Abstract
Several outbreaks of Riemerella anatipestifer in commercial geese occurred within a short time period. A serious disease was recognized in the affected birds, mainly characterized by depression and severe neurologic disturbances. The morbidity ranged from 20 to 30% and the mortality from 5 to 20%. Generally, the clinical signs started at the age of 8 to 10 days. Post-mortem examination revealed fibrinous pericarditis, perihepatitis and airsacculitis in all birds. Some of the birds also had synovitis of the tibio-tarsal joints and oedematous swelling of the subcutaneous tissues around these joints and metatarsus. Histology revealed a characteristic severe inflammation with heterophilic granulocytes in different organs. Bacteriological investigation was made from several organs and R. anatipestifer could be isolated from all birds investigated. The identification of these clinical isolates, done for the first time by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry, confirmed the aetiology. Sequence analysis showed 100% similarity between the clinical isolates, indicating a common source of infection.
Introduction
Riemer (Citation1904) described for the first time an epizootic infectious disease in geese which was named “septicemia anserum exsudativa”. The causative agent of this disease is Riemerella anatipestifer, a non-motile Gram-negative and non-spore-forming rod, belonging to the rRNA superfamily V (Hendrickson & Hilbert, Citation1932; Segers et al., Citation1993). Since then, however, outbreaks have been nearly exclusively reported from domestic ducks and turkeys (Graham et al., Citation1938; Zehr & Ostendorf, Citation1970; Helfer & Helmboldt, Citation1977; Ziedler et al., Citation1984; Smith et al., Citation1987; Cooper, Citation1989; Leavitt & Ayroud, Citation1997; Yu et al., Citation2008; Fulton & Rimler, Citation2010). Furthermore, R. anatipestifer was found as a pathogen in wild birds, especially waterfowl (Bruner et al., Citation1970; Karstad et al., Citation1970; Munday et al., Citation1970; Eleazer et al., Citation1973; Wobeser & Ward, Citation1974; Wyffels & Hommez, Citation1990). Finally, some authors stated that R. anatipestifer is not a primary pathogen in geese (Pierce & Vorhies, Citation1973). These facts were leading to the impression that Riemerella infections in geese are rare and if occurring they are of minor economic importance (Sandhu, Citation2003).
In ducks, turkeys and wild birds, clinical signs of an infection as well as pathological lesions are well described. Ante-mortem signs in these bird species include mainly nasal discharge, dyspnoea, swollen sinuses, neurologic disturbances and diarrhoea (Jortner et al., Citation1969; Leibovitz, Citation1972; Sandhu, Citation2003). The most obvious gross lesion reported is fibrinous exudate involving serosal surfaces in general (Sandhu, Citation2003). Additionally, these bacteria could also be found in the upper respiratory tract of clinically healthy birds (Donahue & Olson, Citation1969; Ryll et al., Citation2001).
Isolation and identification of R. anatipestifer are crucial for a definitive diagnosis, but special growth requirements for initial isolation as well as phenotypic diversities of strains could hamper the diagnosis (Leibovitz, Citation1972; Hinz et al., Citation1998). Furthermore, Pickrell (Citation1966) showed that the isolation rate is also dependent on the phase of disease. The present investigation reports several outbreaks of R. anatipestifer in commercial goose flocks, including clinical signs, gross pathological and microscopic lesions. Furthermore, for the first time, clinical isolates of R. anatipestifer were identified by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS).
Materials and Methods
Case history and clinical signs
From June to July 2012 a total of seven submissions of domestic geese were received by the Clinic for Avian, Reptile and Fish Medicine for post-mortem investigations (). In all cases the same clinical signs of affected birds were reported: depression and severe neurologic disturbances, namely ataxia, birds lying on their sides and unable to stand up, paddling movements of feet and retrocollis. The morbidity ranged from 20 to 30% and mortality from 5 to 20%. Generally, the clinical signs started at the age of 8 to 10 days. It was documented that the birds originated from the same parent flock and the same hatchery.
Table 1. Submissions of live domestic geese and carcasses for necropsy in June and July 2012.
Necropsy
All birds (n=20) were necropsied in a routine manner. Gross pathological lesions were recorded, and organ samples were taken for histological investigation and polymerase chain reaction (PCR).
