Abstract
Despite significant conservation intervention, the kiwi (Apteryx spp.) is in serious population decline. To increase survival in the wild, conservation management includes rearing of young birds in captivity, safe from introduced mammalian predators. However, an increase in density of immunologically naïve kiwi increases the risk of exposure to disease, including coccidia. Intestinal coccidiosis has recently been described in the kiwi, and although extra-intestinal coccidiosis was first recognized in kiwi in 1978, very little is known about this disease entity. This study used archived histological tissues and reports from routine necropsies to describe the pathology of naturally occurring extra-intestinal coccidiosis. At least 4.5% of all kiwi necropsied during 1991 to 2011 (n=558) were affected by extra-intestinal coccidiosis, and it is estimated that it caused death in 0.9 to 1.2% of kiwi in the study group. Four forms were recognized: renal, hepatic, and, less commonly, splenic and pulmonary. At necropsy, renal coccidiosis was associated with miliary white streaks and foci through the kidneys, renomegaly, and renal pallor or congestion. Renal meronts and gametocytes were confined to the distal convoluted tubules and collecting ducts, and were associated with renal tubular necrosis and tubular obstruction. Hepatic miliary pinpoint foci were present throughout the hepatic parenchyma associated microscopically with macromeronts measuring 304×227 µm. In two cases, clusters of splenic meronts were identified, and a similar lesion was identified in the pulmonary interstitium of another case. Juvenile, captive kiwi were most often affected with extra-intestinal coccidiosis, illustrating an increased expression of disease with population manipulation for conservation purposes.
Introduction
Kiwi are a group of closely related ratites endemic to New Zealand. Four of five species of kiwi are classified as threatened with extinction, mainly due to low recruitment of young into wild populations (Basse et al., Citation1999; Miskelly et al., Citation2008). Predation by introduced stoats (Mustela erminea) and, to a lesser extent, cats (Felis catus) is the biggest threat to young kiwi (McLennan et al., Citation1996; Colbourne et al., Citation2005). Conservation management of kiwi includes the close monitoring of populations using radio-tagged birds, and a captive rearing programme aimed at improving the chances of recruitment of young kiwi into wild populations. This programme, dubbed “Operation Nest Egg”, was developed in 1994 and is centred on the collection of kiwi eggs or chicks from the wild (Colbourne et al., Citation2005). Kiwi are safely reared in captivity or in predator-free forest “crèches” until they are large enough to protect themselves from stoats on release back to the wild (Colbourne et al., Citation2005). Kiwi raised through this programme have a 65% chance of survival to adulthood (Anon., Citation2012), compared with less than 5% of birds from unmanaged wild populations (Colbourne et al., Citation2005). However, intensive rearing of young kiwi increases the likelihood of exposure to those pathogens that are usually associated with high-density livestock situations, including coccidia (McDougald, Citation2003; Morgan et al., Citation2012).
Most coccidial species infecting avian hosts occur within the intestinal tract, with enteric coccidiosis being recently described in the brown kiwi (Apteryx mantelli) (Morgan et al., Citation2012). Some coccidia develop in extra-intestinal sites, usually in the renal epithelial cells and less frequently in other locations (Yabsley, Citation2008). Renal, hepatobiliary and pancreatic coccidiosis have been previously reported in mainly young, captive kiwi (Thompson & Wright, Citation1978; Boardman, Citation1995; Hartley, Citation1995; Alley et al., Citation2004).
Renal coccidiosis is frequently reported in species from several orders of birds, and although the specific identity of renal coccidia infecting kiwi is unknown, species of Eimeria have been identified as the primary cause of renal coccidiosis in other avian hosts (Gajadhar et al., 1983b; Leighton & Gajadhar, Citation1986; Gajadhar & Leighton, Citation1988; Yabsley et al., Citation2002). There have been few successful experimental studies on renal coccidiosis, which Yabsley (Citation2008) attributed to difficulties in sporulation of renal oocysts. Nevertheless, it is presumed that, like intestinal coccidia, species of renal Eimeria have a short direct lifecycle (Yabsley, Citation2008). Transmission of coccidia occurs via ingestion of sporulated oocysts from the environment. Although it is uncertain how renal coccidia species are transported from the gastrointestinal tract to the kidney (Wobeser, Citation1997), it has been suggested that sporozoites directly invade renal epithelial cells to initiate merogony followed by gametogony (Yabsley, Citation2008).
In contrast, hepatic coccidiosis has been reported infrequently in avian species. Meronts and gametocytes of Eimeria species have been described in the bile-duct epithelium of a single Magpie-lark (Grallina cyanoleuca) (Reece, Citation1989), and migration of intravenously inoculated Eimeria tenella sporozoites to the liver has been demonstrated in immunosuppressed chickens and chicken embryos (Long, Citation1970, Citation1971). Oocysts of Eimeria adenoeides, which usually inhabits the intestinal tract of turkeys, have also been recorded in the liver of a 5-week-old turkey poult (Critchley et al., Citation1986). Eimeria reichenowi and Eimeria gruis have been documented to cause disseminated coccidiosis affecting multiple visceral organs, including the liver, in several species of cranes (Grus spp.) (Novilla & Carpenter, Citation2004; O'Brien et al., Citation2011). In affected birds, granulomatous nodules containing developing meronts formed on multiple serosal and mucosal surfaces, and gametogony occurs within the intestinal and respiratory tracts (Novilla & Carpenter, Citation2004).
The aims of the present study were to describe the pathology and endogenous morphology of naturally occurring extra-intestinal coccidiosis in the kiwi, and to estimate the incidence and impact of the infection in kiwi.
Materials and Methods
Huia database review
Archived records from Huia, the National Wildlife Pathology Database, of kiwi necropsied at our laboratory between 1991 and 2011 (n=558) were searched to identify cases of extra-intestinal coccidiosis. A full necropsy report was available, including the presence or absence of coccidia in extra-intestinal tissues and the primary cause of death as determined by the pathologist. In most cases, a range of tissues including the liver, kidney, lung and spleen were examined histologically, usually with haematoxylin and eosin (H&E) stain, and results were discussed in the necropsy report. However, it should be noted that not all reports discussed findings from all tissues.
