Abstract
Subunit vaccines capable of inducing antibody against both infectious bursal disease virus (IBDV) and H9 subtype avian influenza virus (AIV) were developed. The VP2 protein of IBDV was used as a cargo protein to display a 12-amino-acid immunodominant epitope derived from the N-terminal M2 extracellular domain (nM2e) of the H9 subtype AIV. Two chimaeric proteins were constructed by insertion of one copy of the nM2e into the PBC region (VP2BCnM2e(H9)) or by fusing four copies of nM2e to the carboxyl terminal (VP2-4nM2e(H9)) of VP2. Genes that encoded the VP2 chimaeras were subsequently cloned into a baculovirus vector and expressed in Spodoptera frugiperda cells. The recombinant proteins were used to vaccinate chickens at day 0 and again after 4 weeks. Blood was collected at 2-week intervals after primary and secondary vaccination to detect the antibody titre against VP2 or the nM2e via indirect enzyme-linked immunosorbent assay. Virus neutralization tests were also performed to measure anti-IBDV or anti-H9 AIV neutralizing antibodies in chick embryo fibroblasts. Oropharyngeal and cloacal swabs were collected 3, 5 and 7 days post H9 subtype AIV infection for virus isolation. Vaccination with VP2-4nM2e(H9) induced higher levels of antibody responses against IBDV or H9 subtype AIV, and provided better protection against an IBDV virulent challenge compared with vaccination with VP2BCnM2e(H9) vaccine, the wild-type VP2 subunit vaccine or the IBDV subunit commercial vaccines. Both chimaeric VP2 vaccines showed poor efficacy in inhibiting H9 virus replication post challenge. In summary, chimaeric proteins that contain the nM2e epitope were able to induce both IBDV and H9 subtype AIV-neutralizing antibody responses.
Introduction
Infectious bursal disease, also known as Gumboro disease, is responsible for major economic losses in the poultry industry worldwide (Wyeth & Cullen, Citation1976; Dobos et al., Citation1979). Infectious bursal disease is a highly contagious, immunosuppressive disease of immature chickens caused by a birnavirus. Infectious bursal disease virus (IBDV) infects the precursors of antibody-producing B cells in the bursa of Fabricius. The genome of IBDV contains two segments of double-stranded RNAs within a non-enveloped capsid (Dobos et al., Citation1979). The smaller segment encodes the viral polymerase VP1 while the larger segment encodes the polyprotein precursor pVP2-VP4-VP3 and a separate small protein, VP5 (Rong et al., Citation2005). The polyprotein precursor is processed into three structural proteins, VP2, VP3 and VP4 (Coulibaly et al., Citation2005). VP2 has been found to contain at least four neutralizing epitopes of IBDV, and recombinant VP2 confers protection against virulent infectious bursal disease in young chickens (Macreadie et al., Citation1990; Pitcovski et al., Citation1996; Pitcovski et al., Citation2003; Rong et al., Citation2005; Perozo et al., Citation2008). VP2 is folded into three different domains (named base B, shell S and projection P) (Coulibaly et al., Citation2005). The expression of VP2 forms dodecahedral subviral particles, which contain 20 VP2 trimers that expose four loops of the P domains (named PBC, PDE, PFG and PHI) on the surface of the particles (Coulibaly et al., Citation2005). It has been shown previously that the PBC region tolerated an insert of a 12-amino-acid antigenic epitope of foot-and-mouth disease virus type O, without disrupting the assembly of the subviral particles. The recombinant VP2 vaccine elicited a strong neutralizing antibody response in immunized mice (Rémond et al., Citation2009). These studies suggest that VP2 is a suitable cargo molecule for the presentation of foreign epitopes.
Avian influenza not only causes a significant economic loss to the poultry industry, but also poses threats to health of mammalian species, including humans and pigs (Lin et al., Citation2000; Peiris et al., Citation2001; Saito et al., Citation2001; Cong et al., Citation2007). Numerous studies have demonstrated that H9N2 influenza viruses are endemic in terrestrial poultry in different Asian countries (Xu et al., Citation2007; Zhang et al., Citation2008; Zhang et al., Citation2009; Sun et al., Citation2010; Wu et al., Citation2010). In addition, severe outbreaks of H9N2 avian influenza virus (AIV) infection are associated with other diseases or environments that cause high morbidity and mortality in turkey and chicken (Alexander, Citation2000; Kishida et al., Citation2004). Although several inactivated H9N2 influenza vaccines have been developed to control these outbreaks in China and other countries in southeastern Asia (Zhang et al., Citation2009; Wu et al., Citation2010), it is unclear whether these vaccines are effective against potential H9 subtype influenza mutants. Thus, novel influenza vaccines that confer protection against a broad-spectrum of virus variants are urgently needed to prepare for a potential pandemic.
