conjunctivitis caused by M. gallisepticum in house finches, confounds what was previously considered a straightforward interpretation of mycoplasmal conjunctivitis in passerine species.
Cryptosporidia are intracellular protozoan parasites in the phylum Apicomplexa that infect epithelial cells. They are pathogens of many vertebrate species and most frequently infect neonatal and young animals (Jervis et al., Citation1966; Rehg et al., Citation1979; Tzipori & Campbell, Citation1981), and some species are human pathogens (Ungar, Citation1990; Mead, Citation1999; Joachim, Citation2004). Cryptosporidia have been reported in birds of different orders, such as Galliformes, Columbiformes, Passeriformes, Struthioniformes, Falconiformes and Anseriformes, in which they may induce respiratory and intestinal diseases (Lindsay et al., Citation1987, Citation1990; Sreter & Varga, Citation2000; van Zeeland et al., Citation2008; Molina-Lopez et al., Citation2010). Sinusitis has been reported as a consequence of cryptosporidial infection of the upper respiratory tract of turkeys (Glisson et al., Citation1984), and the nasal cavity was one of several tissues, including the trachea and bronchi and bursa of Fabricius, that was affected in cryptosporidiosis of quail (Coturnix coturnix) (Tham et al., Citation1982). In addition to the bursa of Fabricius, intestines and cloaca, cryptosporidia were found, albeit to a lesser extent, in tissue scrapings of the conjunctiva, infraorbital sinus, trachea and lung from naturally infected ducks and geese (Richter et al., Citation1994). High prevalences of cryptosporidia were reported in conjunctival epithelia of young geese (Anser anser f. domestica) with mild epithelial hyperplasia and focal infiltration of adjacent lamina propria by lymphocytes and heterophils (Chvala et al., Citation2006). Cryptosporidium baileyi was associated with upper respiratory tract infections in three captive mixed-breed falcons (van Zeeland et al., Citation2008) and with ocular and nasal lesions in otus owls (Otus scops) in a rehabilitation facility (Molina-Lopez et al., Citation2010).
Lymphoplasmacytic inflammation with follicular lymphoid hyperplasia and epithelial hyperplasia in conjunctivae, and also nasal turbinates and trachea, is typical of M. gallisepticum infection in house finches (Luttrell et al., Citation1996, Citation1998). This pattern of response has been reproduced experimentally in domestic canaries (Serinus canaria domestica) as a model of natural M. gallisepticum infection in house finches and the resultant disease in wild passerine species (Hawley et al., Citation2011). Ocular and circumocular lesions associated with M. sturni isolation in a European starling differed from those of M. gallisepticum infection in house finches, however, by an inflammatory infiltrate with heterophils and by ulceration of conjunctival epithelium, which was interpreted as representing an acute or sub-acute condition (Frasca et al., Citation1997). Since its original identification (Forsyth et al., Citation1996), M. sturni has been identified in a modest number of wild bird species with and without gross lesions of conjunctivitis (Ley et al., Citation1998; Wellehan et al., Citation2001; Pennycott et al., Citation2005); however, histopathologic description of the chronic condition associated with isolation and identification of M. sturni has not been obtained. Additionally, an isolate of M. sturni did not produce conjunctivitis in experimentally inoculated house finches (Ley et al., Citation2010), indicating an inability of some strains of M. sturni to generate the lymphoid and epithelial lesions observed routinely in M. gallisepticum infection of certain, key, passerine species. Infection with Cryptosporidium spp. in the respiratory tract of chickens, turkeys and quail can account for varying degrees of epithelial hyperplasia and inflammatory infiltrates of lymphocytes, macrophages and heterophils in the lamina propria, similar to that observed in mycoplasmal infections (Tham, et al., Citation1982; Glisson, et al., Citation1984; Fletcher et al., Citation2008). The complicating issue in these cliff swallows is concurrent infection by M. sturni and a species of Cryptosporidium. Consequently, the causality of the lesions seen in these specimens may be more complicated than mycoplasmosis or cryptosporidiosis alone. Concurrent mycoplasmal and cryptosporidial infection of an avian host is not unique to this case. M. gallisepticum and Cryptosporidium spp. infections have been reported previously in Japanese quail (C. coturnix japonica), wherein inflammation accompanied by follicular lymphoid hyperplasia was reported in oculofacial respiratory tissues (Murakami et al., Citation2002), in a presentation similar to that of the cliff swallows in the present report.