Individual animal details were available from the database including age at the time of death, either as an exact age or a standardised age classification (chick, juvenile, sub-adult, adult). For this study, in keeping with previous publications: chicks were classified as less than 25 days old (McLennan, Citation1988); juveniles were aged between 26 days and 18 months old; and adults were those greater than 18 months old (Basse et al., Citation1999), with sub-adults included in the adult category. In total, 553/558 kiwi on the database had known ages, with 20% (n=113) chicks, 39% (n=218) juveniles, and 40% (n=222) adults.
Habitat classifications included wild, captive and crèche, and of those with recorded habitats (n=550) 64% (n=354) were wild birds, 30% (n=166) were captive and 4% (n=24) were from predator-free crèches. Where species was recorded (n=554), 76% (n=421) were brown kiwi (A. mantelli). Of the other four species, 9% (n=50) were tokoeka (Apteryx australis), including 46 Haast tokoeka (A. australis “Haast”), 7% (n=38) were rowi (Apteryx rowii), 6% (n=34) were great spotted kiwi (Apteryx haastii), and 2% (n=10) were little spotted kiwi (Apteryx owenii). Other details collated included the primary cause of death (as determined by the pathologist); gross renal and/or hepatic findings; and results of any faecal oocysts counts performed post-mortem.
Renal tissues
To test the accuracy of diagnosis of renal coccidiosis, a subset of 94 of the 558 kiwi necropsied were arbitrarily selected if paraffin-embedded tissues were available. Renal tissues were re-cut at 4 µm thickness and stained with periodic acid Schiff (PAS) as well as H&E. Each entire section of PAS-stained kidney was examined under light microscopy at 100× magnification and various coccidial stages were identified and quantified for each field of view. Sizes of renal tissues available ranged from five to 58 fields of view (mean = 22.6), and the numbers of coccidial stages were counted and a value per field of view was obtained. Dimensions of coccidial organisms were measured, and sections stained with H&E were examined for any histopathological changes associated with the presence of coccidia.
Hepatic tissues
To identify the frequency of cases of hepatic coccidiosis overlooked at the time of necropsy, liver sections from a subset of the total 558 cases seen at our laboratory were re-examined for the presence of hepatic coccidia (n=24). The selection criteria for these included availability of hepatic tissues and the pre-identification of coccidia in any tissues during the necropsy. The number of liver sections examined was determined by their availability and varied from one to six sections per case. Coccidial stages were measured as for renal tissues.
Results
Details of all cases of extra-intestinal coccidiosis diagnosed at our laboratory between 1991 and 2011 are shown in . Overall, extra-intestinal coccidiosis was detected in a total of 4.5% (n=25/558) of cases.
Table 1. Details of all cases of extra-intestinal coccidiosis in kiwi (Apteryx spp.) diagnosed at Massey University (1991 to 2011) using histological methods.
Renal coccidiosis
Original necropsy reports from the Huia database revealed a total of 15/558 (2.7%) cases of renal coccidiosis. Of the 94 kiwi (a subset of the overall 558 birds from the Huia database) selected for re-examination, renal coccidiosis was identified in 15/94 cases (16%). Four of these cases had not been diagnosed with renal coccidiosis during the original necropsy (Cases 1932, 1976, 2598, 3101). This indicated that the detection of renal coccidiosis at routine necropsy may be less than 73.3%. Conversely, renal coccidiosis was not detected in one case re-examined although it had been originally diagnosed (Case 3275).
The revised data revealed that a total of 19 cases of renal coccidiosis were present in kiwi necropsied between 1991 and 2011. Mostly juveniles (18/19) and one chick were affected. Of those with exact ages known, all were between 3 and 28 weeks old with a median age of 13 weeks. No adults were affected. Most affected birds were captive (58%, n=11/19), with 11% (n=2/19) in predator-free crèches and 32% (n=6/19) free-living. Brown kiwi were the most commonly infected (84%, n = 16/19), with one case (5%) being detected in a Haast tokoeka and two further cases (11%) in great spotted kiwi.
Renal coccidiosis was the primary cause of death in 16% (n=3/19) of the birds with renal coccidia. In one case (Case 2197) nephritis was considered the primary cause of mortality, with renal coccidiosis as the secondary diagnosis. It is plausible that the nephritis was actually caused by the presence of renal coccidial organisms.
Clinical signs associated with extra-intestinal coccidiosis are recorded in . A clinical history was available for 6/19 cases diagnosed; however, many of these cases were complicated by concurrent disease, including other forms of coccidiosis. The most common clinical sign associated with renal coccidiosis was a reduction in appetite or complete inappetance for a duration of 12 h to 7 days prior to death (4/6 cases). Other signs included haematochezia (n=1/6), weight loss (n=1/6), depression ( n=1/6), dehydration (n=1/6), respiratory changes (n=1/6) and tachycardia (n=1/6).
Table 2. Clinical signs reported in kiwi (Apteryx spp.) associated with various forms of extra-intestinal coccidiosis.
Gross renal pathology was observed in 42% (n=8/19) of cases with histological evidence of renal coccidiosis. The remaining cases did not demonstrate any gross pathological changes. Observed changes included renal pallor (50%, n=4/8), renomegaly (50%, n=4/8), miliary white streaks and foci (38%, n=3/8), and dark or congested kidneys (25%, n=2/8) (a).