Bacteriology
Swabs for bacteriological investigation were taken aseptically from the heart, liver, intestine, brain, air sacs, lungs, hock joints and cutaneous oedema (metatarsus) of diseased birds. The samples were streaked out directly on Columbia (COS) agar containing 5% sheep blood (BioMeriéux, Vienna, Austria), McConkey agar (Bertoni, Vienna, Austria), Schaedler (SCS) agar containing 5% sheep blood (BioMeriéux) and Sabouraud–Gentamicin–Chloramphenicol (SGC2) agar (BioMeriéux). From each organ sample, two COS agar plates were inoculated: one was incubated aerobically at 37°C for 24 h, the other one microaerobically (Genbox microaer; BioMeriéux). The inoculated McConkey agars were incubated aerobically at 37°C, SCS agars anaerobically (Genbox anaer; BioMeriéux) at 37°C and SGC2 agars aerobically at 42°C. Cotton-wool swabs were obtained from the pharyngeal and nasal mucous membranes, respectively, of 50 breeder birds, and were transported in Portagerm® Amies Agar (BioMeriéux) to the laboratory. These swabs were smeared on COS agar plates that were incubated at 37°C, microaerobically for 24 h.
Preliminary identification of Riemerella strains was based on phenotypic characteristics (growth, shape, motility), Gram staining, oxidase and catalase reactions.
Histology
Samples from the livers, air sacs, hearts, brains and cutaneous oedema were fixed for at least 24 h in 3.5% neutral buffered formalin and thereinafter embedded in paraffin. Tissue slices of 4 µm thickness were prepared using the microtome Microm HM 360 (Microm Laborgeräte GmbH, Walldorf, Germany) and mounted on glass slides. Dewaxing and dehydration of the tissues was performed followed by routine staining using haematoxylin and eosin.
Detection of viral DNA and RNA by PCR and DNA sequence analysis of bacteria
DNA from liver samples and bacterial strains was prepared using the DNeasy Blood and Tissue Kit (Qiagen, Vienna, Austria) according to the manufacturer's instructions. For the detection of goose parvovirus, the primers previously described by Limn et al. (Citation1996) were used. PCR was carried out in 25 µl reaction mixture consisting of 1× PCR buffer (Invitrogen, Vienna, Austria) supplemented by 1.5 mM MgCl2 (Invitrogen), 0.2 mM dNTP mix (Invitrogen), 1.0 µl of each primer (primers were used in 10 pmol/µl concentration), 1.5 u Taq DNA Polymerase (Invitrogen) and 5.0 µl prepared DNA as template. The reaction mixture was subjected to an initial denaturation at 94°C for 4 min, followed by 40 cycles of 94°C for 1 min, 48°C for 1 min and 72°C for 1.30 min. Thereafter, the samples were maintained at 72°C for 4 min for the final extension step. PCR amplification of the ompA gene was done from one isolate per each submission number according to the protocol of Yu et al. (Citation2008). The resulting fragments were analysed by 1.5% agarose gel electrophoresis and staining with ethidium bromide (Sambrook et al., Citation1989). OmpA PCR products were excised from the gel and purified by the QIAquick Gel Extraction Kit (Qiagen). Direct fluorescence-based sequencing was performed by LGC Genomics GmbH (Berlin, Germany). Assembly and analyses of sequences were performed with Accelrys Gene, version 2.5 (Accelrys, San Diego, USA) and Lasergene v7 (DNASTAR Inc., Madison, USA) software packages. GenBank database searches of obtained sequences were carried out with the BlastN search algorithm with default settings. The sequence of the ompA gene obtained in this study was deposited to the EMBL database under accession number HF563642. RNA from brain samples was extracted with the RNeasy kit (Qiagen) according to the manufacturer's instructions. The investigation for avian influenza virus was done according to the protocol of Spackman et al. (Citation2003) using Stratagene Mx3000P. For the detection of Newcastle disease virus (APMV-1) the protocol of Creelan et al. (Citation2002) was followed. PCR was carried out using the OneStep RT-PCR kit (Qiagen) according to the manufacturer's instructions. The resulting fragments were analysed by 2% agarose gel electrophoresis and staining with ethidium bromide (Sambrook et al., Citation1989).
Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry
Isolates that were used for ompA PCR were also investigated by MALDI-TOF MS. Sample preparation for MALDI-TOF MS using a Microflex LT instrument (Bruker Daltonic GmbH, Leipzig, Germany) was performed as previously described in detail (Alispahic et al., Citation2010). The parameter settings were as follows: 20.08 kV, IS2 16.77 kV, lens 7.03 kV, detector gain 1634 V. Then 240 laser shots in 40 shot steps (with a 60 Hz nitrogen laser from different positions of the target spot) were summarized and each spot was measured twice automatically with AutoXecute acquisition control software (Flex control 3.3; Bruker Daltonic). Bacterial acid soluble proteins were extracted using formic acid (70%) and acetonitrile according to the standard protocol from Bruker Daltonic. One microlitre of each bacterial extract was spotted four times onto the MALDI target plate and air dried. Afterwards, 2 µl matrix solution (α-cyano-4-hydroxycinnamic acid in 50% acetonitrile/2.5% trifluor acetic acid) were overlaid on each sample and dried again, and each spot was measured twice. All steps were performed at room temperature. The reference library from Bruker Daltonic was used to identify clinical isolates. The generated peak list was matched against this database using the integrated pattern-matching algorithm of MALDI Biotyper software version 3.0 (Bruker Daltonic GmbH).
Results
Necropsy
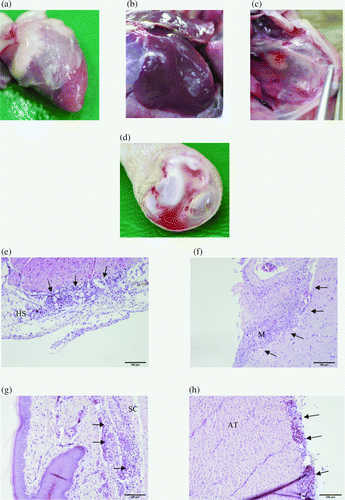
All 20 birds investigated showed mild to severe fibrinous pericarditis and perihepatitis (a,b). The most severely affected organs were the air sacs with airsacculitis, especially the thoracic air sacs that were filled with organized yellow casts (c). Five birds had serious haemorrhagic synovitis of the tibio-tarsal joints (d), and oedematous swelling of the subcutaneous tissues around these joints and metatarsus could be found. No gross pathological lesions were seen in the brains.
Figure 1. Most common gross pathological lesions and findings of histological examinations: fibrinous pericarditis (1a) and perihepatitis (1b) and airsacculitis of the thoracic air sacs filled with organized yellow casts (1c). 1d: Haemorrhagic synovitis of the tibio-tarsal joints. 1e: Heart sac (HS) showing infiltration of mononuclear cells and heterophilic granulocytes. 1f: Investigation of the brain (M, meninges) revealed meningitis characterized by the same inflammatory cells. The subcutis (SC) was predominantly infiltrated by heterophils (1g) and in the Achilles tendon (AT) mononuclear cells and heterophilic granulocytes were found (1h). The location of inflammatory cells is indicated by arrows.
Bacteriology
Colonies of R. anatipestifer were smooth and non-pigmented. They developed on COS agar within 24 h under aerobic conditions, but the growth was improved by microaerobic incubation. The cells were Gram-negative, non-motile rods. Oxidase and catalase reactions were positive. From all 20 birds Riemerellacould be isolated from the heart, air sacs and brain and, when affected, from tibio-tarsal joints and cutaneous oedema. Additionally, from 18 and 10 birds these bacteria could also be isolated from the liver and lung, respectively. From the intestine, R. anatipestifer could be cultivated only once. In one bird, beside R. anatipestifer, Aspergillus fumigatus was found in the lungs and air sacs. No other pathogenic bacteria were isolated from the birds. R. anatipestifer could not be isolated from pharyngeal and nasal swabs taken from the parent flock.
Histology
Severe inflammation with heterophilic granulocytes and mononuclear cells were found in the livers, air sacs, hearts (e) and brains (f). In the case of cutaneous oedema, infiltration of mostly heterophils could be found in the subcutis (g) with involvement of the Achilles tendon (h). Bacteria could not be located by histology.
PCR and sequence analysis
None of the organ samples investigated was positive for goose parvovirus, avian influenza virus or Newcastle disease virus. From all Riemerella isolates a product of 1119 base pairs of the ompA gene was amplified. The sequence analysis demonstrated that all Riemerella isolates were 100% identical. Furthermore, the database search indicated association with the genotype I as defined by Yu et al. (Citation2008).
Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry
Six field isolates investigated were identified as R. anatipestifer based on the reference database. Identification of one isolate (submission number 7) was not reliable due to a low log(score). The log(scores) are presented in .