The ectodomain of the M2 protein (M2e) is an attractive target for developing a universal vaccine. M2e is 23 amino acids long and its sequence is remarkably conserved. M2e is sparingly present on the virus particles, but is abundant on virus-infected cells. Therefore, M2e within inactivated AIV vaccines is poorly immunogenic at eliciting antibody against itself (Fiers et al., Citation2004). Liu et al. (Citation2004; Zou et al., Citation2005) reported that the EVETPIRN sequence (amino acids 6 to 13) at the N-terminus of M2e induced a highly specific antibody titre in mice after a second immunization, and significantly increased the survival rate of mice in a subsequent challenge assay. These studies suggest that the N-terminus of M2e (nM2e) is a potentially useful peptide for the development of broad-spectrum influenza virus vaccines.
In this study, VP2-nM2e chimaeras were produced by introducing either one or four copies of a 12-amino-acid peptide derived from the nM2e of the H9 subtype of AIV into the VP2 protein. Chimaeric proteins were identified that effectively displayed the nM2e epitope. These were able to induce neutralizing antibody responses to both IBDV and H9 subtype AIV and to protect chickens against IBDV infection.
Materials and Methods
Polymerase chain reaction amplification of the VP2 gene
Total RNA of IBDV vaccine strain B87 (accession number DQ906921) was prepared as a template, and the full-length VP2 coding gene was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) as previously described (Rong et al., Citation2005), using primers (Synthesized by GenScript Corp., Nanjing, China) that corresponded to the conserved 5′ and 3′ ends of the VP2 open reading frame (). The PCR conditions were as follows: 5 min at 94°C; 30 cycles of 30 sec at 94°C, 30 sec at 62°C and 50 sec at 72°C; and then 10 min at 72°C . The resulting full-length VP2 fragment was inserted into the plasmid pMD19-T vector (TaKaRa, Dalian, China) for DNA sequencing.
Table 1. Nucleotide sequences of primers used for the construction of chimaeric VP2 genes.
Synthesis of the 4nM2e gene and construction of plasmid vector
The sequences of M2e from the H9 subtype AIVs from GenBank were aligned with an H9 subtype commercial vaccine strain (A/chinken/NanJing/02/2001, NJ02/01) to yield the consensus amino acid sequence of the N-terminus of M2e (amino acids 2 to 13, SLLTEVETHTRN), which was termed nM2e. The 4nM2e DNA sequence was commercially synthesized (GenScript Corp.) and subcloned into pUC57 vector. The 4nM2e sequence encodes amino acids 428 to 441 of the IBDV B87 strain, a KK linker, four copies of nM2e, a stop codon and a Sal I restriction endonuclease, and has the following sequence: 5′-GGAGGTGGCCGACCTCAACTCTCCCCTGAAAATTGCAGGAGCAAAGAAGAGTCTTCTAACCGAGGTCGAAACGCACACCAGAAACAGTCTTCTAACCGAGGTCGAAACGCACACCAGAAACAGTCTTCTAACCGAGGTCGAAACGCACACCAGAAACAGTCTTCTAACCGAGGTCGAAACGCACACCAGAAACTAACGTCGAC-3′.
Construction of chimaeric VP2 genes
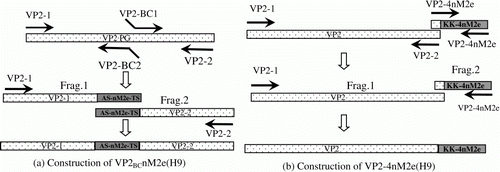
As shown in , a single copy of the H9 nM2e gene was inserted within the PBC loop (amino acids 222 to 223, PG) or four copies of nM2e were fused to the carboxy terminus of VP2 by fusion PCR using a series of appropriate oligonucleotides (). Each of the recombinant VP2 genes was constructed by two rounds of PCR. Fragments 1 and 2 were obtained after the first round of PCR and cloned into the pMD19-T vector (TaKaRa); their insertion was confirmed by sequencing. Fragments 1 and 2 were then digested from the pMD19-T vector by their corresponding restriction enzymes. The two digestion products were used as templates in the second round of PCR to amplify the complete recombinant VP2 gene with the corresponding primers. The resulting plasmids were confirmed by sequencing.
