Abstract
In five experiments, each consisting of four or six groups with seven or 14 brown laying hens per group, birds were inoculated with an Escherichia coli strain, isolated from a layer with the E. coli peritonitis syndrome (EPS) by different routes between 23 and 33 weeks of age. Aerosol-exposed hens inhaled 105.1 to 106.2 colony-forming units per hen; hens inoculated by other routes received 107.6 to 109.1 colony-forming units per hen. In one experiment, one-half of the birds of each group were injected intraperitoneally with sterile egg yolk simultaneously with E. coli. Dead and surviving birds were necropsied and bacteriological examination of the bone marrow was performed. The percentage of birds with EPS that died was 89 (159/179). Nearly all dead birds showed septicaemia (155/159 = 97%), while most had septicaemia and peritonitis (126/159 = 79%). Surviving hens with EPS (20/179 = 11%) showed chronic peritonitis and inactive ovaries. Taking all experiments together, exposure of hens by the intravenous, intratracheal and intraperitoneal routes induced EPS in 84% (41/49), 80% (55/69) and 76% (16/21), respectively, while aerosol and intravaginal exposure resulted in EPS percentages of 57% (32/56) and 49% (28/57), respectively. Except for orally inoculated groups (7/56 = 13% EPS), in all other groups the EPS rates differed significantly (P <0.01) from those of the placebo-exposed groups (0/42). Neither the age of hens nor the presence of free yolk in the abdomen influenced the EPS rate. The results of the present study are suggestive of the respiratory and vaginal origin of EPS in the field.
Introduction
Coliform salpingitis/peritonitis/salpingoperitonitis (SPS) is a common condition in laying hens, which has been observed since the beginning of poultry farming. Birds with salpingitis show an inflamed oviduct that is frequently distended, thin-walled and filled with laminated, malodorous, caseous exudate consisting of fibrin, granulocytes, yolk and shell material, which is often referred to as egg concrements. The spread of Escherichia coli into the abdominal cavity through the compromised oviduct wall results in concurrent peritonitis (salpingoperitonitis) with accumulation of caseating exudate in the abdominal cavity. This exudate often has the appearance of coagulated yolk and therefore the condition is commonly named “egg peritonitis”. Peritonitis in the absence of salpingitis can also occur, but is uncommon (Jordan et al., Citation2005; Landman & Cornelissen, Citation2006; Barnes et al., Citation2008). Internal laying may accompany salpingitis and as a consequence free yolk may be present in the abdominal cavity, which may favour the occurrence of peritonitis (Gross & Siegel, Citation1959). Although the pathogenesis of SPS is still obscure, three infection routes have been proposed: ascendance of bacteria from the cloaca into the oviduct as intensive egg production and associated estrogenic activity predispose hens to salpingitis by relaxing the sphincter between vagina and cloaca; translocation of bacteria from the air sacs; and translocation of bacteria from the intestines to the oviduct and/or abdominal cavity. The first route seems to be the most important in the pathogenesis of SPS (Landman & Cornelissen, Citation2006; Barnes et al., Citation2008). The incidence of SPS is generally low and as a consequence its economic impact is limited because mortality rates are low and egg production and egg quality are not affected or are only slightly affected (Bisgaard & Dam, Citation1981; Jordan et al., Citation2005; Landman & Cornelissen, Citation2006; Stokholm et al., Citation2010).
From the mid-1990s onwards, however, the incidence and severity of E. coli peritonitis in layer flocks seems to have substantially increased in the USA and in many European countries including the Netherlands. The disease, which is further referred to as E. coli peritonitis syndrome (EPS), occurs predominantly from the start of egg production to peak production, and, in contrast to SPS, acute mortality soars up to 10% in combination with severe septicaemia and fibrinous polyserositis lesions. The polyserositis lesions from which E. coli can be isolated include pericarditis, perihepatitis, oophoritis and peritonitis. Splenomegaly, congested lungs and ruptured ovarian follicles are often observed, the latter resulting in deposition of yolk in the peritoneal cavity. A decrease in egg production and an increase in downgraded eggs of a few percent may be present. Flock and house associations are often observed with recurrent outbreaks within the same flock and in successive flocks in the same house (Dhillon & Jack, Citation1996; Zanella et al., Citation2000; Vandekerchove et al., Citation2004a; Landman & Cornelissen, Citation2006; Raviv et al., Citation2007). E. coli strains isolated from diseased flocks predominantly belonged to the O78 serotype and usually possessed F11 fimbriae and flagella (Vandekerchove et al., Citation2004a) or belonged to the O111 serotype (Zanella et al., Citation2000).