Table 2. Log(scores) of field isolates based on the Bruker Daltonic database.
Discussion
This case report documents several outbreaks of an infection caused by R. anatipestifer in commercial goose flocks. The clinical picture was mainly characterized by depression and severe neurologic disturbances, signs also reported in ducks, turkeys and wild birds infected with R. anatipestifer (Jortner et al., Citation1969; Karstad et al., Citation1970; Leibovitz, Citation1972; Eleazer et al., Citation1973; Frommer et al., Citation1990). Diarrhoea, a common clinical sign in ducks (Pickrell, Citation1966), was not seen in the affected geese. Morbidity and mortality ranged from 20 to 30% and from 5 to 20%, respectively. For ducks, reported mortalities varied from 5 to 75% and morbidity was usually higher (Sandhu, Citation2003), whereas in turkeys lower morbidity and mortality rates—namely up to 5% and 1%, respectively—were found (Frommer et al., Citation1990). As expected from a classical severe infection in young birds the signs started at the age of around 8 to 10 days. This is in agreement with the findings in ducks, which are reported to be highly susceptible at 1 to 8 weeks of age (Sandhu, Citation2003), whereas turkeys were affected at 10 to 13 weeks of age (Zehr & Ostendorf, Citation1970; Frommer et al., Citation1990).
The main gross pathological findings were fibrinous pericarditis, perihepatitis and airsacculitis, characteristic lesions that were also found in other birds (Jortner et al., Citation1969; Karstad et al., Citation1970; Zehr & Ostendorf, Citation1970; Leibovitz, Citation1972; Smith et al., Citation1987; Leavitt & Ayroud, Citation1997). Other common but variable lesions in the affected geese were haemorrhagic synovitis of tibio-tarsal joints and subcutaneous oedema around the metatarsus. Such lesions were also demonstrated in intravenously infected turkeys and naturally infected wild waterfowl (Karstad et al., Citation1970; Cooper & Charlton, Citation1992). Asplin (Citation1955) and Leibovitz (Citation1972) stated that an important route of infection of R. anatipestifer might be wounds of the skin, particularly of the feet. In the actual cases in which joints and feet were affected two birds had minor foot pad lesions, but in all other birds no obvious skin lesions were found. However, the respiratory tract is also seen as a main route of infection (Leibovitz, Citation1972), which might be the route of entrance in the present cases due to severe airsacculitis in all birds investigated.
Actual reports and data of outbreaks of R. anatipestifer infection in geese are not available. This lack of information might be due to the fact that R. anatipestifer is difficult to isolate from infected birds, particularly if they are past the acute stage of the disease as was shown in ducks (Pickrell, Citation1966). Therefore, it is recommended that samples from several organs need to be cultured and that the initial isolation should be done under microaerobic conditions (Leibovitz, Citation1972). This approach usually goes beyond routine diagnostics. In the present investigation, R. anatipestifer could be isolated from all birds from the heart, air sacs and brain, and when affected from tibio-tarsal joints and cutaneous oedema. The isolates developed also under aerobic conditions but the colonies were much smaller than the colonies grown microaerobically. Such small colonies can easily be missed. Furthermore, identification of R. anatipestifer faces several hindrances: traditional methods are often inconclusive because of inconsistent reactions and phenotypic diversity (Hinz et al., Citation1998); and published PCR tests so far are not specific for this species (Christensen & Bisgaard, Citation2010). Therefore, genetic methods such as gene sequencing or protein profiling should be used (Rubbenstroth et al., Citation2012). Protein profiling by means of mass spectrometry has been shown already to be a fast and reliable method for the identification of well-described Riemerella reference strains (Rubbenstroth et al., Citation2011, Citation2012). Hence, in the present investigation MALDI-TOF MS was used for the first time to identify clinical isolates of R. anatipestifer, and six strains could be identified to species level. Anyhow, in case of one isolate only genus identification was possible. Several serotypes of these bacteria are known (Brogden et al., Citation1982; Sandhu & Leister, Citation1991) and Yu et al. (Citation2008) observed differences in R. anatipestifer genotypes and serotypes. The fact that the Bruker database contained only the type strain of R. anatipestifer (DSM 15868T) did not allow further description of individual isolates by MALDI-TOF MS. Therefore, in the future it will be necessary to include more reference strains in such databases.