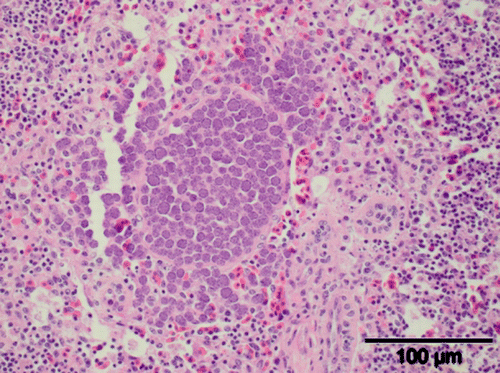
Figure 1. 1a: Partially eviscerated juvenile brown kiwi (A. mantelli; Case 1976) with kidneys in situ demonstrating renal pallor and renomegally associated with a heavy burden of coccidia. BW, body wall; CaL, caudal pole left kidney; CaR, caudal pole right kidney; CrL, cranial pole left kidney; CrR, cranial pole right kidney; R, transected rectum. 1b: Low-power view illustrating coccidial organisms (arrow) within collecting ducts of the medullary cone in the kidney of a juvenile brown kiwi (H&E stain). Scale bar = 200 µm. 1c: Coccidial oocysts causing dilation and obstruction of a collecting duct in the kidney of a juvenile brown kiwi (H&E stain). Scale bar = 20 µm. 1d: Renal collecting ducts of a juvenile brown kiwi demonstrating gametocytes within epithelial cells (H&E stain). Scale bar = 20 µm. 1e: Immature meronts causing tubular dilation within collecting duct epithelial cells in the kidney of a juvenile brown kiwi (H&E stain). Scale bar = 20 µm. 1f: Mature meronts with radially projecting merozoites within a renal collecting duct of a juvenile brown kiwi (H&E stain). Scale bar = 20 µm.
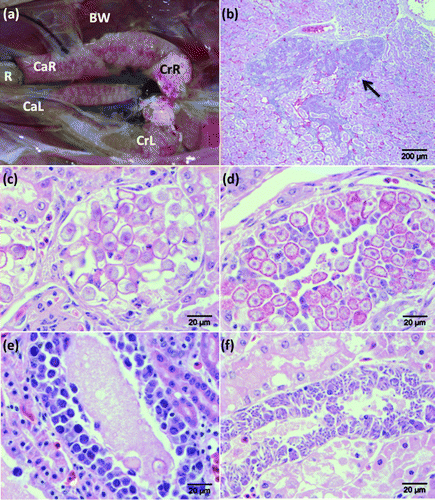
Histological lesions associated with coccidia were most apparent in collecting ducts within the distal convoluted tubules and medullary cones of the renal parenchyma (b). Distal tubular lesions were localized, and often appeared in zones, with immature meronts evident in one area, and gametocytes in another. In the affected areas, the normal architecture of distal convoluted tubules was severely disrupted, with dilated lumina that were often obstructed by a combination of oocysts, sloughed epithelial cells, and inflammatory cells. Many affected tubules contained uric acid tophi and/or protein globules, with or without associated coccidial organisms. The cuboidal epithelium of the distal convoluted tubules was often attenuated or obliterated and replaced with developing gametocytes, and less frequently meronts. Epithelial cell necrosis was often seen associated with coccidial organisms, and in other tubules necrosis occurred without direct association with parasites. Tubular nephritis was seen in distal tubules with or without the presence of parasites and epithelial hyperplasia was observed in the worst affected cases. Proximal tubules were unaffected.
Affected collecting ducts were often very dilated with large numbers of luminal oocysts (c). Tubular epithelial cells often contained large numbers of developing gametocytes or meronts (Figures 1d,e,f), which severely disrupted the epithelium and caused superficial epithelial necrosis to the extent that, in some areas, only a few basal cells of the ducts remained. There was evidence of localized epithelial hyperplasia in some cases, with increased numbers of small epithelial cells and occasional mitotic figures. Interstitial fibrosis of the medullary cone was seen in several cases. This was usually accompanied by interstitial nephritis with moderate numbers of infiltrating lymphocytes, macrophages and granulocytes, which were migrating within ducts. The most severe renal infection occurred in a 3-week-old Haast tokoeka (A. australis). In this case, there was a localized interstitial lymphoid response of predominantly lymphocytes together with occasional plasma cells and macrophages. Multinucleate giant cells were occasionally present within tubules of the medullary papillae together with occasional granulocytes. Plugs of mucus were frequently evident within markedly dilated collecting tubules in association with sloughed and proliferating epithelial cells. In a few severely affected cases, gametocytes extended into the ureters and were associated with small numbers of submucosal granulocytes and lymphocytes and proliferation of capillary endothelial cells.
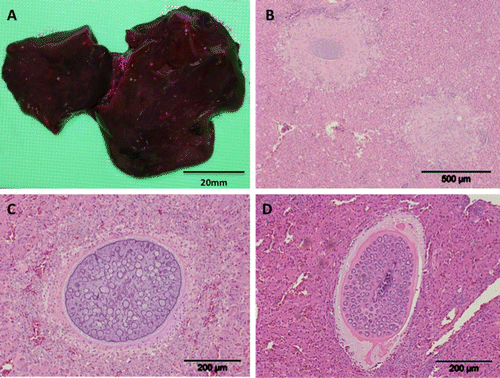
Figure 2. 2a: Pinpoint white serosal foci associated with coccidiosis in the liver of a brown kiwi chick (Case 2586). Scale bar = 20 mm. 2b: Focal areas of necrosis within the liver of a juvenile brown kiwi associated with coccidial meronts (H&E stain). Scale bar = 500 µm. 2c: An immature macromeront within the liver of a juvenile brown kiwi (H&E stain). Scale bar = 200 µm. 2d: An alternative form of macromeront surrounded by a thick eosinophilic capsule within the liver of a brown kiwi chick (H&E stain). Scale bar = 200 µm.
Renal oocysts were identified in all cases; gametocytes were found in 27% (n=4/15) of cases, and small numbers of immature and/or mature meronts were identified in 20% (n=3/15) of cases. Quantitative analysis in the re-examined cases identified a range of renal oocysts and/or gametocytes from 1.9 to 367 per field of view. Three cases were significantly higher than others, with counts of 235, 271 and 367 coccidial organisms per field of view (Cases 2520, 2586 and 1976, respectively). Quantification of coccidia from the kidneys of the juvenile Haast tokoeka (Case 4467) diagnosed with renal coccidiosis was subsequently performed, which revealed a count of 421 per field of view. Of these, three cases (Cases 2520, 2586 and 4467) were considered to have died from renal coccidiosis, and all showed miliary white accumulations on the serosal surface of the kidneys. The primary cause of death in the other case (Case 1976) was enteric coccidiosis, inferring that this infection was more significant than the renal disease. Overall, evidence suggests the methodology of quantification of renal coccidiosis used in the present study is appropriate to evaluate severity of infection.