Pathogenesis studies on EPS are scarce and fragmentary, and involvement of yolk in the onset of the disease has not been studied at all. Raviv et al. (Citation2007) and Zanella et al. (Citation2000) reproduced the syndrome by inoculation of laying hens with virulent E. coli strains intratracheal (i.t.) and via the intramuscular route, respectively. Oro-nasal inoculation reproduced the syndrome in only two of eight layers (Zanella et al., Citation2000).
As a contribution to the knowledge of the pathogenesis of EPS in layers, in this study five experiments were performed in which commercial brown egg-producing layers ranging in age from 23 to 33 weeks were inoculated by both artificial and natural routes with an E. coli strain isolated from a bird that died due to EPS. Placebo-inoculated hens were included in each of the experiments. Moreover, in one of the experiments one-half of the birds of each group were inoculated intraperitoneally (i.p.) with egg yolk just before E. coli inoculation. Morbidity and mortality were recorded daily and all dead birds were necropsied immediately At the end of the experiments, 2 to 4 weeks after E. coli inoculation, surviving hens were killed and subjected to gross post-mortem examination as well. To assess septicaemia, bacteriological examination of the bone marrow of all birds was performed. E. coli re-isolates were compared for genomic identity with the E. coli inoculation strain by pulsed-field gel electrophoresis (PFGE).
Materials and Methods
Hens and management
Egg-producing brown layers of various ages as presented in were obtained from commercial flocks. The Mycoplasma gallisepticum, Mycoplasma synoviae and Salmonella Enteritidis free status of the flocks had been assessed by serology at the end of the rearing period. Nobilis® M. gallisepticum and Nobilis® M. synoviae antigen (Intervet B.V., Boxmeer, the Netherlands) were used for the rapid plate agglutination test to detect antibodies against these mycoplasma species (Timms, Citation1967), while S. Enteritidis serology was performed using an enzyme-linked immunosorbent assay test (Van Zijderveld et al., Citation1993). Groups of experimental hens (seven or 14 hens per group) were housed in separate negative-pressure high-efficiency particulate air isolators with a volume of 1.34 m3 each (Beyer & Eggelaar, Utrecht, the Netherlands). The isolator temperature, relative humidity and ventilation rate were 18 to 20°C, 50 to 60% and approximately 30 m3/h, respectively. Light was supplied for 16 hours per day. Birds were fed ad libitum a standard commercial layer diet and had free access to drinking water. The hens were left to acclimatize for 1 week before the experiments started.
Table 1. E. coli peritonitis syndrome in egg-producing brown layers after E. coli administration by different routes
Experimental design
The experimental design is outlined in . Five experiments were performed. Experiment 1 consisted of six groups of 14 hens each. One-half of the birds from each group was each given 25 ml bacteriological sterile egg yolk i.p. immediately before E. coli inoculation at 23 weeks of age. Five groups were inoculated with E. coli either intravenously (i.v.), i.p., i.t., intravaginally (IVAG) or orally. The sixth group served as a placebo-treated control group. Hens of this group were i.p. placebo inoculated with buffered peptone water (BPW).
Experiment 2 was a replicate of Experiment 1 with two modifications: none of the birds were given egg yolk and the inoculation age was 26 weeks. Experiment 3 consisted of three E. coli-inoculated groups (n = 14) and a placebo (BPW) i.t. inoculated control group (n=7). The E. coli groups were either inoculated i.t. or exposed to an E. coli aerosol once or four times with intervals of 2 hours at 28 weeks of age. In each of Experiments 4 and 5, five groups of hens (n=14) were inoculated with E. coli i.v., i.t., IVAG or by the oral or aerosol (once) route. In both experiments a negative control group (n=7) was i.t. placebo (BPW) inoculated. The difference between these experiments was the age at inoculation: 23 weeks in Experiment 4 and 33 weeks in Experiment 5. EPS was assessed by gross post-mortem examination and bacteriological analysis of the bone marrow of the femur. Hens that died were examined immediately, while surviving birds were analysed at the end of the experiments (2 to 4 weeks after inoculations). The latter hens were stunned using a mixture of carbon dioxide and oxygen and were bled by incision of the vena jugularis. Bacteriological examination was performed as described under “E. coli culture”. The E. coli inoculum strain and all re-isolates were stored at −80°C to assess their genetic relatedness using PFGE.