All cases had in common that the birds originated from the same parent flock and the same hatchery. Additionally, the sequence analysis of ompA gene revealed a 100% similarity between the strains isolated from diseased birds, indicating a common source of infection. Glünder & Hinz (Citation1989) isolated R. anatipestifer from embryonated goose eggs, suggesting vertical transmission. But in such cases the rate of hatching was reduced, which was not the fact in the actual case report. It is also known that older geese do not contract clinical signs (Sandhu, Citation2003). However, the investigation of samples from the parent flock remained negative for R. anatipestifer. In addition to geese the hatchery also hatches ducks. Interestingly, the disease in goslings occurred only on farms to which the birds were transported with trucks that were also used for the delivery of ducklings. Ryll et al. (Citation2001) isolated R. anatipestifer from the upper respiratory tract of clinically healthy ducklings, and Riemer (Citation1904) found differences in the pathogenicity when geese and ducks were infected with the same Riemerella strain. In the present study no outbreak of the disease was reported from ducks hatched at the same time as the diseased geese. This might suggest that the ducks were asymptomatic carriers of this pathogen. For future investigation, sampling of ducklings will be advisable and necessary with regard to epidemiology.
The present investigation reports several outbreaks of R. anatipestifer infections in commercial geese. In contrast to the generally held view, the actual data demonstrate that these bacteria are able to induce a serious disease in geese, similar to clinical and post-mortem findings seen in other bird species. Furthermore, more than 100 years after the first description by Riemer (Citation1904), the actual bacteriological and histological data confirm his conclusions that R. anatipestifer is able to induce septicaemia in geese.
Acknowledgements
Excellent technical assistance was contributed by Patricia Wernsdorf and Silvia Aufischer-Gritsch. This work was partly done within the CEPO (Centre of Excellence for Poultry) project, funded by the European Regional Development Fund, Cross-border Cooperation Programme Austria-Hungary 2007–2013.
References
- Alispahic , M. , Hummel , K. , Jandreski-Cvetkovic , D. , Nöbauer , K. , Razzazi-Fazeli , E. , Hess , M. and Hess , C. 2010 . Species-specific identification and differentiation of Arcobacter, Helicobacter and Campylobacter by full-spectral matrix-associated laser desorption/ionization time of flight mass spectrometry . Journal of Medical Microbiology , 59 : 295 – 301 . doi: 10.1099/jmm.0.016576-0
- Asplin , F.D. 1955 . A septicaemic disease of ducklings . The Veterinary Record , 67 : 854 – 858 .
- Brogden , K.A. , Rhoades , K.R. and Rimler , R.B. 1982 . Serologic types and physiologic characteristics of 46 avian Pasteurella anatipestifer cultures . Avian Diseases , 26 : 891 – 896 . doi: 10.2307/1589877
- Bruner , D.W. , Angstrom , C.I. and Price , J.I. 1970 . Pasteurella anatipestifer infection in pheasants. A case report . Cornell Veterinarian Journal , 60 : 491 – 494 .
- Christensen , H. and Bisgaard , M. 2010 . Phylogenetic relationships of Riemerella anatipestifer serovars and related taxa and an evaluation of specific PCR tests reported for R. anatipestifer . Journal of Applied Microbiology , 108 : 1612 – 1619 . doi: 10.1111/j.1365-2672.2009.04558.x
- Cooper , G.L. 1989 . Pasteurella anatipestifer infections in California turkey flocks: circumstantial evidence of a mosquito vector . Avian Diseases , 33 : 809 – 815 . doi: 10.2307/1591165
- Cooper , G.L. and Charlton , B.R. 1992 . Spondylitis in turkeys associated with experimental Pasteurella anatipestifer infection . Avian Diseases , 36 : 290 – 295 . doi: 10.2307/1591503
- Creelan , J.L. , Graham , D.A. and McCullough , S.J. 2002 . Detection and differentiation of pathogenicity of avian paramyxovirus serotype 1 from field cases using one-step reverse transcriptase-polymerase chain reaction . Avian Pathology , 31 : 493 – 499 . doi: 10.1080/0307945021000005860
- Donahue , J.M. and Olson , L.D. 1969 . Survey of wild ducks and geese for Pasteurella spp . Bulletin of Wildlife Disease Association , 5 : 201 – 205 .