Figure 1. Schematic diagram of the construction of the VP2BCnM2e(H9) and VP2-4nM2e(H9) chimaeric genes. 1a: Two amino acids, PG, in the PBC domain of IBDV VP2 proteins were mutated and inserted with one copy of nM2e combined with amino acids AS and TS as linker by fusion PCR with corresponding oligonucleotides. 1b: Four copies of nM2e with linker KK fusion to the carboxy terminus of IBDV VP2 by fusion PCR with corresponding oligonucleotides.
Cloning and expression of the chimaeric VP2 gene in Spodoptera frugiperda cells
To generate recombinant baculoviruses (rBVs), wild-type (wt) and chimaeric VP2 genes were cloned into the pFastBac 1 plasmid using the Bac-to-Bac® baculovirus expression system following the manufacturer's instructions (Invitrogen, Carlsbad, California, USA) and previous reports (Rémond et al., Citation2009).
Spodoptera frugiperda (Sf9) cells at a density of 2×106 cells/ml were infected at a multiplicity of infection of 3.0 plaque-forming units per cell with rBVs that encoded wt or chimaeric VP2. Cell were collected and frozen at 72 h post infection, before being lysed by sonication. The cell lysates were pelleted by centrifugation (47,400×g, 30 min at 4°C) and then resuspended in phosphate-buffered saline that contained a protease inhibitor leupeptin (GE Healthcare, Piscataway, New Jersey, USA) at a concentration of 2 µg/ml.
Confirmation of the expression of chimaeric VP2 proteins
The soluble extracts of cells transfected with rBVs were analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% polyacrylamide gels (Laemmli, Citation1970). Membranes were probed with chicken anti-IBDV or anti-nM2e hyperimmune serum. The hyperimmune serum against IBDV VP2 was prepared from chickens that received four injections of commercial IBDV VP2 subunit vaccine (0.3 ml/dose). The hyperimmune serum against AIV nM2e was obtained from chickens receiving four injections of vaccine, with each dose of vaccine containing 300 µg of the synthetic two-copy nM2e peptide (SLLTEVETHTRNSLLTEVETHTRN) conjugated with 300 µg keyhole limpet haemocyanin carrier protein and formulated in water-in-oil emulsion with Marcol 52 mineral oil (ESSO, Paris, France). The wt and recombinant VP2 proteins were tested by electron microscopy with negative staining; however, no virus-like particles were observed.
Preparation of vaccines
The wt and chimaeric VP2 proteins were prepared from Sf9 cells infected with rBVs as described in the previous sections ‘Cloning and expression of the chimaeric VP2 gene’ and ‘Confirmation of the expression of chimaeric VP2 proteins’, and the titre of the VP2 proteins was analysed by agar-gel precipitation assay, according to the method previously described (Rong et al., Citation2005). The protein solution was added into Marcol 52 mineral oil (ESSO) until the agar-gel precipitation antigen titre of VP2 proteins was 1/32, to yield a water-in-oil emulsion vaccine (v/v 1:2) (Rong et al., Citation2005). The processes of vaccine preparation were similar to the method used to generate the IBDV VP2 subunit commercial vaccine (YEBIO Bioengineering Co. Ltd, Qingdao, China), in which VP2 genes was derived from the JZ 3/02 virus strain and cloned into the pET 28a vector before being expressed in Escherichia coli. The antigen payload of vaccine in this study was also similar to the IBDV subunit commercial vaccine. The H9 subtype AIV inactivated vaccine was obtained from Nanjing Tianbang Bio-industry Co., Ltd (Nanjing, China), in which the vaccine strain was NJ02/01. The production processes for the Bac control vaccine were also similar to those of the recombinant VP2 vaccine, with the slight modification of using the treated empty baculovirus transfected Sf9 cells as the antigen solution.
Vaccination
Seven groups of 20 specific pathogen free 14-day-old White Leghorn chickens (Gallus gallus domesticus) were included in this trial. Fourteen-day-old chickens from each group received two injections (0.3 ml) of the specific vaccine subcutaneously at day 0 and day 28. The groups and numbers of chickens used in the trial are listed in . All birds were bled on days 14, 28, 42 and 56 post primary vaccination. At 60 days post primary vaccination, birds in each group were separated into two subgroups and challenged with IBDV or H9 AIV according to the procedures described below.
