Abstract
Low-pathogenic avian influenza virus (H9N2) is circulating in the poultry industry of many countries in the Middle East and Asia, causing serious economic damage. In this study the clinical signs, antibody response, viral shedding and efficacy of oil emulsion vaccines in Chukar partridges were investigated until 9 days post inoculation (d.p.i.). Seventy-five Chukar partridges (Alectoris chukar) were divided randomly in three groups of challenged (Group C), vaccinated and challenged (Group VC) and control (non-vaccinated and non-challenged [Group NC]), 25 birds/group. Groups C and VC were inoculated with 0.4 ml allantoic fluid containing 107 median embryo infective dose/bird of A/Chicken/Iran/772/1998(H9N2) avian influenza virus. Clinical signs, antibody response, viral shedding and vaccine efficacy were evaluated and compared among these groups over 9 days. Clinical signs such as coughing and sneezing with depression and decreased feed and water consumption were observed in Group C. In Group VC only a slight decrease in food and water consumption was observed. Both Groups C and VC showed maximum antibody titre at 9 d.p.i. At 1 d.p.i. the virus was detected from all tissues in challenged group, but the virus was not detected from the spleen and caecal tonsil of Group VC. Group C showed the longest period of viral shedding in the trachea and kidney.
Introduction
Avian influenza (AI) is a viral disease that infects both domestic and wild birds. The disease can result in a range of signs from a subclinical infection up to 100% mortality. Based on the pathogenicity of AI viruses in avian species, these viruses are pathotyped into highly pathogenic AI viruses and low-pathogenic AI viruses (Capua & Alexander, Citation2006). The virus is transmitted by both direct and indirect contact between infected and susceptible birds (Easterday et al., Citation1997). AI viruses have been isolated from many species of birds (Swayne & Halvorson, Citation2008) such as chickens, turkeys, Muscovy ducks, geese, quail, guinea fowl, partridges, including chukars, pheasants and ostriches (Alexander, Citation2008). Frequent infection of humans with some AI viruses (H5N1, H7N7, H7N3, and H9N2) from poultry has been observed, and therefore AI viruses present a public health threat as a zoonotic pathogen, although the risk is considered low (Subbarao et al., Citation1998; Peiris et al., Citation1999; Koopmans et al., Citation2004). Since the first report of H9N2 virus isolation from turkeys in North America in 1966, more attention has been paid to the virus in recent years. The H9N2 AI subtype is circulating in the commercial poultry industry in the Middle East and Asian countries, causing high economic losses (Guo et al., Citation1999; Peiris et al., Citation1999; Lin et al., Citation2000; Butt et al., Citation2005). In Iran, the epidemic of H9N2 AI was first reported in 1998 (Nili & Asasi, Citation2003). In some of the infected farms the mortality rate was as high as 65%. This virus could potentially reassort with a human influenza A virus to produce the next human influenza pandemic. The origin of new pandemic viruses is not clearly known, although it appears that it can be caused by a completely new virus being introduced into the human population or by a reassortment event between the circulating human strain and another influenza virus (Wright et al., Citation2007). Considering a recent report by the World Health Organization, H9 together with H5 and H7 subtypes are among the leading candidates for future influenza pandemics (OIE, Citation2008). The recurring presence of H9N2 infections in humans has raised concerns about the possibility of H9N2 viruses evolving into pandemic strains.
On rare occasions, low-pathogenic AI viruses have been isolated from free-living non-aquatic birds in the orders Piciformes, Passeriformes (sparrows, starlings,), Columbiformes (doves and pigeons), and Galliformes (pheasant and partridge) (Stallknecht, Citation1998; Stallknecht et al., Citation2007). Nevertheless, to date, most experimental studies on AI are based on either chickens, turkeys or waterfowl species, while investigation into the ability of influenza A viruses to replicate in minor poultry species is scarce (Perkins & Swayne, Citation2001; Humberd et al., Citation2006; Jeong et al., Citation2009), and numerous aspects of the epidemiology of both low-pathogenic AI viruses and highly pathogenic AI viruses in free-range raised poultry and game birds still remain unclear. In recent years Chukar partridge breeding has been getting more popular in Iran, so it would sensible to evaluate the pathogenesis of H9N2 AI virus in this species. The Chukar partridge is a species that is kept in live markets for weeks because of its higher price and it has been well documented that partridges can be infected with AI viruses (Easterday & Tumova, Citation1972; Perkins & Swayne, Citation2001, Citation2003; Bertran et al., Citation2011).