Morphological measurements of extra-intestinal coccidial stages are summarized in . Renal oocysts were invariably oval in shape, with mean dimensions of 17.2×12.4 µm in tissue sections (). Macrogametocytes measured 13.2×10.1 µm, and microgametocytes were 14.5×10.9 µm. Immature meronts were identified by the presence of a deeply basophilic blastophore measuring 7.9×6.8 µm. Mature meronts were characterized by the presence of short slender merozoites projecting radially from these blastophores. Merozoites measured 7.9×1.6 µm.
Table 3. Morphometrics of various extra-intestinal coccidial stages identified in this study.
Hepatic coccidiosis
Hepatic coccidiosis was identified in a total of 10/558 (1.8%) kiwi submitted for post-mortem examination between 1991 and 2011. In the 24 cases (a subset of the total 558 cases available) where hepatic tissues were re-examined, eight cases were positive, three of which were not diagnosed on initial examination, indicating that the sensitivity of routine hepatic histology was less than 62.5%. All birds diagnosed with hepatic coccidiosis were brown kiwi, with the majority of birds from captivity (80%, n=8/10) and the rest from the wild (20%, n=2/10). Ages were variable. Most (70%, n = 7/10) were juveniles, with known ages ranging from 8 weeks to 18 months, and 30% (n=3/10) were adult birds.
Hepatic coccidiosis was the primary cause of death in two birds, although one case also had enteric coccidiosis listed as the primary cause of death. A clinical history was available for 60% (n=6/10) of the birds diagnosed with hepatic coccidiosis. Of these six birds, one demonstrated no clinical signs prior to death (17%), while 66% (n=4/6) of birds showed a reduction in food intake or complete inappetance. Other clinical signs included diarrhoea (n=1/6), haematochezia (n=2/6), depression/lethargy (n=2/6), respiratory distress (n=1/6), and poor body condition (n=1/6). Many of these birds had concurrent disease, which included renal and enteric coccidiosis.
Gross hepatic pathology was observed in 50% (n=5/10) of cases. Most (80%, n=4/5) showed multifocal white pinpoint (<1 mm) lesions on the serosal surface and within the parenchyma of the liver (a). Hepatic congestion was noted in the remaining case.
Immature and mature meronts measuring a mean of 304×227 µm, were identified within the hepatic parenchyma surrounded by a variable zone of necrosis (b). Meronts were closely associated with blood vessels and may have been were located within endothelial cells. There was a mild inflammatory response surrounding the meronts, including macrophages and lymphocytes. There was also a mild periportal cholangitis including hyperplasia of bile ductules, although meronts were not found within these structures. Immature meronts were characterized by a basophilic membrane that infolded towards the centre, and each infold hosted a blastophore-like structure (c). Within the body of the macromeront, numerous blastophores were present, which increased in size as the macromeront matured. Mature meronts were characterized by merozoites radially projecting from a central blastophore. In one case, there was an additional morphologically distinctive form of mature meront. This was a similar size to the others (399×237 µm) but consisted of blastophores with projecting merozoites embedded in a homogeneous eosinophilic matrix (d). In addition, a thick eosinophlic capsule surrounded the meront, with an outer zone of loose, slightly basophilic mucinous material suggestive of mucopolysaccharides. The presence of red blood cells adjacent to the capsule margin suggested these meronts were located within a blood vessel wall.
Splenic and pulmonary coccidiosis
Splenic coccidiosis was detected in two kiwi necropsied at our laboratory between 1991 and 2011. In one of these cases (Case 2589), concurrent infections with hepatic, intestinal and renal coccidiosis were observed; and in the other (Case 4929), no other forms of coccidia were seen. In this case, associated clinical signs included a reduced appetite and weight loss. On histology, splenic coccidiosis was seen as a large cluster of meronts. These measured 8.4×6.7 µm and were located adjacent to a blood vessel ().
Figure 4. Pulmonary histopathology of a juvenile brown kiwi illustrating coccidial meronts (H&E stain). Scale bar = 100 µm.
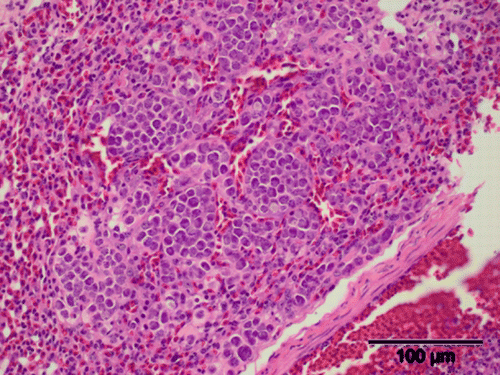
Pulmonary coccidiosis was also identified in a single case that had concurrent enteric and hepatic coccidia (Case 5133). In the 12 h leading up to its death, this bird was lethargic and inappetant, progressing to lateral recumbency after 12 h. Gross pulmonary changes included a 4 to 6 mm diameter area of haemorrhage on the ventral surface of each lung lobe. Meronts measured 9.6×7.1 µm and were arranged in a large cluster approximately 300 µm in diameter (Figure 4). These were similar in appearance to those found within the spleen.
Discussion
This study has found that extra-intestinal coccidial infection is common in the kiwi. Four forms of extra-intestinal coccidia were found: renal, hepatic, and, less commonly, splenic and pulmonary. Although renal coccidiosis is common in a number of avian species, naturally occurring hepatic coccidiosis is infrequently reported in avian hosts. Splenic and pulmonary coccidial organisms have been reported as part of disseminated visceral coccidiosis in cranes (Novilla & Carpenter, Citation2004). Pancreatic stages as reported by Hartley (Citation1995) were not found in this study.