Table 2. Protection efficacy of recombinant VP2 vaccine.
Infectious bursal disease virus challenge study
One-half of the chickens in each group were challenged with 0.1 ml of 10 median bursa infective dose of the challenge strain, IBDV BC 6/85 (China strain characterized as virulent IBDV from the China Institute of Veterinary Drug Control, Beijing) by the intranasal route. Chicken were observed clinically for 4 days, and after this observation period all surviving chickens were killed humanely and subjected to checking for gross lesions in the bursa of Fabricius.
H9 avian influenza virus challenge study
The remainder of the chickens in each group were challenged with 0.1 ml of 107.0 median embryo infectious dose of the NJ02/01 strain by the intranasal route and observed clinically for 14 days. Oropharyngeal and cloacal swab samples were collected at 3, 5 and 7 days post challenge, or collected when chickens died within the clinical observation period. Virus isolation was performed from the swab samples, as described previously (Tang et al., Citation2009).
Indirect enzyme-linked immunosorbent assay
nM2e-based indirect enzyme-linked immunosorbent assay
An indirect enzyme-linked immunosorbent assay (iELISA) was carried out to characterize the accessibility of the nM2e epitope on the surface of the chimaeric VP2 protein. Briefly, a 96-well Costar microplate (Corning Inc., Steuben County, New York, USA) was coated with 100 µl of 5 µg/ml synthetic two-copy nM2e peptides overnight at 4°C. After washing, the plate was blocked by 1% bovine serum albumin in phosphate-buffered saline–Tween 20 (0.05% solution) for 3 h at 37°C. The presence of the nM2e epitope was revealed by incubation with pooled antiserum samples derived from each vaccination group (400-fold dilution in phosphate-buffered saline) for 1 h, followed by detection with a horseradish peroxidase conjugated goat-anti-chicken Ig (Southern Biotech Inc., Birmingham Alabama, USA).
VP2-based indirect enzyme-linked immunosorbent assay
An iELISA was performed according to the same procedures as described for the nM2e-based iELISA except that the wt VP2 proteins isolated from the rBV-infected Sf9 cells were used as the source of the coating antigen. Only the agar-gel precipitation titre of the stock solution of VP2 proteins reached 1/32, and was then diluted 50-fold before it was used as the coating antigen.
Virus neutralization tests
Titration of the antibody against the VP2 proteins
The individual sera and pooled sera from each vaccination group were examined by cytopathic effect-based median tissue culture infective dose to determine the titre of the antibody against the VP2 proteins. For the virus neutralization test (VNT), briefly, each sample of serum was heat-inactivated for 0.5 h at 56°C. Two-fold serial dilutions of antiserum, ranging from 1×10−2 to 6.4×10−3, were mixed with an equivalent volume of the B87 virus (200 median tissue culture infective dose), and the mixture was incubated for 1 h at 37°C. A total volume of 100 µl of the virus–serum mixtures was inoculated into confluent monolayers of chicken embryo fibroblasts (CEFs) in 96-well plates. After incubation for 96 to 120 h, cytopathic effects were examined and the neutralizing antibody titre was determined as the highest dilution of serum that completely inhibited virus growth in three out of four repeat tests for each sample.
Titration of the antibody against the nM2e epitope
Only pooled sera were used to test the titre of the antibody against the nM2e epitope, but the test was performed as described above in this section. The reference strain used in the assay was H9N2 NJ02/01, and 5 µg/ml l-l-(tosylamido-2-phenyl) ethyl chloromethyl ketone-treated trypsin was added to the culture medium.
Results
Construction of the chimaeric VP2 gene
Three baculovirus constructs encoding two chimaeric forms and one wt form of the VP2 of IBDV were generated. The size of the VP2 open reading frame of IBDV of the B87 virus is 1343 base pairs. VP2BCnM2e(H9) baculoviruses encode a chimaeric VP2 with a 12-amino-acid peptide (SLLTEVETHTRN) derived from the nM2e of H9 AIV antigenic epitope and two adapters (AS and TS) inserted within the BC loop (a). In the VP2-4nM2e(H9) baculoviruses, an adaptor (KK) and four copies of the nM2e were fused to the carboxyl terminus of VP2 (b).