Humberd et al. (Citation2006) conducted a study to investigate the replication and transmission of AI A viruses in Chukar partridge but the investigation of the replication was made only on the basis of tracheal and cloacal swabs. Surprisingly, there is no study about the pathogenicity of the AI H9N2 subtype in different organs of Chukar partridge (Alectoris chukar). Further, little research is available on vaccines in Chukar partridges, quail, pheasants, guinea fowl, ratites, and zoo birds. The objective of the present study is to investigate the pathobiology of AI H9N2 in Chukar partridge and to evaluate the efficacy of oil emulsion vaccination in this bird.
Materials and Methods
Experimental birds and trials
Seventy-five Chukar partridges (A. chukar), 165 days old, which were serologically negative for AI viruses, were used in this experiment. Birds were divided randomly into three groups of 25 birds, as follows: challenged (Group C), vaccinated and challenged (Group VC), and control or non-vaccinated and non-challenged (Group NC) groups. Birds were reared separately in the Animal Research Unit of the Veterinary School of Shiraz University and received feed and water ad libitum during the experiment.
Challenge trial
The virus isolate used in this study was A/Chicken/Iran/772/1998(H9N2). The isolate was propagated twice in 9-day-old to 11-day-old embryonated chicken eggs. The median embryo infective dose was calculated according to the Reed & Muench (Citation1938) formula. Birds in Group VC were vaccinated with 0.3 ml/bird inactivated AI (H9N2) vaccine (Pasouk Biological Research and Manufacturing Company, Tehran, Iran) on day 165 via the subcutaneous route. All birds in Groups C and VC were then challenged separately intranasally and ocularly with 0.2 ml allantoic fluid containing 107 median embryo infective dose/bird of the H9N2 virus on day 177 ().
Table 1. Experimental design for evaluating the pathogenesis and protection against challenge strain A/Chicken/Iran/772/1998(H9N2).
Growth performance and signs
Following challenge, birds were monitored daily for overt clinical signs and mortality.
Serum samples
Serum samples from 20 birds in total were randomly collected and tested before inoculation (vaccination and challenge) to ensure that the birds were serologically negative for AI H9N2 virus. Blood samples were collected for haemagglutination inhibition tests using partridge red blood cells according to the Manual of Standards for Diagnostic Test (OIE, Citation2008) using antigen provided by Pasouk Biological Research and Manufacturing Company. At days 1, 3, 6 and 9 post challenge, four birds from each group were subjected to blood sampling to determine the antibody titres of AI virus. Blood was centrifuged at 150×g for 10 min and then sera samples were frozen at −20°C until use. One-way analysis of variance was used to show statistical significance of serology results.
Reverse transcriptase-polymerase chain reaction extraction
Before viral inoculation and at days 1, 3, 6 and 9 post challenge, four birds from each group were euthanized. Samples from the trachea, lungs, spleen, kidneys, caecal tonsil and faeces swab were aseptically collected for virus detection by reverse transcriptase-polymerase chain reaction (RT-PCR) assay. All samples were immediately stored at −70°C until used. RNX solution (Cinnagen, Tehran, Iran) was used for total RNA extraction. About 100 mg of each tissue sample was homogenized with 1 ml RNX and then 200 µl chloroform was added into the mixture and centrifuged at 16,090×g at 4°C for 10 min. The aqueous top phase was then added to an equal volume of isopropanol and centrifuged at 16,090×g at 4°C for 10 min. After the washing step, the dried RNA pellet was completely resuspended in 50 µl distilled water. All RNA solution was stored at −70°C until used.
PCR protocol
The cDNA was synthesized using an AccuPowder® RTPreMix kit (BioNeer Corporation, Daejeon, South Korea) according to the manufacturer's instruction. The primer AIMCD was specific for M1 protein gene and is shown in (Ward et al., Citation2004). Five microlitres of total RNA and 10 pmol of M1-specific primer were used for cDNA preparation.
Table 2. RT-PCR primers and sequences.