Inocula
Egg yolk was obtained from specific pathogen free eggs after disinfecting their shells with an ethanol (70%) spray. The egg shell was broken into two halves by hitting a disinfected table edge. The egg yolk was separated from the egg white by transferring the egg content back and forth between the two egg shell halves, allowing the egg white to escape. Sterilized bottles coupled to a funnel were used as recipients. Approximately 1.5 l of egg yolk was collected and stored in a refrigerator until use. Egg yolk was administered after reaching room temperature.
The sterility of sampled egg yolk material was assessed by means of standard bacteriological analysis.
In one-half of the birds of each group in Experiment 1, 25 ml bacteriological sterile egg yolk was injected per hen i.p. just prior to E. coli inoculation ().
E. coli culture
E. coli (chicken/NL/Dev/SP01404Cou/05) isolated from the bone marrow (femur) of a dead brown layer with EPS originating from a commercial layer flock with high mortality due to this syndrome was used. To obtain the isolate, the opening of the sagitally cut bone physis was sterilized with a hot scalpel blade after which a bone marrow sample was collected with a wire. A sheep-blood agar plate (K004P090; Biotrading, Mijdrecht, the Netherlands) was then inoculated, subsequently incubated overnight at 37°C and thereafter visually inspected for purity. Biochemical identification of colonies was performed using the indole and β-glucuronidase test. Frozen Protect beads (TS70E; Biotrading) containing the E. coli strain (−70°C) were rolled on a sheep-blood agar plate (K004P090; Biotrading). After overnight incubation at 37°C, the plates were visually inspected for purity. Colonies were then scraped off and suspended in 3 ml physiologic salt solution until a concentration of 0.5 McFarland (108 colony-forming units [CFU]/ml) was obtained. A volume of 0.1 ml of this suspension was mixed with 90 ml of 0.1% glucose broth (1000 ml purified water, 5 g Lab Lemco [Oxoid LP0029; Oxoid, Badhoevedorp, the Netherlands], 10 g bacteriological peptone [Oxoid LP0-037], 5 g NaCl [VWR 1.06404.1000; Merck Eurolab B.V., Amsterdam, the Netherlands] and 1 g glucose [VWR 1.08342.1000; Merck]), which was incubated for 17 h at 37°C. E. coli concentrations, which were assessed by means of bacterial counts according to international standards (ISO7402, Citation1985), ranged from 108.6 to 109.1 CFU/ml. Inoculation i.v. of hens for Experiment 1 was performed with a 1:10 in glucose broth-diluted E. coli culture; for aerosol exposures, 1:2 in BPW diluted E. coli cultures were used. In all other cases, undiluted culture was administered. The E. coli dose was given in a volume of 1.0 ml per hen for all inoculation routes, except for aerosol exposures where each time a volume of 100 ml diluted E. coli culture was used per isolator. During applications, the E. coli inocula were kept on melting ice. The inoculated E. coli doses ranged from 107.6 to 109.1 CFU per hen, while the inhaled doses following aerosol exposure ranged from 105.6 to 106.2 CFU per hen (Table 1).
Inoculations
Inoculation i.v. was performed in the vena ulnaris using a 2 ml syringe coupled to a 23 G needle after disinfecting the skin with 70% ethanol. Inoculation i.p. was performed after also disinfecting the inoculation site with 70% ethanol. Hens were restrained in a hanging position after which the abdominal wall was lifted slightly using the thumb and forefinger positioned halfway between the end of the sternum and the vent following the median line. Lifting the abdominal wall was performed to avoid injection in the intestines. The egg yolk was inoculated using a 50 ml syringe coupled to an 18 G needle, while other inoculation i.p. was performed using the same devices as for inoculation i.v. Egg yolk and other inocula were administered after each other at the same site. A 2 ml syringe coupled to a knobbed curved stainless steel cannula (article number 14186; AUV Group, Cuijk, the Netherlands) was used for inoculation i.t., orally and IVAG. For inoculation IVAG, a mild prolapse of the cloaca was induced until the vaginal orifice became visible.