- Eleazer , T.H. , Blalock , H.G. , Harrell , J.S. and Derieux , W.T. 1973 . Pasteurella anatipestifer as a cause of mortality in semiwild pen-raised mallard ducks in South Carolina . Avian Diseases , 17 : 855 – 857 . doi: 10.2307/1589055
- Frommer , A. , Bock , R. , Inbar , A. and Zemer , S. 1990 . Muscovy ducks as a source of Pasteurella anatipestifer infection in turkey flocks . Avian Pathology , 19 : 161 – 163 . doi: 10.1080/03079459008418664
- Fulton , R.M. and Rimler , R.B. 2010 . Epidemiologic investigation of Riemerella anatipestifer in a commercial duck company by serotyping and DNA fingerprinting . Avian Diseases , 54 : 969 – 972 . doi: 10.1637/9087-092409-Case.1
- Glünder , G. and Hinz , K.H. 1989 . Isolation of Moraxella anatipestifer from embryonated goose eggs . Avian Pathology , 18 : 351 – 355 . doi: 10.1080/03079458908418608
- Graham , R. , Brandly , C.A. and Dunlap , G.L. 1938 . Studies on duck septicemia . Cornell Veterinarian , 28 : 1 – 8 .
- Helfer , D.H. and Helmboldt , C.F. 1977 . Pasteurella anatipestifer infection in turkeys . Avian Diseases , 21 : 712 – 715 . doi: 10.2307/1589432
- Hendrickson , J.M. and Hilbert , K.F. 1932 . A new and serious septicemic disease in young ducks with a description of the causative organism, Pfeifferella anatipestifer, N.S . Cornell Veterinarian , 22 : 239 – 252 .
- Hinz , K.H. , Ryll , M. , Köhler , B. and Glünder , G. 1998 . Phenotypic characteristics of Riemerella anatipestifer and similar micro-organisms from various hosts . Avian Pathology , 27 : 33 – 42 . doi: 10.1080/03079459808419272
- Jortner , B.S. , Porro , R. and Leibovitz , L. 1969 . Central-nervous system lesions of spontaneous Pasteurella anatipestifer infection in ducklings . Avian Diseases , 13 : 27 – 35 . doi: 10.2307/1588411
- Karstad , L. , Lusis , P. and Long , J.R. 1970 . Pasteurella anatipestifer as a cause of mortality in captive wild waterfowl . Journal of Wildlife Diseases , 6 : 408 – 413 .
- Leavitt , S. and Ayroud , M. 1997 . Riemerella anatipestifer infection of domestic ducklings . Canadian Veterinarian Journal , 38 : 113
- Leibovitz , L. 1972 . A survey of the so-called “anatipestifer syndrome” . Avian Diseases , 16 : 836 – 851 . doi: 10.2307/1588765
- Limn , C.K. , Yamada , T. , Nakamura , M. and Takehara , K. 1996 . Detection of Goose parvovirus genome by polymerase chain reaction: distribution of Goose parvovirus in Muscovy ducklings . Virus Research , 42 : 167 – 172 . doi: 10.1016/0168-1702(95)01310-5
- Munday , B.L. , Corbould , A. , Heddleston , K.L. and Harry , E.G. 1970 . Isolation of Pasteurella anatipestifer from black swan (Cygnus artratus) . Australian Veterinary Journal , 46 : 322 – 325 . doi: 10.1111/j.1751-0813.1970.tb07910.x
- Pickrell , J.A. 1966 . Pathologic changes associated with experimental Pasteurella anatipestifer infection in ducklings . Avian Diseases , 10 : 281 – 288 . doi: 10.2307/1588273
- Pierce , R.L. and Vorhies , M.W. 1973 . Pasteurella anatipestifer infection in geese . Avian Diseases , 17 : 868 – 870 . doi: 10.2307/1589058
- Riemer . 1904 . Kurze Mitteilung über eine bei Gänsen beobachtete exsudative Septikämie und deren Erreger [Short communication on the occurrence of septicemia anserum exsudativa in geese and its causative agent.] . Zentralblatt der Bakteriologischen Abteilung 1 , 37 , 641 – 648 .