PCR was performed to amplify a 450-base-pair fragment of the M1 protein gene of AI virus using M protein genes primers (CN1, CN2) () designed at the Laboratory of Virology, School of Veterinary Medicine, Shiraz University, Iran. The reaction was accomplished in a total volume of 20 µl containing 2 µl of 10× PCR buffer, 0.6 µl MgCl2 (50 mM stock), 0.4 µl dNTP (10 mM), 1 u Taq DNA polymerase, 5 µl cDNA and 10 pmol of the primers CN1 and CN2. The reaction mixture was subjected to 94°C for 5 min and 35 cycles of 94°C for 35 sec, 54.9°C for 45 sec and 72°C for 45 sec, followed by a final extension at 72°C for 5 min (Mohammadi et al., Citation2010). The PCR products were separated in 1% agarose gel and visualized under ultraviolet light.
Results
Clinical signs, gross lesions and mortality
Prior to the experiment, birds in all groups were healthy and did not show any clinical signs. Also, after virus inoculation no mortality was seen in any group. Birds in Group NC showed no clinical signs, gross lesions or mortality. Birds in Group C showed clinical signs including coughing and sneezing with depression and decreased feed and water consumption at 5, 6 and 7 days post inoculation (d.p.i.). In Group VC a slight decrease of food and water consumption was observed on day 6 post inoculation and disappeared at 8 d.p.i. The only lesion was hyperaemia in the trachea and small intestine at 6 d.p.i. in two birds of Group C.
Serological findings
Blood sampling prior to the experiment did not show any previous exposure to H9N2 AI virus. The results of the haemagglutination inhibition test on serum samples collected on days 1, 3, 6 and 9 post inoculation are presented in .
Table 3. Mean antibody titres of partridges (log 2).
The mean antibody titre was increased at 6 d.p.i. in Group C and reached 5.75 at 9 d.p.i. The mean antibody titre in Group VC was 4.25 on day 1 post inoculation and 5.75 at 9 d.p.i. There was no significant increase of the antibody titre against H9N2 AI virus in the control group (Group NC) chicks (P>0.05).
Virus detection in tissues at different days post infection
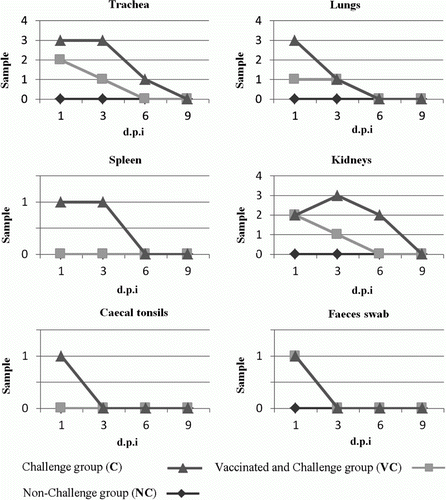
The presence of the virus genome was checked in all samples obtained from the inoculated and control birds at different d.p.i. The results of viral genome detection measured by RT-PCR are presented in and . It should be noted that samples collected before viral inoculation and in Group NC were negative for viral genome detection. As indicated in , at 1 d.p.i. the virus was detected from all tissues in Group C but not from the spleen and caecal tonsil of Group VC. At day 3 post inoculation, most of the trachea and kidney tissues of Group C were RT-PCR-positive, of which three out of four were positive in both organs. At 6 d.p.i. the virus was detected from just one sample of the trachea and two samples from the kidney in Group C. In Group VC on days 6 and 9 post inoculation and in Group C at day 9 post inoculation, the virus detection was negative. Overall, the highest detection of H9N2 virus was from the trachea and kidneys (10/32) and the lowest was from the caecal tonsil (1/32).
Table 4. Detection of the virus genome in various organs in Chukar partridges in different groups following inoculation of A/Chicken/Iran/772/1998(H9N2).
Discussion
Generally it is accepted that low-pathogenic AI virus infections are limited to the respiratory and gastrointestinal tract. Recent studies show that H9N2 AI viruses can also be isolated from the kidney (Mosleh et al., Citation2009). Non-aquatic species have not been considered reservoirs of low-pathogenic AI viruses, and generally infection in these species is thought to occur via spillover from infected domestic poultry (Stallknecht et al., 1998). There are reports that indicate partridge could be a source of fowl plague (Nettles et al., Citation1985), but none of the previous studies have investigated the infection dynamics of H9N2 AI viruses in Chukar partridge and the effect of influenza vaccine in this species.