Aerosol generation and characterization
The E. coli aerosol was generated using a spray head with an orifice diameter of 0.5 mm (Walther Pilot I spray-head; Walther Spritz- and Lackiersysteme, Wuppertal, Germany) coupled to an air compressor (Mecha Concorde, type 7SAX, 1001, 10 bar/max; SACIM, Verona, Italy). The E. coli culture (100 ml 1:2 in BPW-diluted E. coli culture) was nebulized in each isolator subjected to aerosol treatment at a pressure of 2 bar and an air yield of 30 l/min, resulting in a flow of 42 ml/min. The droplet spectrum of the aerosol was assessed as described (Landman et al., Citation2004): Dv(0.1) = 2.7 µm, Dv(0.5) = 9.9 µm and Dv(0.9) = 30.7 µm. From the start of the aerosol generation until 30 min after ending, the isolator ventilation was switched off. During aerosolization the relative humidity of the isolator air raised to 100% within a few minutes (Corbanie et al., Citation2008). Immediately and 10 min after ending the aerosol generation, air sampling was performed as described (Landman et al., Citation2004) in order to calculate the bacterial concentrations per m3 of isolator air and subsequently the dose uptake of the birds by inhalation. Directly after the aerosol generation and 10 min later, E. coli concentrations in isolator air ranged from 107.6 to 108.6 CFU/m3 and from 107.3 to 107.4 CFU/m3, respectively.
Inhaled E. coli aerosol dose
The number of E. coli bacteria inhaled per hen over the period from the start of aerosolization until 30 min after the end of it, was estimated based on a ventilation rate of the hens of 513 ml/min (Gleeson et al., Citation1985). For the calculation of the uptake of bacteria, the following assumptions were made: all airborne E. coli-containing particles measured by air sampling are inhalable; the increase and decrease of the E. coli concentration is linear; 30 min after E. coli culture nebulization, the airborne E. coli concentrations are considered “0”; and E. coli particle retention in the birds is 100% (Yadin, Citation1980). The calculated inhaled E. coli dose varied from 105.1 to 105.7 CFU per hen after a single aerosol exposure and was 106.2 CFU per hen after exposure to four aerosols.
Pulsed-field gel electrophoresis analysis
The PFGE technique of contour-clamped homogeneous electric fields (CHEF) was used for genomic typing of re-isolates from Experiments 3, 4 and 5 (five re-isolates per experimental group) (Centers for Disease Control and Prevention, Citation1999). Genomic DNAs were digested in agarose plugs with XbaI (10 U; Roche Diagnostics, Mannheim, Germany). The resulting fragments were resolved by CHEF-PFGE with a CHEF DR-III apparatus (Bio-Rad Laboratories, Richmond, California, USA) at a constant voltage of 200 V for 20 h at 13°C and a linearly ramped pulse time of 2.2 to 54.2 sec. The fingerprints generated were processed using Bionumerics software (Applied Maths, Kortrijk, Belgium). Isolates were considered “indistinguishable” if 100% of the fragments were identical.
Statistical analysis
Differences in the number of hens with EPS between groups were analysed using Fisher's exact test (User's manual Statistix 8.2 for Windows®, Citation2010). Differences were considered significant if P<0.01.
Ethics
Experiments were approved by the Institutional Animal Experimental Committee, DEC-Consult Foundation, according to Dutch law on experimental animals (Wet op de dierproeven).
Results
EPS occurring in this study was characterized by bacteraemia (E. coli was isolated from bone marrow) and septicaemia (swelling of spleen, liver and kidneys), and/or peritonitis. The peritonitis was characterised by the occurrence of profuse hyperaemia of the peritoneum, fibrin clots and purulent exudate in the abdominal cavity. Moreover, pericarditis, perihepatitis, airsacculitis and pneumonia were observed frequently in birds with EPS (in 24.6%, 8.4%, 33.0% and 11.7% of birds with EPS, respectively). Incidentally, salpingitis, vaginitis and arthritis were seen (in 1.7%, 0.6% and 1.7% of hens with EPS, respectively).