Overall, extra-intestinal coccidial infection occurred in 4.5% of kiwi submitted to Massey University for necropsy between 1991 and 2011. Re-examination of both renal and hepatic histological sections revealed a number of previously undetected cases, and it is therefore likely that routine necropsy may overlook focal or minor infections. The use of PAS staining of renal tissue greatly enhanced the ability to detect oocysts and gametocytes, and should be used routinely for renal histology in kiwi. Since 2005, PAS staining of renal tissue has been part of the routine necropsy of kiwi at Massey University, inferring better detection of renal coccidiosis since this date. Multiple sections of all tissues affected by extra-intestinal coccidia (kidney, liver, spleen, lung) are recommended to increase the chances of finding coccidial stages. A significant limitation of the present study was that, because all kiwi sampled were dead, this may not be a representative group of kiwi for establishing a true prevalence of extra-intestinal coccidiosis in kiwi. As this study shows, most kiwi do not die from extra-intestinal coccidiosis, and the true prevalence of extra-intestinal coccidiosis may be higher than seen in this study.
Identification of renal coccidiosis in only young kiwi agrees with findings in other avian species, where disease caused by renal coccidia is usually limited to naïve, young birds (Gajadhar et al., Citation1983a; Wobeser, Citation1974, Citation1997). Although it is known that infection with renal Eimeria spp. in other avian hosts results in the development of immunity, little is known about host immunity to Eimeria species developing at other extra-intestinal sites (Spalding et al., Citation2008; Yabsley, Citation2008). Hepatic coccidiosis seen in this study affected kiwi of various ages, including adults. Similarly, coccidiosis affecting multiple viscera, including the liver, has been seen in a naturally infected adult whooping crane, although usually this disease is seen in juvenile cranes (Novilla et al., Citation1989).
As well as individual susceptibility to infection, transmission of coccidia between individuals and subsequent disease are influenced by a number of other factors, including host concentration and distribution of oocysts within the environment (Spalding et al., Citation2008). Intensive production in the poultry industry means chickens are kept at higher density than their wild counterparts, increasing the infection pressure of diseases such as coccidia (McDougald, Citation2003). Similarly, Operation Nest Egg increases the population density of young susceptible kiwi, and in the present study captive birds accounted for the majority of cases of extra-intestinal coccidiosis, and all mortalities relating to this disease. This is similar to other free-ranging avian populations, where renal coccidiosis is rarely an issue, although outbreaks of disease may be seen with an increase in density of birds such as that occurring during breeding, or other stressors such as habitat degradation (Yabsley, Citation2008).
Although brown kiwi are the most common species reared in captivity through Operation Nest Egg, an increasing number of other species, including Haast tokoeka, rowi and great spotted kiwi, are being reared through this programme, and in some instances more than one species of kiwi are reared in the same Operation Nest Egg facility. Oocysts have evolved to withstand many environmental conditions, and even with good hygiene practices there will be a significant risk of transfer of coccidia between individuals and therefore between species in these situations. Although coccidia are well known to be highly host specific, it is not known whether kiwi coccidia have the ability to transfer between closely related species of kiwi.
Extra-intestinal coccidiosis was the primary cause of death in five to seven (0.9 to 1.2%) birds in this study. It should be noted that the majority of deaths occurred between 2003 and 2005, and at the time these deaths triggered a review of husbandry techniques and an increased awareness and improvement in diagnosis and treatment of coccidiosis in captive young kiwi (personal observations). Many infections detected in this study were also complicated by concurrent disease, making it difficult to ascertain the exact impact of extra-intestinal coccidia on kiwi. As occurs in other host species, extra-intestinal coccidiosis probably increases susceptibility to other diseases (Novilla & Carpenter, Citation2004).
Renal coccidiosis was the most common form of extra-intestinal coccidiosis in this study. A clinical history was only available for birds in captivity at the time of death, and of these a reduction in appetite was the most consistent clinical sign. Clinical signs of renal coccidiosis in other avian species are rarely observed because most reports are from surveys of wild birds (Yabsley, Citation2008). Signs that have been observed in individual birds or experimental infections with renal coccidiosis have included weakness, depression, blood-tinged milky-white droppings or diarrhoea, inappetance, emaciation, and, in flighted species, an inability to fly (Montgomery et al., Citation1978; Gajadhar et al., Citation1983b).
There are few gross changes reported in the kidneys in other avian species with mild to moderate renal coccidial infections, (Tuggle & Crites, Citation1984). This was also the case in the present study, and the most significant renal changes seen at necropsy generally correlated with the most severe infections. Although direct impression smears of the kidneys were not taken during necropsy, it is most likely that the white serosal lesions observed contained oocysts and accumulations of urates as seen in other avian species (Gajadhar et al., Citation1982; Tuggle & Crites, Citation1984; Wobeser, Citation1997).
Some reports of renal coccidiosis in other avian species claim that only sexual stages of Eimeria were found in the kidney and it was suggested that asexual reproduction occurred elsewhere (Wobeser, Citation1974; Nation & Wobeser, Citation1977; Obendorf & McColl, Citation1980; Franson & Derkson, Citation1981; Gomis et al., Citation1996; Wobeser, Citation1997). However, other reports describe asexual stages within the renal tubular epithelium, providing evidence that at least one asexual phase of development of some Eimeria spp. occurs within the kidney (Montgomery et al., Citation1978; Cawthorn & Stockdale, Citation1982; Yun et al., Citation2000; Yabsley et al., Citation2002). As the kidney is a site of both merogony and gametogony in the kiwi, it is plausible that the entire lifecycle of a renal species of coccidia may occur at this site. It is also possible that one or more asexual stage of reproduction occurs at sites other than the renal tract, for example the intestinal tract. Many avian host species harbour both intestinal and renal coccidia, and, to date, renal species of avian Eimeria are reported to be distinct from those found in the intestine (Gajadhar et al., Citation1983b). Owing to the strict site specificity of Eimeriid coccidia in mammals and birds (Ball et al., Citation1989), it is extremely unlikely that one species of kiwi coccidia can undergo a single phase of gametogony in both the intestine and kidneys. Furthermore, all renal stages in this study are morphologically distinct in size from earlier descriptions of intestinal coccidia (Morgan et al., Citation2012).