In this study, few or no clinical signs were observed in the birds monitored throughout this study. These results are in agreement with those that Humberd et al. (Citation2006) and Bertran et al. (Citation2011) reported previously. The clinical signs in experimental studies with chickens are mostly milder than in birds under field conditions (Swayne & Slemons, Citation1994; Pourbakhsh et al., Citation2000; Nili & Asasi, Citation2003) due to co-infection with other pathogens such as Escherichia coli or a second virus such as infectious bronchitis virus and/or in combination with a poor climate.
Our results indicate that predominant infection was for the respiratory organs and kidney, in which detection of the virus genome continued until 6 d.p.i. in the challenged group (Group C). Humberd et al. (Citation2006) detected H9N2 virus in the trachea of experimentally infected Chukar partridges for 7 days. The results of our study are also similar to the finding of Manjili et al. (Citation2011) in specific pathogen free (SPF) chickens, as H9N2 virus was detected in the lung and kidney for 7 d.p.i. in this study. Kwon et al. (Citation2008) reported that H9N2 viral antigen was detected in 100% of samples of caecal tonsils of SPF chickens on day 5 post inoculation. Hablolvarid et al. (Citation2004) recovered viral antigen from the caecal tonsil at 10 d.p.i. in SPF chickens. In contrast to the previous studies in chickens, in the present study detection of the virus genome from the caecal tonsil was just from one sample on day 1 post inoculation in Group C. Faeces samples that were obtained to measure the virus shedding were only positive at 1 d.p.i. in both Groups C and VC. In agreement with our results, Humberd et al. (Citation2006) did not detect any viral antigen from cloacal samples of Chukar partridge. In contrast, in chickens, Mosleh et al. (Citation2009) recovered viral RNA from faeces on day 6 (4/5) post inoculation, and Kwon et al. (2008) detected virus antigens in cloacal swabs on days 5 and 7 post inoculation. One of the possible explanations for this difference could be a difference in affinity of this virus for the upper respiratory system and gastrointestinal tract of Chukar partridges compared with chickens.
Perkins & Swayne (Citation2001) reported that initial detection of antigen of highly pathogen H5N1 AI virus in several tissues from Chukar partridge has a significant delay in comparison with chickens, turkeys, Japanese and Bobwhite quail, guinea fowl and pheasants. However, in the present study, detection of genome of low-pathogenic H9N2 AI virus was initiated at 1 d.p.i. and lasted until 6 d.p.i. In comparison with the chicken, initial detection of H9N2 virus genome was at 3 d.p.i. and lasted longer (Manjili et al., Citation2011; Tavakkoli et al., Citation2011).
One can conclude that the vaccination of Chukar partridges was not able to prevent virus replication after challenge, but it did result in a reduction in the virus replication and clinical signs.
References
- Alexander , D.J. 2008 . Orthomyxoviridae –avian influenza . M. Pattison , P.F. McMullin , J.M. Bradbury , & D.J. Alexander In Poultry Diseases , 6th edn (pp. 317 – 332 ). Philadelphia, PA: W.B. Saunders .
- Bertran , K. , Pérez-Ramírez , E. , Busquets , N. , Dolz , R. , Ramis , A. , Darji , A. , Abad , F.X. , Valle , R. , Chaves , A. , Vergara-Alert , J. , Barral , M. , Höfle , U. and Majó , N. 2011 . Pathogenesis and transmissibility of highly (H7N1) and low (H7N9) pathogenic avian influenza virus infection in red-legged partridge (Alectoris rufa) . Veterinary Research , 42 : 24 doi: 10.1186/1297-9716-42-24
- Butt , K.M. , Smith , G.J. , Chen , H. , Zhang , L.J. , Leung , Y.H. , Xu , K.M. , Lim , W. , Webster , R.G. , Yuen , K.Y. , Peiris , J.S. and Guan , Y. 2005 . Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003 . Journal of Clinical Microbiology , 43 : 5760 – 5767 . doi: 10.1128/JCM.43.11.5760-5767.2005
- Capua , I. and Alexander , D.J. 2006 . The challenge of avian influenza to the veterinary community . Avian Pathology , 3 : 189 – 205 . doi: 10.1080/03079450600717174
- Easterday , B.C. , Hinshaw , V.S. and Halvorson , D.A. 1997 . “ Avian influenza ” . In Diseases of Poultry , 10th edn , Edited by: Calnek , B.W. , Barnes , H.J. , Beard , C.W. , McDougald , L.R. and Saif , Y.M. 583 – 605 . Ames : Iowa State Press .