The clinical history suggests that CLSW-4 was the index case and source of infection(s) for the other three birds at Lindsay Wildlife Museum, perhaps from contact with carriers of M. sturni and/or Cryptosporidium spp. such as European starlings and English house sparrows (Lindsay Wildlife Museum has a policy of not rehabilitating introduced species). During the course of their care and treatment, the swallows displayed signs of increased respiratory effort. There was evidence of rhinitis and sinusitis, which could have reduced to some degree the flow of air through the upper respiratory passages; however, we do not know whether additional lesions were present in the tracheas, lungs and air sacs, since only the heads of each swallow were submitted for histopathological examination, although there were no gross lesions in these tissues.
While it is possible that one dose of prednisone may have precipitated the worsening of clinical signs, or may have facilitated or exacerbated the concurrent cryptosporidial infection, the swallows in this case did receive oral erythromycin for 14 days. A macrolide antibiotic was used in the successful treatment of owls with C. baileyi infection (Molina-Lopez et al., Citation2010), and macrolide antibiotics have been used in the treatment of Cryptosporidium spp. infections of the intestine of humans with some positive effects (Uip et al., Citation1998). Nevertheless, clinical signs eventually worsened in these swallows despite erythromycin treatment. One possible reason for the poor response to antimicrobial therapy in this case could have been resistance on the part of the Cryptosporidium sp., leading to incomplete clearance and then resurgence after steroid administration.
The individual or combined roles that M. sturni and Cryptosporidium spp. infections played in the development and severity of disease in these swallows, and the impacts of these infections on cliff swallow abundance, require further study. Identification of both infections in these cliff swallows with gross lesions comparable with those of conjunctivitis caused by M. gallisepticum underscores the need to obtain histopathological correlates for gross and microbiological findings when documenting the occurrence of Mycoplasma spp. infections in novel hosts. These relationships and the resulting disease caused by either or both of these agents could be complex, and also potentially have a notable impact, particularly as emerging infections in a colonial species that may not yet have adapted to them.
Acknowledgements
The present work was funded through NSF-EF Grant number 0622705 to A. Dhondt under the NSF-NIH Ecology of Infectious Diseases programme. The authors thank the hospital staff at Lindsay Wildlife Museum Hospital for care of the swallows while in rehabilitation, especially homecare by Patricia Orlowski. Technical assistance from Sile Huyan and Judith McLaren is greatly appreciated. The authors thank Laura Monarski for assistance with grossing of specimens and Alexandra Young for digital artistry. Thanks to Arnaud Van Wettere for critical review of the manuscript, and Arnaud Van Wettere and Melissa West for assistance with DNA sequence analyses.
References
- Altizer , S. , Hochachka , W.M. and Dhondt , A.A. 2004 . Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches . Journal of Animal Ecology , 73 : 309 – 322 .
- Altschul , S.F. , Gish , W. , Miller , W. , Myers , E.W. and Lipman , D.J. 1990 . Basic local alignment search tool . Journal of Molecular Biology , 215 : 403 – 410 .
- Brown , C.R. and Brown , M.B. 1986 . Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota) . Ecology , 67 : 1206 – 1218 .
- Chvala , S. , Fragner , K. , Hackl , R. , Hess , M. and Weissenbock , H. 2006 . Cryptosporidium infection in domestic geese (Anser anser f. domestica) detected by in-situ hybridization . Journal of Comparative Pathology , 134 : 211 – 218 .
- Dhondt , A.A. , Tessaglia , D.L. and Slothower , R.L. 1998 . Epidemic mycoplasmal conjunctivitis in house finches from eastern North America . Journal of Wildlife Diseases , 34 : 265 – 280 .
- Fischer , J.R. , Stallknecht , D.E. , Luttrell , P. , Dhondt , A.A. and Converse , K.A. 1997 . Mycoplasmal conjunctivitis in wild songbirds: the spread of a new contagious disease in a mobile host population . Emerging Infectious Diseases , 3 : 69 – 72 .
- Fletcher , O.J. , Abdul-Aziz , T. and Barnes , H.J. 2008 . “ Respiratory system ” . In Avian Histopathology , 3rd edn , Edited by: Fletcher , O.J. 128 – 163 . Jacksonville , FL : American Association of Avian Pathologists .
- Forsyth , M.H. , Tully , J.G. , Gorton , T.S. , Hinckley , L. , Frasca , S. Jr , van Kruiningen , H.J. and Geary , S.J. 1996 . Mycoplasma sturni sp. nov., from the conjunctiva of a European starling (Sturnus vulgaris) . International Journal of Systematic Bacteriology , 46 : 716 – 719 .