Expression of the recombinant VP2 proteins
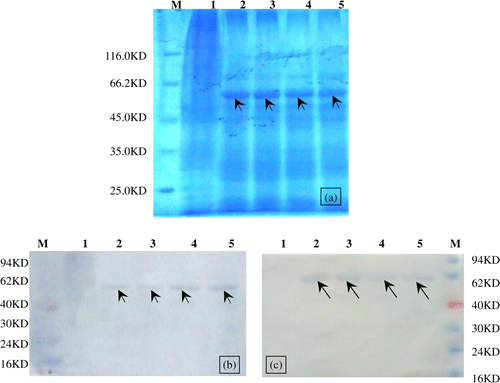
The expression of the recombinant VP2 proteins in Sf9 cells was detected by an analysis of the cell lysates by 12% SDS-PAGE with Coomassie blue staining (a) and western blotting (b,c). The molecular weights of the recombinant VP2 proteins were about 48 kDa for VP2BCnM2e(H9) and 53 kDa for VP2-4nM2e(H9).
Figure 2. SDS-PAGE and western blotting analysis of chimaeric VP2 proteins. 2a: Coomassie blue R250-stained SDS-PAGE gels showing the expression of chimaeric VP2 proteins. 2b, 2c: Incubation with hyperimmune serum against VP2 and nM2e, respectively. Lane M, protein marker; lane 1, soluble extracts of Sf-9 cells; lanes 2 and 3, VP2BCnM2e(H9); lanes 4 and 5 VP2-4nM2e(H9).
Detection of antibody titre post vaccination
Anti-IBDV serum antibodies were detected by iELISA and VNT. The ability of recombinant VP2 to stimulate antibodies production was shown by iELISA (a). The levels of maternally-derived antibodies against IBDV were barely detectable in pre-vaccination birds. Four weeks post primary vaccination, the antibody titre against VP2 in chickens vaccinated with VP2-4nM2e(H9) was approximately the same as in chickens from the wt-VP2 vaccination group, but was higher than the titre in the groups inoculated with VP2BCnM2e(H9) or the IBDV subunit commercial vaccine. In contrast, the antibody titre was hardly detectable in the control groups, including Bac, H9 AIV or non-immunization blank controls.
Figure 3. Antibody titres of pooled serum samples from each vaccination group measured by iELISA or VNT. 3a: The iELISA antibody titre against IBDV VP2 using wt VP2 proteins as coating antigen. 3b: The iELISA antibody titre against AIV nM2e epitope using the two-copy nM2e peptides as coating antigen. 3c, 3d: The antibody titre against IBDV or H9 AIV evaluated by VNT in CEFs, respectively. The neutralizing index is expressed as the reciprocal of the dilution of serum required to inhibit the cytopathic effect. Pooled serum samples were obtained pre vaccination (pre-vac), 2 weeks post primary vaccination (2W.P.P.V), 4 weeks post primary vaccination (4W.P.P.V), 2 weeks post secondary vaccination (2W.P.S.V) and 4 weeks post secondary vaccination (4W.P.S.V).
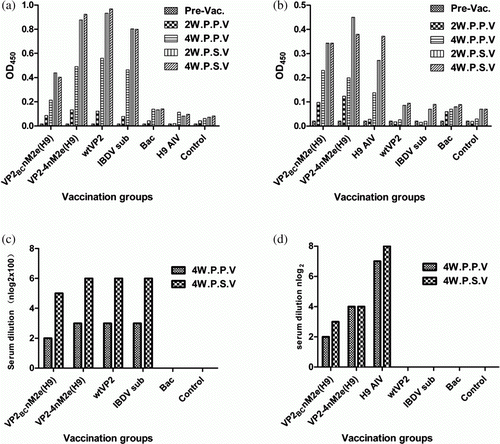
To assess the efficiency of the recombinant VP2 vaccines in eliciting antibody-mediated inhibition of IBDV infection, the titres of virus neutralization antibodies in serum pools from each vaccination group were measured in CEF (c). VNT antibody titres in all serum pools were lower than 1:800 at 4 weeks after primary vaccination, which indicated a poor level of protection against IBDV infection. However, at 4 weeks after secondary vaccination, chickens in the VP2-4nM2e(H9), wt-VP2 and IBDV subunit commercial vaccine groups had induced antibody titres up to 1:6400. Antibody titres in these three groups were higher than in the VP2BCnM2e(H9) group. Statistical correlation of the iELISA antibody titre against VP2 with the VNT antibody titre against IBDV tested on CEF at 4 weeks post primary vaccination and at 4 weeks post secondary vaccination was analysed (a,c; Pearson's r=0.95 at both time points).