- Easterday , B.C. and Tumova , B. 1972 . “ Avian influenza ” . In Diseases of Poultry , 6th edn , Edited by: Hofstad , M.S. , Calnek , B.W. , Helmbolt , C.F. , Reid , W.M. and Yoder , H.W. Jr . 670 – 700 . Ames : Iowa State Press .
- Guo , Y. , Li , J. and Cheng , X. 1999 . Discovery of men infected by avian influenza A (H9N2) virus . Zhonghua Shi Yan He Lin Chuang Bing Du XueZaZhi , 13 : 105 – 108 .
- Hablolvarid , M.H. , Sohraby Haghdost , I. , Pourbakhsh , S.A. and Gholami , M.R. 2004 . Histopathological study of intranasally inoculated A/Chicken/Iran/259/1998(H9N2) influenza virus in chicken . Archives of Razi Institute , 58 : 51 – 62 .
- Humberd , J. , Guan , Y. and Webster , R.G. 2006 . Comparison of the replication of influenza A viruses in Chinese ring-necked pheasants and Chukar partridges . Journal of Virology , 5 : 2151 – 2161 . doi: 10.1128/JVI.80.5.2151-2161.2006
- Jeong , O.M. , Kim , M.C. , Kim , M.J. , Kang , H.M. , Kim , H.R. , Kim , Y.J. , Joh , S.J. , Kwon , J.H. and Lee , Y.J. 2009 . Experimental infection of chickens, ducks and quails with the highly pathogenic H5N1 avian influenza virus . Journal of Veterinary Science , 10 : 53 – 60 . doi: 10.4142/jvs.2009.10.1.53
- Koopmans , M. , Wilbrink , B. , Conyn , M. , Natrop , G. , van der Nat , H. , Vennema , H. , Meijer , A. , van Steenbergen , J. , Fouchier , R. , Osterhaus , A. and Bosman , A. 2004 . Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands . The Lancet , 363 : 587 – 593 . doi: 10.1016/S0140-6736(04)15589-X
- Kwon , J.S. , Lee , H.J. , Lee , D.H. , Lee , Y.J. , Mo , I.P. , Nahm , S.S. , Kim , M.J. , Lee , J.B. and Park , S.Y. 2008 . Immune response and pathogenesis in immunocompromised chickens in response to infection with H9N2 low pathogenic avian influenza virus . Virus Research , 133 : 187 – 194 . doi: 10.1016/j.virusres.2007.12.019
- Lin , Y.P. , Shaw , M. , Gregory , V. , Cameron , K. , Lim , W. , Klimov , A. , Subbarao , K. , Guan , Y. , Krauss , S. , Shortridge , K. , Webster , R. , Cox , N. and Hay , A. 2000 . Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates . Proceedings of the National Academy of Sciences of the United States of America , 97 : 9654 – 9658 . doi: 10.1073/pnas.160270697
- Manjili , S.A. , Haghdoost , I.S. , Mortazavi , P. , Habibi , H. , Iashini , H. and Saberfar , E. 2011 . Detection of H9N2 avian influenza virus in various organs of experimentally infected chickens . African Journal of Microbiology Research , 5 : 5826 – 5830 .
- Mohammadi , A. , Masoudian , M. , Nemati , Y. and Seifi , S. 2010 . Serological and RT-PCR assays for detection of avian influenza of domestic pigeons in Kavar area (Fars province, Iran) . Bulgarian Journal of Veterinary Medicine , 13 : 117 – 121 .
- Mosleh , N. , Dadras , H. and Mohammadi , A. 2009 . Evaluation of H9N2 avian influenza virus dissemination in various organs of experimentally infected broiler chickens using RT-PCR . Iranian Journal of Veterinary Research , 10 : 12 – 20 .