- Frasca , S. Jr , Hinckley , L. , Forsyth , M. H. , Gorton , T.S. , Geary , S.J. and Van Kruiningen , H.J. 1997 . Mycoplasmal conjunctivitis in a European starling . Journal of Wildlife Diseases , 33 : 336 – 339 .
- Glisson , J.R. , Brown , T.P. , Brugh , M. , Page , R.K. , Kleven , S.H. and Davis , R.B. 1984 . Sinusitis in turkeys associated with respiratory cryptosporidiosis . Avian Diseases , 28 : 783 – 790 .
- Hartup , B.K. , Bickal , J.M. , Dhondt , A.A. , Ley , D.H. and Kollias , G.V. 2001a . Dynamics of conjunctivitis and Mycoplasma gallisepticum infections in house finches . The Auk , 118 : 327 – 333 .
- Hartup , B.K. , Dhondt , A A. , Sydenstricker , K.V. , Hochachka , W.M. and Kollias , G.V. 2001b . Host range and dynamics of mycoplasmal conjunctivitis among birds in North America . Journal of Wildlife Diseases , 37 : 72 – 81 .
- Hartup , B.K. , Kollias , G.V. and Ley , D.H. 2000 . Mycoplasmal conjunctivitis in songbirds from New York . Journal of Wildlife Diseases , 36 : 257 – 264 .
- Hawley , D.M. , Grodio , J. , Frasca , S. , Kirkpatrick , L. and Ley , D.H. 2011 . Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease . Avian Pathology , 40 : 321 – 327 .
- Hochachka , W.M. and Dhondt , A.A. 2000 . Density-dependent decline of host abundance resulting from a new infectious disease . Proceedings of the National Acadamy of Sciences of the USA , 97 : 5303 – 5306 .
- Hong , Y. , Garcia , M. , Levisohn , S. , Savelkoul , P. , Leiting , V. , Lysnyansky , I. , Ley , D.H. and Kleven , S.H. 2005 . Differentiation of Mycoplasma gallisepticum strains using amplified fragment length polymorphism and other DNA-based typing methods . Avian Diseases , 49 : 43 – 49 .
- Jervis , H.R. , Merrill , T.G. and Sprinz , H. 1966 . Coccidiosis in the guinea pig small intestine due to a Cryptosporidium . American Journal of Veterinary Research , 27 : 408 – 414 .
- Joachim , A. 2004 . Is Cryptosporidium a zoonotic agent? . Wiener Klinische Wochenschrift, 116 Supplement , 4 : 2 – 6 .
- Kleven , S.H. 2008 . “ Mycoplasmosis ” . In A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens , 5th edn , Edited by: Dufour-Zavala , L. 59 – 64 . Athens , GA : American Association of Avian Pathologists .
- Leiting , V.A. and Kleven , S.H. 2000 . Preparation of a heterogeneous conjugate to detect Mycoplasma iowae by immunofluorescence . Avian Diseases , 44 : 697 – 700 .
- Ley , D.H. , Anderson , N. , Dhondt , K.V. and Dhondt , A.A. 2010 . Mycoplasma sturni from a California house finch with conjunctivitis did not cause disease in experimentally infected house finches . Journal of Wildlife Diseases , 46 : 994 – 999 .
- Ley , D.H. , Berkhoff , J.E. and Levisohn , S. 1997 . Molecular epidemiologic investigations of Mycoplasma gallisepticum conjunctivitis in songbirds by random amplified polymorphic DNA analyses . Emerging Infectious Diseases , 3 : 375 – 380 .
- Ley , D.H. , Berkhoff , J.E. and McLaren , J.M. 1996 . Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis . Avian Diseases , 40 : 480 – 483 .
- Ley , D.H. , Geary , S.J. , Berkhoff , J.E. , McLaren , J.M. and Levisohn , S. 1998 . Mycoplasma sturni from blue jays and northern mockingbirds with conjunctivitis in Florida . Journal of Wildlife Diseases , 34 : 403 – 406 .
- Lierz , M. , Hagen , N. , Harcourt-Brown , N. , Hernandez-Divers , S.J. , Luschow , D. and Hafez , H.M. 2007 . Prevalence of mycoplasmas in eggs from birds of prey using culture and a genus-specific mycoplasma polymerase chain reaction . Avian Pathology , 36 : 145 – 150 .
- Lindsay , D.S. , Blagburn , B.L. and Hoerr , F.J. 1990 . Small intestinal cryptosporidiosis in cockatiels associated with Cryptosporidium baileyi-like oocysts . Avian Diseases , 34 : 791 – 793 .