VNT antibody titres against IBDV in individual chicken sera at 4 weeks post secondary vaccination were also measured (). VNT antibody titres of all chickens were above 1:6400 in the VP2-4nM2e(H9) and wtVP2 groups. In the VP2BCnM2e(H9) group, only seven chickens yield titres above 1:6400, whereas the titres of the remaining three chickens were 1:3200. In the commercial IBDV subunit vaccinated group, seven chickens had titres of 1:6400, with the remaining three chickens yielding titres of 1:3200. No VNT antibody titre was detected in the sera of chickens from the empty Bac control or the challenge control groups. The mean VNT antibody titres determined from individual chickens in each vaccination group at 4 weeks post secondary vaccination were similar to the pooled serum titres of the corresponding group (data not shown).
Serum antibodies against the nM2e epitope were measured by nM2e-based iELISA (b). Antibody titres of chickens vaccinated with the VP2-4nM2e(H9), VP2BCnM2e(H9) and H9 AIV vaccines gradually increased after the second vaccination when compared with the empty Bac control and commercial IBDV subunit vaccinated groups. Additionally, the efficacy of the VP2-4nM2e(H9) vaccine was slightly better than the VP2BCnM2e(H9) and H9 AIV vaccines.
The levels of the nM2e epitope that induced neutralizing antibodies against H9 AIV were measured to assess the immune response level. As shown in d, the results of the VNT showed that the VP2-4nM2e(H9), VP2BCnM2e(H9) and H9 AIV vaccines were able to inhibit H9 subtype infection in CEF. The inhibitory effect of VP2-4nM2e(H9) was slightly higher than for the VP2BCnM2e(H9) vaccine, but lower than for the H9 inactivated whole virus vaccine.
Protection against IBDV and H9N2 subtype AIV challenges
No chickens died during the IBDV challenge study. The degree of protection against the virulent IBDV challenge was assessed by examining the gross lesions in the bursa of Fabricius in all chickens in each experimental group. No chickens in the unvaccinated control group were free of infection. The infected bursas became swollen and/or yellowish in colour. Eight out of 10 chickens in the empty Bac control group presented similar signs to the chickens in the unvaccinated control group, whereas the remaining two chickens developed severe petechial haemorrhages in the thigh and pectoral muscles (data not shown). Only one out of 10 chickens in both the VP2-4nM2e(H9) and the wt-VP2 vaccine groups showed slight oedema of the bursal mucosa. Four out of 10 chickens had mild oedema and sporadic haemorrhage within the bursa mucosa in both the VP2BCnM2e(H9) and IBDV subunit commercial vaccine groups ().
No chicken died during the 14-day observation period in the H9 virus challenge study. The number of chickens showing depression and anorexia at 3 days post infection in the VP2BCnM2e(H9), VP2-4nM2e(H9) and IBDV subunit commercial vaccine groups were eight, seven and nine, respectively. The signs lasted for 2 to 4 days. All chickens in the wt-VP2, Bac and challenge control groups showed signs of depression and anorexia, in a similar manner to those in the chimaeric VP2 groups. None of the chickens in the H9 commercial vaccine group showed any clinical signs ().
Oropharyngeal and cloacal swab samples were collected at 3, 5 and 7 days post H9 subtype AIV challenge for virus isolation. The virus isolation results indicated that four out of 10 birds in the H9 commercial vaccine group and all birds in the other six groups were not free from infection 3 days post challenge. All swab samples in the wtVP2, IBDV commercial vaccine, Bac and challenge control groups, nine out of 10 samples in the VP2BCnM2e(H9) vaccine group, eight out of 10 samples in the VP2-4nM2e(H9) vaccine group and four out of 10 samples in the H9 commercial vaccine group were positive for viral isolation at day 5 post challenge. All swab samples in the wtVP2, Bac and challenge control groups, and eight out of 10 samples in the VP2BCnM2e(H9) and VP2-4nM2e(H9) vaccination groups, were positive for virus isolation at 7 days post challenge. Only one out of 10 samples was positive for virus isolation in the H9 commercial vaccine group at 7 days post challenge.