- Nettles , V.F. , Wood , J.M. and Webster , R.G. 1985 . Wildlife surveillance associated with an outbreak of lethal H5N2 avian influenza in domestic poultry . Avian Diseases , 29 : 733 – 741 . doi: 10.2307/1590665
- Nili , H. and Asasi , K. 2003 . Avian influenza (H9N2) outbreak in Iran . Avian Diseases , 47 : 828 – 831 . doi: 10.1637/0005-2086-47.s3.828
- Office International Des Epizooties (OIE) . 2008 . Avian influenza . In Manual of Diagnostic Tests and Vaccines for Terrestrial Animcals (Mammals, Birds and Bees) . 6th edn (pp. 465 – 481 ) Paris , France .
- Peiris , M. , Yuen , K.Y. , Leung , C.W. , Chan , K.H. , Ip , P.L. , Lai , R.W. , Orr , W.K. and Shortridge , K.F. 1999 . Human infection with influenza H9N2 . The Lancet , 354 : 916 – 917 . doi: 10.1016/S0140-6736(99)03311-5
- Perkins , L.E. and Swayne , D.E. 2001 . Pathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species . Veterinary Pathology , 38 : 149 – 164 . doi: 10.1354/vp.38-2-149
- Perkins , L.E. and Swayne , D.E. 2003 . Comparative susceptibility of selected avian and mammalian species to a Hong Kong-origin H5N1 high-pathogenicity avian influenza virus . Avian Diseases , 47 : 956 – 967 . doi: 10.1637/0005-2086-47.s3.956
- Pourbakhsh , S.A. , Khodashenas , M. , Kianizadeh , M. and Goodarzi , H. 2000 . Isolation and identification of avian influenza virus H9N2 subtype . Archives of Razi Institute , 51 : 27 – 38 .
- Reed , L.J. and Muench , H. 1938 . A simple method of estimating fifty percent endpoint . American Journal of Epidemiology , 27 : 493 – 497 .
- Stallknecht , D.E. 1998 . Ecology and epidemiology of avian influenza viruses in wild bird populations: waterfowl, shorebirds, pelicans, cormorants, etc . In D.E. Swayne & R.D. Slemons In Proceedings of the Fourth International Symposium on Avian Influenza (pp. 61 – 69 ). Richmond , VA : US Animal Health Association .
- Stallknecht , D.E. , Nagy , E. , Hunter , D.B. and Slemons , R.D. 2007 . “ Avian influenza ” . In Infectious Diseases of Wild Birds , Edited by: Thomas , N.J. , Hunter , D.B. and Atkinson , C.T. 108 – 130 . Ames , IA : Blackwell .
- Subbarao , K. , Klimov , A. , Katz , J. , Regnery , H. , Lim , W. , Hall , H. , Perdue , M. , Swayne , D. , Bender , C. , Huang , J. , Hemphill , M. , Rowe , T. , Shaw , M. , Xu , X. , Fukuda , K. and Cox , N. 1998 . Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness . Science , 279 : 393 – 396 . doi: 10.1126/science.279.5349.393
- Swayne , D.E. and Halvorson , D.A. 2008 . “ Influenza ” . In Diseases of Poultry , 12th edn , Edited by: Saif , Y.M. , Barnes , H.J. , Glisson , J.R. , Fadly , A.M. , McDougald , L.R. and Swayne , D.E. 153 – 184 . Ames : Blackwell Publishing .
- Swayne , D.E. and Slemons , R.D. 1994 . Comparative pathology of a chicken-origin and two duck-origin influenza virus isolates in chickens: the effect of route of inoculation . Veterinary Pathology , 31 : 237 – 245 . doi: 10.1177/030098589403100211
- Tavakkoli , H. , Asasi , K. and Mohammadi , A. 2011 . Effectiveness of two H9N2 low pathogenic avian influenza conventional inactivated oil emulsion vaccines on H9N2 viral replication and shedding in broiler chickens . Iranian Journal of Veterinary Research , 12 : 214 – 221 .
- Ward , C.L. , Dempsey , M.L. , Ring , C.J.A. , Kempson , R.E. , Zhang , L. , Gor , D. , Snowden , B.W. and Tisdale , M. 2004 . Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement . Journal of Clinical Virology , 29 : 179 – 188 . doi: 10.1016/S1386-6532(03)00122-7
- Wright , P.F. , Neumann , G. and Kawaoka , Y. 2007 . “ Orthomyxoviruses ” . In Fields Virology , 5th edn , Edited by: Knipe , D.M. and Howley , P.M. Vol. 2 , 1691 – 1740 . Philadelphia : Lippincott Williams & Wilkins .