In total, 179 hens showed EPS. During the experiments, 89% (159/179) of the birds with EPS had died (up to 2 to 4 weeks after E. coli inoculation). E. coli was isolated from the bone marrow of dead birds only; nearly all dead hens showed septicaemia (155/159 = 97%), while the great majority had septicaemia and peritonitis (126/159 = 79%).
Mortality occurred between 0 and 8 days after E. coli exposure. Mean death time±standard deviation was lowest in groups exposed to E. coli i.p., IVAG and i.v. (1.6±1.1 [n=13], 1.6±1.1 [n=22] and 1.7±1.5 [n=41] days, respectively) and highest in groups exposed by the oral and aerosol routes (3.3±1.5 [n=4] and 4.0±1.8 [n=30] days, respectively). The mean death time of birds inoculated i.t. was in between: 2.3±1.4 (n=43) days.
The septicaemic form of EPS (i.e. sepsis without visible peritonitis lesions) occurred in 51% (21/41) and 23% (5/22) of dead hens of the i.v. and IVAG inoculated groups, respectively. In groups exposed to E. coli by other routes, the percentages of birds showing the septicaemic form of EPS was zero or very low. Dead birds had productive or degenerated (flaccid and corrugated yellow follicles) ovaries.
Twenty hens with EPS survived (20/179 = 11%). E. coli could not be isolated from their bone marrow, but they all showed chronic peritonitis lesions, had inactive ovaries and frequently were emaciated.
The number of birds with EPS per exposure group is presented in . None of the birds in the placebo groups developed EPS. Except for two groups, no significant differences (P ≥0.01) in the number of hens with EPS were observed between groups exposed to E. coli by the same route in the various experiments. Also no significant differences were found within groups between birds that received egg yolk or not.
Exposure of hens to E. coli i.v., i.t. or i.p. overall induced EPS in 84%, 80% and 76% of hens, respectively, while exposure by aerosol or inoculation IVAG resulted in EPS percentages of 57% and 49%, respectively. Oral inoculation showed the lowest percentage of EPS-affected birds (13%) ().
The percentage of birds with EPS in groups treated i.v., i.t. or i.p. did not differ significantly (P ≥0.01). The aerosol and IVAG exposed groups did not differ significantly from each other regarding the number of birds with EPS, but they differed significantly from the i.v. and i.t. treated hens. The placebo-treated birds differed significantly from all other groups except the orally inoculated groups.
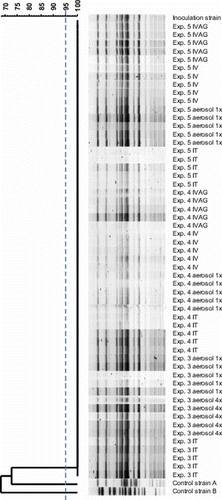
Pulsed-field gel electrophoresis
In contrast to control isolates, all re-isolates showed the same banding pattern as the parent strain and were therefore considered genetically indistinguishable ().
Figure 1. Restriction endonuclease digestion patterns obtained by PFGE of re-isolated E. coli from Experiments 3, 4 and 5 (n=5 re-isolates per experimental group; two re-isolates of orally inoculated birds of Experiment 4 were not tested) were considered genetically indistinguishable compared the inoculum strain. The two control isolates A and B originating from two different field outbreaks of EPS appeared to be genetically unrelated to the former isolates. IVAG, i.v., aerosol 1×, i.t. and aerosol 4× denote the inoculation or exposure routes by order of appearance in the figure, which are intravaginal, intravenous, aerosol once, intratracheal and aerosol four times, respectively. The dendrogram was constructed based on Dice similarity comparison of fragment profiles followed by UPGMA analysis.