- Rubbenstroth , D. , Hotzel , H. , Knobloch , J. , Teske , L. , Rautenschlein , S. and Ryll , M. 2011 . Isolation and characterization of atypical Riemerella columbina strains from pigeons and their differentiation from Riemerella anatipestifer . Veterinary Microbiology , 147 : 103 – 112 . doi: 10.1016/j.vetmic.2010.06.008
- Rubbenstroth , D. , Ryll , M. , Hotzel , H. , Christensen , H. , Knobloch , J.K. , Rautenschlein , S. & Bisgaard , M. 2012 . Description of Riemerella columbipharyngis sp. nov., isolated from the pharynx of healthy domestic pigeons (Columba livia f. domestica), and emended description of the genus Riemerella, Riemerella anatipestifer and Riemerella columbina . International Journal of Systematic and Evolutionary Microbiology , 280 – 297 .
- Ryll , M. , Christensen , H. , Bisgaard , M. , Christensen , J.P. , Hinz , K.H. and Köhler , B. 2001 . Studies on the prevalence of Riemerella anatipestifer in the upper respiratory tract of clinically healthy ducklings and characterization of untypable stains . Journal of Veterinary Medicine Series B , 48 : 537 – 546 . doi: 10.1046/j.1439-0450.2001.00471.x
- Sambrook , J. , Fritsch , E.F. & Maniatis , T. 1989 . Commonly used techniques in molecular cloning . In J. Sambrook D.W. Russell Molecular Cloning. A Laboratory Manual , 2nd ed E3 – E4 . New York , NY : Cold Spring Harbor Laboratory Press .
- Sandhu , T.S. 2003 . “ Riemerella anatipestifer infection ” . In Diseases of Poultry , 12th ed. , Edited by: Saif , Y.M. , Aly , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 758 – 764 . Ames : Iowa State Press .
- Sandhu , T.S. and Leister , M.L. 1991 . Serotypes of “Pasteurella” anatipestifer isolates from poultry in different countries . Avian Pathology , 20 : 233 – 239 . doi: 10.1080/03079459108418760
- Segers , P. , Mannheim , W. , Vancanneyt , M. , De Brandt , K. , Hinz , K.H. , Kersters , K. and Vandamme , P. 1993 . Riemerella anatipestifer gen. nov., com. nov., the causative agent of septicemia anserum exsudativa, and ist phylogenetic affiliation within the Flavobacterium-Cytophaga rRNA homology group . International Journal of Systematic Bacteriology , 43 : 768 – 776 . doi: 10.1099/00207713-43-4-768
- Smith , J.M. , Frame , D.D. , Cooper , G. , Bickford , A.A. , Yan Ghazikhanian , G. and Kelly , B.J. 1987 . Pasteurella anatipestifer infection in commercial meat-type turkeys in California . Avian Diseases , 31 : 913 – 917 . doi: 10.2307/1591053
- Spackman , E. , Senne , D.A. , Bulaga , L.L. , Myers , T.J. , Perdue , M.L. , Garber , L.P. , Lohman , K. , Daum , L.T. and Suarez , D.L. 2003 . Development of real-time RT-PCR for the detection of avian influenza virus . Avian Diseases , 47 : 1079 – 1082 . doi: 10.1637/0005-2086-47.s3.1079
- Wobeser , G. and Ward , G.E. 1974 . Pasteurella anatipestifer infection in migrating whistling swans . Journal of Wildlife Diseases , 10 : 466 – 470 .
- Wyffels , R. and Hommez , J. 1990 . Pasteurella anatipestifer isolated from respiratory lesions in partridges (Perdix perdix) in captivity . Vlaams Diergeneeskundig Tijdschrift , 59 : 105 – 106 .
- Yu , C.Y. , Liu , Y.W. , Chou , S.J. , Chao , M.R. , Wenig , B.C. , Tsay , J.G. , Chiu , C.H. , Wu , C.C. , Lin , T.L. , Cang , C.C. and Chu , C. 2008 . Genomic diversity and molecular differentiation of Riemerella anatipestifer associated with eight outbreaks in five farms . Avian Pathology , 37 : 273 – 279 . doi: 10.1080/03079450802056546
- Zehr , W.J. and Ostendorf , J. 1970 . Pasteurella anatipestifer in turkeys . Avian Diseases , 14 : 557 – 560 . doi: 10.2307/1588619
- Ziedler , K. , Heidrich , R. , Hanke , P. and Sobanski , E. 1984 . Zum Auftreten der Moraxella septicaemiae-Infektion bei Pekingenten und Cairina moschata (Moschusenten) [Occurrence of Moraxella septicaemiae infection in Cairina moschata.] . Monatshefte für Veterinärmedizin , 39 : 299 – 301 .