Discussion
The humoral antibody response plays an important role in protection against IBDV or H9 AIV infection (Vakharia et al., Citation1994; Zhang et al., Citation2008). In this study, the antibody titre against VP2 as determined by iELISA corresponded well with the results of the VNT (Pearson's r=0.95, a,c), as indicated by the results for the VP2-4nM2e(H9) and wt-VP2 groups. Furthermore, the results of both the iELISA and VNT antibody titres correlated well with the results of the virulent IBDV challenge. Importantly, these results were consistent with results of other research studies in which VP2 was expressed in E. coli (Rong et al., Citation2005; Rong et al., Citation2007), yeast (Macreadie et al., Citation1990; Pitcovski et al., Citation2003) or baculovirus (Vakharia et al., Citation1994; Pitcovski et al., Citation1996).
The highly conserved M2e of influenza A virus is one of the most promising targets for the development of universal influenza vaccines. However, new strategies are required to improve the immunogenicity of vaccines based on M2e containing only the N-terminus of the protein (Liu et al., Citation2004; Zou et al., Citation2005). In previous studies, the hepatitis B virus core protein (De Filette et al., Citation2008), cholera toxin subunit A1 (Eliasson et al., Citation2008), TLR5 ligand flagellin (Huleatt et al., Citation2008), Salmonella (Layton et al., Citation2009) or truncated Mycobacterium tuberculosis HSP70 (Ebrahimi et al., Citation2010) have been used as carriers for the presentation of the M2e antigen. One study (Rémond et al., Citation2009) reported that the insertion of an immunodominant epitope from foot-and-mouth disease virus into the PBC region of IBDV VP2 elicited a neutralizing antibody response in the immunized mice. However, there was no information about the influence of the foot-and-mouth disease virus epitope on the immunogenicity of VP2.
In this study, the VP2 protein of IBDV was used as a cargo protein to display the nM2e. The observation of the clinical signs post H9 AIV challenge indicated that these antibody levels had no effect on the inhibition of virus replication in chickens. In addition, the results of H9 virus isolation post challenge suggested that immunization with the vaccine containing the nM2e epitope did not reduce virus shedding. The chimaeric VP2 proteins containing nM2e elicited a low level of VNT antibody, which was consistent with previous studies (Jegerlehner et al., Citation2004; Layton et al., Citation2009). Indeed, haemagglutinin is the most immunogenic antigen of the influenza virus. The inactivated H9 commercial vaccine containing haemagglutinin is more immunogenic than the subunit vaccines and induced a higher antibody response than in the other groups.
The antibody responses and the results of IBDV challenge in the VP2BCnM2e(H9) group indicated that the insertion of one copy of the nM2e within the PBC region may destroy the integrity and slightly influence the immunogenicity of VP2. However, fusion of four copies of the nM2e to the carboxyl terminus of the VP2 protein had no significant adverse effect on the immunogenicity of VP2 when compared with that of wt-VP2.
In this study, three recombinant baculovirus constructs were generated to express VP2 or chimaeric VP2 proteins containing the nM2e immunodominant epitope derived from the H9 subtype AIV. The expression products of these constructs elicit an immune response in chickens. The chimaeric VP2 protein containing four copies of the nM2e at the carboxyl terminus was shown to be more immunogenic than the chimaeric VP2 protein with the insertion of one copy of the nM2e within the PBC region.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 31201904) and the Independent Innovation of Agricultural Sciences Program of Jiangsu Province (No. CX(11)4072, No. CX(10)450 and No. CX (10)216).
References
- Alexander , D.J. 2000 . A review of avian influenza in different bird species . Veterinary Microbiology , 74 : 3 – 13 . doi: 10.1016/S0378-1135(00)00160-7
- Cong , Y.L. , Pu , J. , Liu , Q.F. , Wang , S. , Zhang , G.Z. , Zhang , X.L. , Fan , W.H. , Brown , E.G. and Liu , J.H. 2007 . Antigenic and genetic characterization of H9N2 swine influenza viruses in China . Journal of General Virology , 88 : 2035 – 2041 . doi: 10.1099/vir.0.82783-0
- Coulibaly , F. , Chevalier , C. , Gutsche , I. , Pous , J. , Navaza , J. , Bressanelli , S. , Delmas , B. and Rey , F.A. 2005 . The birnavirus crystal structure reveals structural relationships among icosahedral viruses . Cell , 120 : 761 – 772 . doi: 10.1016/j.cell.2005.01.009
- De Filette , M. , Martens , W. , Smet , A. , Schotsaert , M. , Birkett , A. , Londoño-Arcila , P. , Fiers , W. and Saelens , X. 2008 . Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies . Vaccine , 26 : 6503 – 6507 . doi: 10.1016/j.vaccine.2008.09.038
- Dobos , P. , Hill , B.J. , Hallett , R. , Kells , D.T. , Becht , H. and Teninges , D. 1979 . Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes . Journal of Virology , 32 : 593 – 605 .