Discussion
EPS in the field is characterized by acute mortality, septicaemia and/or peritonitis (Dhillon & Jack, Citation1996; Zanella et al., Citation2000; Vandekerchove et al., Citation2004a, Citationb; Landman & Cornelissen, Citation2006) and was successfully induced in the present study in egg-producing commercial brown layers. Exposure of the hens to an E. coli strain isolated from the bone marrow of a bird from a field case of EPS by both artificial (i.v. and i.p.) and natural infection routes (i.t., aerosol and IVAG) resulted in high percentages of EPS (varying from 49 to 84%). After oral exposure only 13% of the hens developed EPS, which was not significantly different (P ≥0.01) from the placebo-exposed control hens, and thus translocation of E. coli from the intestines to the oviduct and/or abdominal cavity does not seem to be important. Although groups of aerosol-exposed birds inhaled an E. coli dose (105.1 to 106.2 CFU per bird), which was approximately 1000 times lower than the dose of the IVAG inoculated groups (108.7 to 108.9 CFU per hen), the EPS rates of these groups did not differ significantly (P ≥0.01) (57% in aerosol-exposed groups versus 49% in IVAG inoculated groups). This observation may be explained considering the local defence mechanisms of the air capillary region in comparison with that of the vagina. Once bacteria pass beyond the trachea and the bronchi, the mucociliary clearance as well as innate immunity with antimicrobial peptides and the adaptive immunity with local immunoglobulins are no longer the principal barrier. Air capillaries depend largely on macrophages for bacterial clearance. In case the limited capacity for clearance by macrophages is overwhelmed E. coli can relatively easily enter the vascular system. (Kothlow & Kaspers, Citation2008; López, Citation2012). In contrast, the vagina is supplied with mucociliary clearance as well as elements of both innate (e.g. antimicrobial peptides) and adaptive immunity (e.g. T and B lymphocytes) (Wigley et al., Citation2008; Foster, Citation2012). Therefore, the threshold to establish E. coli septicaemia through the avian vagina might be much higher. Nevertheless, our results are suggestive of both the respiratory and vaginal origin of EPS in the field. This suggestion is in agreement with research data from other scientists (Vandekerchove et al., Citation2004a; Raviv et al., Citation2007).
In the present study, three distinct forms of EPS were found: the septicaemic form (only signs of sepsis were seen and E. coli was isolated from the bone marrow), the acute peritonitis form (besides signs of sepsis, acute fibrinous peritonitis was also observed and E. coli was isolated from bone marrow) and the chronic peritonitis form (i.e. chronic peritonitis was found without signs of sepsis and E. coli was not isolated from the bone marrow). The septicaemic form of EPS was predominantly found in groups of hens with the lowest mean death time; that is, the i.v. and IVAG groups (mean death time was 1.7 and 1.6 days, respectively), where mortality due to septicaemia probably occurred before the development of peritonitis. The i.p. inoculated groups of hens also showed a low mean death time (1.6 days), but showed the acute peritonitis form instead of the septicaemic form of EPS. The direct deposition of massive numbers of E. coli bacteria on the peritoneum probably led to the quick development of acute peritonitis in combination with sepsis. All hens with either form of EPS lost their economic value as the septicaemic and acute peritonitis form resulted in mortality and hens with chronic peritonitis, despite survival at least during the experimental period, were unproductive.
In general, healthy broiler chickens older than approximately 2 weeks will overcome experimentally induced colibacillosis within a short time span (Dwars et al., Citation2009), in contrast to broilers with an E. coli superinfection after infection with respiratory virus such as infectious bronchitis virus (Dwars et al., Citation2009) and Newcastle disease virus or mycoplasmas (Barnes et al., Citation2008). In the present study, EPS was reproduced experimentally in laying hens in the absence of any trigger. Moreover, neither the age of the laying hens (age at inoculation varied from 23 to 33 weeks), and thus the phase of their egg production period, nor the presence of free yolk in the abdominal cavity seemed to play a significant role in the pathogenesis of EPS. The foregoing illustrates the primary nature of the E. coli strain involved, probably because this strain has the ability to invade the bloodstream rapidly and overwhelm the bird's immune response. In agreement with our results, Vandekerchove et al. (Citation2004b) also showed the occurrence of primary pathogenic E. coli strains. Based on field data using serology they concluded that EPS was not associated with the respiratory pathogens of infectious bronchitis virus, Newcastle disease virus, avian pneumovirus, M. gallisepticum, M. synoviae and Ornithobacterium rhinotracheale. Although Raviv et al. (Citation2007) claimed a possible role for M. synoviae as a complicating factor in EPS, this claim seems doubtful because the numbers of hens with EPS in their various experimental groups were very low. Moreover a statistical significant difference (P <0.05) between birds infected with E. coli alone and those exposed to E. coli and M. synoviae was not found. Significant difference (P<0.05) was only reported between the negative control group and birds with the double infection.