The most common clinical sign associated with hepatic coccidiosis was a reduction in appetite, as was the case with renal coccidiosis. Other clinical signs included diarrhoea, haematochezia, dehydration and depression. The magpie-lark with hepatic coccidiosis was found moribund shortly before death (Reece, Citation1989), and clinical signs exhibited with disseminated visceral coccidiosis in experimentally infected cranes include anorexia, weakness, emaciation and diarrhoea (Novilla et al., Citation1989). Naturally infected cranes did not demonstrate clinical signs (Novilla & Carpenter, Citation2004). Gross hepatic changes associated with coccidial lesions in the present study on the kiwi are typical of those seen in hepatic coccidial infections in other avian and mammalian host species (Critchley et al., Citation1986; Reece, Citation1989; Dai et al., Citation1991; Canfield & Hartley, Citation1992; Pakandl, Citation2009; O'Brien et al., Citation2011; Wessels et al., Citation2011). Most reports of hepatobiliary coccidiosis in other host species, including the magpie-lark and experimentally immunosuppressed chickens, occurred within the biliary duct epithelium (Clark, Citation1970; Long, Citation1970, Citation1971; Lee & Long, Citation1972; Levine & Ivens, Citation1972; Collins et al., Citation1988; Reece, Citation1989; Dai et al., Citation1991; Brunnert et al., Citation1992; Schafer et al., Citation1995; Williams et al., Citation1996; Pakandl, Citation2009). Lesions within hepatic tissue outside the biliary system have not been described previously in natural infections of any avian species, although they have been reported in wallabies and in a single goat (Canfield & Hartley, Citation1992; Mahmoud et al., Citation1994). Experimental intravenous inoculation of chick embryos with E. tenella sporozoites resulted in the development of meronts in mainly fibrocytes of the liver connective tissue and, less frequently, in endothelial cells and hepatocytes, with occasional gametocytes also found in hepatocytes (Lee & Long, Citation1972).
Only asexual stages were identified in the liver of kiwi, whereas in the magpie-lark both meronts and gametocytes were found within the biliary system (Reece, Citation1989). This suggests that gametogony may occur at a distant site in the kiwi, as occurs in cranes (Spalding et al., Citation2008). Given that hepatic coccidiosis was invariably associated with concurrent enteric coccidiosis (where enteric tissues were available for examination), it is feasible that gametogony occurs in the intestine. Alternatively, it is possible that gametogony had yet to occur in all kiwi livers examined, although this scenario is less likely.
The close association of meronts to hepatic blood vessels seen in the present study suggests at least some of the dissemination of sporozoites from the intestine may occur haematogeneously. The route of migration in other hepatic species of coccidia is unclear; however, both lymphatic and haematogenous spread have been implicated (Smetana, Citation1933; Horton, Citation1967; Pakandl, Citation2009). Asexual and sexual stages of E. reichenowi and E. gruis in cranes occur primarily in macrophages, and it is suggested that these species use this route to penetrate into the blood and/or lymphatic system (Novilla et al., Citation1981).
The findings in this study are in keeping with observations that there are at least two, and possibly three, species of coccidia infecting kiwi (Morgan et al., Citation2012). As in other avian host species, it is plausible that at least one species undergoes complete development within the renal system with excretion of unsporulated oocysts via the ureters. Counter to this hypothesis, however, was the detection of only one morphological form of merogony in the renal system, suggesting that further stages of merogony may occur elsewhere. It remains unclear whether the hepatic, splenic and pulmonary meronts are an essential part of the organism's lifecycle, or an aberrant systemic dissemination of infection from an alternative location, possibly either the kidneys or the intestine. This aberrant migration may be the result of immunosuppression, as demonstrated by the development of intravenously inoculated E. tenella sporozoites in the liver of experimentally immunosuppressed chickens and chicken embryos (Long, Citation1970, Citation1971),
Although introduced mammalian predators are currently considered the key agent in decline of kiwi in the wild, Holzapfel et al. (Citation2008) also suggest that there may be other as yet unrecognized agents of decline of kiwi. Disease may be one of these, and this may be particularly pertinent with an increase in population manipulation for conservation management purposes. Captive rearing of young kiwi changes the epizootiology of natural infections with coccidia, and although wild kiwi appear to be less frequently affected by extra-intestinal coccidiosis, captive conditions increase the likelihood of young kiwi developing severe coccidiosis. Translocation of individual birds from captive rearing houses carries the risk of introducing a novel species of coccidia to a naïve population of wild kiwi, and consideration must be given to management of coccidial burdens prior to re-release of kiwi to wild populations. Further research is required to understand the lifecycles of these extra-intestinal coccidia to enable careful management of this disease in captivity.
Acknowledgements
Thanks to the Department of Conservation and to the kiwi captive rearing facilities for supplying the samples used in this study, and to the many pathologists who performed the original necropsies on the kiwi used in this study and who contributed their results to the Huia database. The diagnosis of pulmonary coccidiosis was provided by Stuart Hunter. Thanks also to Barbara Adlington, Anne Tunnicliffe and Evelyn Lupton for parasitological and histological technical support; and to the Chamberlain family for allowing access to the kiwi on their property. The authors would like to thank the Pacific Vet Avian Research Fund, the Institute of Veterinary, Animal and Biomedical Sciences McGeorge and the Lewis Fitch research funds, and the Institute of Veterinary, Animal and Biomedical Sciences postgraduate research fund for financial assistance.
References
- Alley , M.R. , Gartrell , B.D. and Morgan , K.J. 2004 . Coccidiosis in kiwi, Apteryx mantelli . Kokako , 11 : 29 – 30 .
- Anon 2012 Operation Nest Egg. Retrieved March 13, 2013, from http://www.kiwisforkiwi.org/
- Ball , S.J. , Pittilo , R.M. and Long , P.L. 1989 . Intestinal and extraintestinal life cycles of Eimeriid coccidia . Advances in Parasitology , 28 : 1 – 54 .