- Lindsay , D.S. , Blagburn , B.L. , Hoerr , F.J. and Giambrone , J.J. 1987 . Experimental Cryptosporidium baileyi infections in chickens and turkeys produced by ocular inoculation of oocysts . Avian Diseases , 31 : 355 – 357 .
- Luttrell , M.P. , Fischer , J.R. , Stallknecht , D.E. and Kleven , S.H. 1996 . Field investigation of Mycoplasma gallisepticum infections in house finches (Carpodacus mexicanus) from Maryland and Georgia . Avian Diseases , 40 : 335 – 341 .
- Luttrell , M.P. , Stallknecht , D.E. , Fischer , J.R. , Sewell , C.T. and Kleven , S.H. 1998 . Natural Mycoplasma gallisepticum infection in a captive flock of house finches . Journal of Wildlife Diseases , 34 : 289 – 296 .
- Mead , J.R. 1999 . Recent trends in Cryptosporidium research: workshop summary . Journal of Eukaryotic Microbiology , 46 : 38S – 39S .
- Mikaelian , I. , Ley , D.H. , Claveau , R. , Lemieux , M. and Berube , J.P. 2001 . Mycoplasmosis in evening and pine grosbeaks with conjunctivitis in Quebec . Journal of Wildlife Diseases , 37 : 826 – 830 .
- Molina-Lopez , R.A. , Ramis , A. , Martin-Vazquez , S. , Gomez-Couso , H. , Ares-Mazas , E. , Caccio , S.M. , Leiva , M. and Darwich , L. 2010 . Cryptosporidium baileyi infection associated with an outbreak of ocular and respiratory disease in otus owls (Otus scops) in a rehabilitation centre . Avian Pathology , 39 : 171 – 176 .
- Murakami , S. , Miyama , M. , Ogawa , A. , Shimada , J. and Nakane , T. 2002 . Occurrence of conjunctivitis, sinusitis and upper region tracheitis in Japanese quail (Coturnix coturnix japonica), possibly caused by Mycoplasma gallisepticum accompanied by Cryptosporidium sp. infection . Avian Pathology , 31 : 363 – 370 .
- Pennycott , T.W. , Dare , C.M. , Yavari , C.A. and Bradbury , J.M. 2005 . Mycoplasma sturni and Mycoplasma gallisepticum in wild birds in Scotland . Veterinary Record , 156 : 513 – 515 .
- Rehg , J.E. , Lawton , G.W. and Pakes , S.P. 1979 . Cryptosporidium cuniculus in the rabbit (Oryctolagus cuniculus) . Laboratory Animal Science , 29 : 656 – 660 .
- Richter , D. , Wiegand-Tripp , G. , Burkhardt , E. and Kaleta , E.F. 1994 . Natural infections by Cryptosporidium sp. in farm-raised ducks and geese . Avian Pathology , 23 : 277 – 286 .
- Sreter , T. and Varga , I. 2000 . Cryptosporidiosis in birds-a review . Veterinary Parasitology , 87 : 261 – 279 .
- Tham , V.L. , Kniesberg , S. and Dixon , B.R. 1982 . Cryptosporidiosis in quails . Avian Pathology , 11 : 619 – 626 .
- Tzipori , S. and Campbell , I. 1981 . Prevalence of Cryptosporidium antibodies in 10 animal species . Journal of Clinical Microbiology , 14 : 455 – 456 .
- Uip , D.E. , Lima , A.L.L. , Amato , V.S. , Boulos , M. , Neto , V.A. & Bem David , D. 1998 . Roxithromycin treatment for diarrhoea caused by Cryptosporidium spp. in patients with AIDS . Journal of Antimicrobial Chemotherapy , 41 Suppl B , 93 – 97 .
- Ungar , B.L. 1990 . Enzyme-linked immunoassay for detection of Cryptosporidium antigens in fecal specimens . Journal of Clinical Microbiology , 28 : 2491 – 2495 .
- van Zeeland , Y.R. , Schoemaker , N.J. , Kik , M.J. and van der Giessend , J.W. 2008 . Upper respiratory tract infection caused by Cryptosporidium baileyi in three mixed-bred falcons (Falco rusticolus x Falco cherrug) . Avian Diseases , 52 : 357 – 363 .
- Wellehan , J.F. , Calsamiglia , M. , Ley , D.H. , Zens , M.S. , Amonsin , A. and Kapur , V. 2001 . Mycoplasmosis in captive crows and robins from Minnesota . Journal of Wildlife Diseases , 37 : 547 – 555 .