EPS can easily be discriminated from coliform SPS (“egg peritonitis”), which forms a substantial proportion of the “normal” mortality in flocks of layer hens (Bisgaard & Dam, Citation1981; Jordan et al., Citation2005; Stokholm et al., Citation2010) based on its acute and septicaemic character, its high mortality rates and the rare occurrence of salpingitis. The latter aspect was also observed in laying hens with EPS from commercial flocks presented for post-mortem examination to GD - Animal Health Service the Netherlands (Landman & Cornelissen, Citation2006).
References
- Barnes , H.J. , Nolan , L.K. and Vaillancourt , J.P. 2008 . “ Colibacillosis ” . In Diseases of Poultry , 12 ed. , Edited by: Saif , Y.M. , Fadly , A.M. , Glisson , J.R. , McDougald , L.R. , Nolan , L.K. and Swayne , D.E. 691 – 732 . Ames : Iowa State Press .
- Bisgaard , M. and Dam , A. 1981 . Salpingitis in poultry. II. Prevalence, bacteriology, and possible pathogenesis in egg-laying chickens . Nordisk Veterinaer Medicin , 33 : 81 – 89 .
- Centers for Disease Control and Prevention, N. C. f. I. D., Division of Bacterial and Mycotic Diseases, Foodborne and Diarrheal Diseases Branch . 1999 . One-Day (24–28 h) Standardized Laboratory Protocol for Molecular Subtyping of Escherichia coli O157: H7 by Pulsed-Field Gel Electrophoreses (PFGE) . Atlanta , GA : Centers for Disease Control and Prevention .
- Corbanie , E.A. , Vervaet , C. , Van Eck , J.H. , Remon , J.P. and Landman , W.J. 2008 . Vaccination of broiler chickens with dispersed dry powder vaccines as an alternative for liquid spray and aerosol vaccination . Vaccine , 26 : 4469 – 4476 . doi: 10.1016/j.vaccine.2008.06.055
- Dhillon , A.S. and Jack , O.K. 1996 . Two outbreaks of colibacillosis in commercial caged layers . Avian Diseases , 40 : 742 – 746 . doi: 10.2307/1592290
- Dwars , R.M. , Matthijs , M.G. , Daemen , A.J. , Van Eck , J.H. , Vervelde , L. and Landman , W.J. 2009 . Progression of lesions in the respiratory tract of broilers after single infection with Escherichia coli compared to superinfection with E. coli after infection with infectious bronchitis virus . Veterinary Immunology and Immunopathology , 127 : 65 – 76 . doi: 10.1016/j.vetimm.2008.09.019
- Foster , R.A. 2012 . “ Female reproductive system and mammary gland ” . In Pathologic Basis of Veterinary Disease , 5th ed , Edited by: Zachary , J.F. and McGavin , M.D. 1085 – 1126 . Missouri : Elsevier Mosby .
- Gleeson , M. , Haigh , A.L. , Molony , V. and Anderson , L.S. 1985 . Ventilatory and cardiovascular responses of the unanaesthetized chicken, Gallus domesticus, to the respiratory stimulants etamiphylline and almitrine . Comparative Biochemistry and Physiology Part C: Comparative Pharmacology , 81 : 367 – 374 . doi: 10.1016/0742-8413(85)90021-0
- Gross , W.B. and Siegel , P.B. 1959 . Coliform peritonitis of chickens . Avian Diseases , 3 : 370 – 373 . doi: 10.2307/1587575
- ISO7402 1985 . Microbiology – General Guidance for the Enumeration of Enterobacteriaceae Without Resuscitation—MPN Technique and Colony Count Technique , (1st ed.) . Geneva : International Standard Organisation .