- Basse , B. , McLennan , J.A. and Wake , G.C. 1999 . Analysis of the impact of stoats, Mustela erminea, on northern brown kiwi, Apteryx mantelli, in New Zealand . Wildlife Research , 26 : 227 – 237 . doi: 10.1071/WR97091
- Boardman , W. 1995 . Causes of mortality in North Island brown kiwis at Auckland Zoo 1960–1994 . Kokako , 2 : 11 – 13 .
- Brunnert , S. , Citino , S. , Herron , A. and Altman , N. 1992 . Hepatic coccidiosis in chamois (Rupicapra rupicapra) . Journal of Zoo and Wildlife Medicine , 23 : 276 – 280 .
- Canfield , P. and Hartley , W. 1992 . A survey and review of hepatobiliary lesions in Australian macropods . Journal of Comparative Pathology , 107 : 147 – 167 . doi: 10.1016/0021-9975(92)90032-P
- Cawthorn , R.J. and Stockdale , P.H.G. 1982 . Oocyst production, late-stage merogony, gametogony, and cross-transmission studies of Eimeria bubonis (Protozoa: Eimeriidae) of the great horned owl, Bubo virginiansus . Canadian Journal of Zoology , 60 : 2279 – 2283 . doi: 10.1139/z82-294
- Clark , G. 1970 . Eimeria ahtanumensis n. sp. from the Northwestern fence lizard Sceloporus occidentalis in Central Washington . Journal of Protozoology , 17 : 526 – 530 .
- Colbourne , R.M. , Bassett , S. , Billing , T. , McCormick , H. , McLennan , J.A. , Nelson , A. and Robertson , H.A. 2005 . The Development of Operation Nest Egg as a Tool in the Conservation Management of Kiwi. Science for Conservation , 24 Wellington , , New Zealand : Department of Conservation .
- Collins , J. , Dubey , J. and Rossow , K. 1988 . Hepatic coccidiosis in a calf . Veterinary Pathology , 25 : 98 – 100 . doi: 10.1177/030098588802500118
- Critchley , K.L. , Long , P.L. , Johnson , J. , Fletcher , O.J. and Glisson , J.R. 1986 . Coccidial oocysts in the liver of a turkey poult . Avian Pathology , 15 : 619 – 621 . doi: 10.1080/03079458608436321
- Dai , Y.B. , Menchu , L. , Shenxing , Z. and Aoqi , F. 1991 . Hepatic coccidiosis in the goat . International Journal for Parasitology , 21 : 381 – 382 . doi: 10.1016/0020-7519(91)90045-9
- Franson , J.C. and Derkson , D.V. 1981 . Renal coccidiosis in oldsquaws (Clangula hyemalis) from Alaska . Journal of Wildlife Diseases , 17 : 237 – 239 .
- Gajadhar , A.A. , Cawthorn , R.J. and Rainnie , D.J. 1982 . Experimental studies on the life cycle of a renal coccidium of lesser snow geese (Anser c. caerulescens) . Canadian Journal of Zoology , 60 : 2085 – 2092 . doi: 10.1139/z82-267
- Gajadhar , A.A. , Cawthorn , R.J. , Wobeser , G.A. and Stockdale , P.H.G. 1983a . Prevalence of renal coccidia in wild waterfowl in Saskatchewan . Canadian Journal of Zoology , 61 : 2631 – 2633 . doi: 10.1139/z83-345
- Gajadhar , A.A. and Leighton , F. 1988 . Eimeria wobeseri sp. n. and Eimeria goelandi sp. n (Protozoa: Apicomplexa) in the kidneys of herring gulls (Larus argentatus) . Journal of Wildlife Diseases , 24 : 538 – 546 .
- Gajadhar , A.A. , Wobeser , G. and Stockdale , P.H.G. 1983b . Coccidia of domestic and wild waterfowl (Anseriformes) . Canadian Journal of Zoology , 61 : 1 – 24 . doi: 10.1139/z83-001
- Gomis , S. , Didiuk , A.B. , Neufeld , J. and Wobeser , G. 1996 . Renal coccidiosis and other parasitologic conditions in lesser snow goose goslings at Tha-anne River, west coast Hudson Bay . Journal of Wildlife Diseases , 32 : 498 – 504 .
- Hartley , W. 1995 . Some interesting avian cases in the Taronga Zoo pathology collection . Kokako , 2 : 11
- Holzapfel , S.A. , Roberston , H. , McLennan , J. , Sporle , W. , Hackwell , K. and Impey , M. 2008 . Kiwi Recovery Plan 2008–2018 , 71 Wellington , , New Zealand : Department of Conservation .
- Horton , R.J. 1967 . The route of migration of Eimeria stiedae (Lindemann, 1865) sporozoites between the duodenum and bile ducts of the rabbit . Parasitology , 57 : 9 – 17 . doi: 10.1017/S0031182000071857
- Lee , D.L. and Long , P.L. 1972 . An electron microscopical study of Eimeria tenella grown in the liver of the chick embryo . International Journal of Parasitology , 2 : 55 – 58 . doi: 10.1016/0020-7519(72)90034-3
- Leighton , F. and Gajadhar , A.A. 1986 . Eimeria fraterculae sp. n. in the kidneys of Atlantic puffins (Fratercula arctica) from Newfoundland, Canada: species description and lesions . Journal of Wildlife Diseases , 22 : 520 – 526 .
- Levine , N.D. and Ivens , V. 1972 . Coccidia of the Leporidae . Journal of Protozoology , 19 : 572 – 581 .
- Long , P.L. 1970 . Development (schizogony) of Eimeria tenella in the liver of chickens treated with corticosteroid . Nature , 225 : 290 – 291 . doi: 10.1038/225290a0
- Long , P.L. 1971 . Schizogony and gametogony of Eimeria tenella in the liver of chick embryos . Journal of Protozoology , 18 : 17 – 20 .
- Mahmoud , O. , Haroun , E. and Sulman , A. 1994 . Hepato-biliary coccidiosis in a dairy goat . Veterinary Parasitology , 53 : 15 – 21 . doi: 10.1016/0304-4017(94)90012-4
- McDougald , L. 2003 . “ Protozoal infections ” . In Diseases of Poultry , 11th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 973 – 991 . Ames : Iowa State Press .