- Jordan , F.T. , Williams , N.J. , Wattret , A. and Jones , T. 2005 . Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock . The Veterinary Record , 157 : 573 – 577 .
- Kothlow , S. and Kaspers , B. 2008 . “ The avian respiratory immune system ” . In Avian Immunology , 1 ed. , Edited by: Davison , F. , Kaspers , B. and Schat , K.A. 273 – 288 . London : Academic Press .
- Landman , W.J. and Cornelissen , R.A. 2006 . [Escherichia coli salpingitis and peritonitis in layer chickens: an overview] . Tijdschrift voor Diergeneeskunde , 131 : 814 – 822 .
- Landman , W.J.M. , Corbanie , E.A. , Feberwee , A. and Van Eck , J.H.H. 2004 . Aerosolization of Mycoplasma synoviae compared with Mycoplasma gallisepticum and Enterococcus faecalis . Avian Pathology , 33 : 210 – 215 . doi: 10.1080/0307945042000195812
- López , A. 2012 . “ Respiratory system, mediastinum and pleurae ” . In Pathologic Basis of Veterinary Disease , 5th ed. , Edited by: Zachary , J.F. and McGavin , M.D. 458 – 535 . Missouri : Elsevier Mosby .
- Raviv , Z. , Ferguson-Noel , N. , Laibinis , V. , Wooten , R. and Kleven , S.H. 2007 . Role of Mycoplasma synoviae in commercial layer Escherichia coli peritonitis syndrome. Avian Diseases , 51 : 685 – 690 . doi: 10.1637/0005-2086(2007)51[685:ROMSIC]2.0.CO;2
- Stokholm , N.M. , Permin , A. , Bisgaard , M. and Christensen , J.P. 2010 . Causes of mortality in commercial organic layers in Denmark . Avian Diseases , 54 : 1241 – 1250 . doi: 10.1637/9375-041910-Reg.1
- Timms , L. 1967 . Isolation and identification of avian mycoplasma . Journal of Medical Laboratory Technology , 24 : 79 – 89 .
- User's manual Statistix 8.2 for Windows [Computer software] . 2010 . Tallhassee , FL : Analytical Software .
- Van Zijderveld , F.G. , Van Zijderveld-Van Bemmel , A.M. , Brouwers , R.A.M. , De Vries , T.S. , Landman , W.J.M. and De Jong , W.A. 1993 . Serological detection of chicken flocks naturally infected with Salmonella Enteritidis, using an enzyme-linked immunosorbent assay based on monoclonal antibodies against the flagellar antigen . Veterinary Quarterly , 15 : 135 – 137 . doi: 10.1080/01652176.1993.9694391
- Vandekerchove , D. , De Herdt , P. , Laevens , H. and Pasmans , F. 2004a . Colibacillosis in caged layer hens: characteristics of the disease and the aetiological agent . Avian Pathology , 33 : 117 – 125 . doi: 10.1080/03079450310001642149
- Vandekerchove , D. , De Herdt , P. , Laevens , H. , Butaye , P. , Meulemans , G. and Pasmans , F. 2004b . Significance of interactions between Escherichia coli and respiratory pathogens in layer hen flocks suffering from colibacillosis-associated mortality . Avian Pathology , 33 : 298 – 302 . doi: 10.1080/030794504200020399
- Wigley , P. , Barrow , P. and Schat , K.A. 2008 . “ The avian reproductive immune system ” . In Avian Immunology , 1st edn. , Edited by: Davison , F. , Kaspers , B. and Schat , K.A. 289 – 298 . London : Academic Press .
- Yadin , H. 1980 . Aerosol vaccination against Newcastle disease: virus inhalation and retention during vaccination . Avian Pathology , 9 : 163 – 170 . doi: 10.1080/03079458008418399
- Zanella , A. , Alborali , G.L. , Bardotti , M. , Candotti , P. , Guadagnini , P.F. , Martino , P.A. and Stonfer , M. 2000 . Severe Escherichia coli O111 septicaemia and polyserositis in hens at the start of lay . Avian Pathology , 29 : 311 – 317 . doi: 10.1080/03079450050118430