- McLennan , J.A. 1988 . Breeding of North Island brown kiwi, Apteryx australis mantelli, in Hawke's Bay, New Zealand . New Zealand Journal of Ecology , 11 : 89 – 97 .
- McLennan , J. , Potter , M. , Roberston , H. , Wake , G. , Colbourne , R. , Dew , L. , Joyce , L. , McCann , A. , Miles , J. , Miller , P. and Reid , J. 1996 . Role of predation in the decline of kiwi, Apteryx spp., in New Zealand . New Zealand Journal of Ecology , 20 : 27 – 35 .
- Miskelly , C.M. , Dowding , J.E. , Elliott , G.P. , Hitchmough , R.A. , Powlesland , R.G. , Roberston , H. , Sagar , P.M. , Scofield , R.P. and Taylor , G.A. 2008 . Conservation status of New Zealand birds, 2008 . Notornis , 55 : 117 – 135 .
- Montgomery , R.D. , Novilla , M.N. and Shillinger , R.B. 1978 . Renal coccidiosis caused by Eimeria gaviae n. sp. in a common loon (Gavia immer) . Avian Diseases , 22 : 809 – 814 . doi: 10.2307/1589663
- Morgan , K.J. , Alley , M.R. , Pomroy , W.J. , Castro , I. and Howe , L. 2012 . Enteric coccidiosis in the brown kiwi (Apteryx mantelli) . Parasitology Research , 111 : 1689 – 99 . doi: 10.1007/s00436-012-3008-5
- Nation , P.N. and Wobeser , G. 1977 . Renal coccidiosis in wild ducks in Saskatchewan . Journal of Wildlife Diseases , 13 : 370 – 375 .
- Novilla , M.N. and Carpenter , J.W. 2004 . Pathology and pathogenesis of disseminated visceral coccidiosis in cranes . Avian Pathology , 33 : 275 – 280 . doi: 10.1080/0307945042000203371
- Novilla , M.N. , Carpenter , J.W. , Jeffers , T.K. and White , S.L. 1989 . Pulmonary lesions in disseminated visceral coccidiosis of sandhill and whooping cranes . Journal of Wildlife Diseases , 25 : 527 – 533 .
- Novilla , M.N. , Carpenter , J.W. , Spraker , T. and Jeffers , T.K. 1981 . Parenteral development of Eimerian coccidia in sandhill and whooping cranes . Journal of Protozoology , 28 : 248 – 255 .
- Obendorf , D. and McColl , K. 1980 . Mortality in little penguins (Eudyptes minor) along the coast of Victoria . Journal of Wildlife Diseases , 16 : 251 – 259 .
- O'Brien , M. , Brown , M. , Stidworthy , M. , Peirce , M. , Marshall , R. , Honma , H. and Nakai , Y. 2011 . Disseminated visceral coccidiosis in Eurasian granes (Grus grus) in the UK . Veterinary Record , 168 : 216 doi: 10.1136/vr.c6409
- Pakandl , M. 2009 . Coccidia of rabbit: a review . Folia Parasitologica , 56 : 153 – 166 .
- Reece , R. 1989 . Hepatic coccidiosis (Eimeria sp.) in a wild magpie-lark (Grallina cyanoleuca) . Avian Pathology , 18 : 357 – 362 . doi: 10.1080/03079458908418609
- Schafer , K. , Stevenson , G. and Kazacos , K. 1995 . Hepatic coccidiosis associated with hepatic necrosis in a goat . Veterinary Pathology , 32 : 723 – 727 . doi: 10.1177/030098589503200618
- Smetana , H. 1933 . Coccidiosis of the liver of rabbits: experimental study of the mode of infection of the liver by sporozoites . Archives of Pathology , 15 : 330 – 339 .
- Spalding , M.G. , Carpenter , J.W. and Novilla , M.N. 2008 . “ Disseminated visceral coccidiosis in cranes ” . In Parasitic Diseases of Wild Birds , Edited by: Atkinson , C. , Thomas , N.J. and Hunter , D. 181 – 194 . Ames : Wiley Blackwell .
- Thompson , E.J. and Wright , I.G.A. 1978 . Coccidiosis in kiwis . New Zealand Veterinary Journal , 26 : 167 doi: 10.1080/00480169.1978.34530
- Tuggle , B.N. and Crites , J.L. 1984 . Renal coccidiosis in interior Canada geese, Branta canadensis interior Todd, of the Mississippi Valley population . Journal of Wildlife Diseases , 20 : 272 – 278 .
- Wessels , J. , Wessels , M. , Wood , R. and Quayle , J. 2011 . Hepatic coccidiosis in red lechwe (Kobus leche leche) . Veterinary Record , 168 : 388 doi: 10.1136/vr.d2191
- Williams , B. , Chimes , M. and Gardiner , C. 1996 . Biliary coccidiosis in a ferret (Mustela putorius furo) . Veterinary Pathology , 33 : 437 – 439 . doi: 10.1177/030098589603300412
- Wobeser , G. 1974 . Renal coccidiosis in mallard and pintail ducks . Journal of Wildlife Diseases , 10 : 249 – 255 .
- Wobeser , G. 1997 . Diseases of Wild Waterfowl , 2nd ed. , 119 – 124 . New York : Plenum Press .
- Yabsley , M. 2008 . “ Eimeria ” . In Parasitic Diseases of Wild Birds , Edited by: Atkinson , C. , Thomas , N.J. and Hunter , D. 162 – 180 . Ames : Wiley Blackwell .
- Yabsley , M.J. , Gottdenker , N.L. and Fischer , J.R. 2002 . Description of a new Eimeria sp. and associated lesions in the kidneys of double-crested cormorants (Phalocrocorax auritus) . Journal of Parasitology , 88 : 1230 – 1233 .
- Yun , C.H. , Lillehoj , H.S. and Lillehoj , E.P. 2000 . Intestinal immune responses to coccidiosis . Developmental and Comparative Immunology , 24 : 303 – 324 . doi: 10.1016/S0145-305X(99)00080-4