- Ebrahimi , S.M. , Tebianian , M. , Toghyani , H. , Memarnejadian , A. and Attaran , H.R. 2010 . Cloning, expression and purification of the influenza A (H9N2) virus M2e antigen and truncated Mycobacterium tuberculosis HSP70 as a fusion protein in Pichia pastoris . Protein Expression and Purification , 70 : 7 – 12 . doi: 10.1016/j.pep.2009.11.001
- Eliasson , D.G. , Bakkouri , K.E. , Schön , K. , Ramne , A. , Festjens , E. , Löwenadler , B. , Fiers , W. , Saelens , X. and Lycke , N. 2008 . CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine . Vaccine , 26 : 1243 – 1252 . doi: 10.1016/j.vaccine.2007.12.027
- Fiers , W. , De Filette , M. , Birkett , A. , Neirynck , S. and Min Jou , W. 2004 . A “universal” human influenza A vaccine . Virus Research , 103 : 173 – 176 . doi: 10.1016/j.virusres.2004.02.030
- Huleatt , J.W. , Nakaar , V. , Desai , P. , Huang , Y. , Hewitt , D. , Jacobs , A. , Tang , J. , McDonald , W. , Song , L. , Evas , R.K. , Umlauf , S. , Tussey , L. and Powell , T.J. 2008 . Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin . Vaccine , 26 : 201 – 214 . doi: 10.1016/j.vaccine.2007.10.062
- Jegerlehner , A. , Schmitz , N. , Storni , T. and Bachmann , M.F. 2004 . Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity . Journal of Immunology , 172 : 5598 – 5605 .
- Kishida , N. , Sakoda , Y. , Eto , M. , Sunaga , Y. and Kida , H. 2004 . Co-infection of Staphylococcus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza A virus infection in chickens . Archives of Virology , 149 : 2095 – 2104 . doi: 10.1007/s00705-004-0372-1
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 . doi: 10.1038/227680a0
- Layton , S.L. , Kapczynski , D.R. , Higgins , S. , Higgins , J. , Wolfenden , A.D. , Liljebjelke , K.A. , Bottje , W.G. , Swayne , D. , Berghman , L.R. , Kwon , Y.M. , Hargis , B.M. and Cole , K. 2009 . Vaccination of chickens with recombinant Salmonella expressing M2e and CD154 epitopes increases protection and decreases viral shedding after low pathogenic avian influenza challenge . Poultry Science , 88 : 2244 – 2252 . doi: 10.3382/ps.2009-00251
- Lin , Y.P. , Shaw , M. , Gregory , V. , Cameron , K. , Lim , W. , Klimov , A. , Subbarao , K. , Guan , Y. , Krauss , S. , Shortridge , K. , Webster , R. , Cox , N. and Hay , A. 2000 . Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates . Proceedings of the National Academy of Sciences of the USA , 97 : 9654 – 9658 . doi: 10.1073/pnas.160270697
- Liu , W. , Zou , P. and Chen , Y.-H. 2004 . Monoclonal antibodies recognizing EVETPIRN epitope of influenza A virus M2 protein could protect mice from lethal influenza A virus challenge . Immunology Letters , 93 : 131 – 136 . doi: 10.1016/j.imlet.2004.03.003
- Macreadie , I.G. , Vaughan , P.R. , Chapman , A.J. , McKern , N.M. , Jagadish , M.N. , Heine , H.G. , Ward , C.W. , Fahey , K.J. and Azad , A.A. 1990 . Passive protection against infectious bursal disease virus by viral VP2 expressed in yeast . Vaccine , 8 : 549 – 552 . doi: 10.1016/0264-410X(90)90006-8
- Peiris , J.S.M. , Guan , Y. , Markwell , D. , Ghose , P. , Webster , R.G. and Shortridge , K.F. 2001 . Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment? . Journal of Virology , 75 : 9679 – 9686 . doi: 10.1128/JVI.75.20.9679-9686.2001
- Perozo , F. , Villegas , P. , Estevez , C. , Alvarado , I.R. , Purvis , L.B. and Williams , S. 2008 . Protection against infectious bursal disease virulent challenge conferred by a recombinant avian adeno-associated virus vaccine . Avian Diseases , 52 : 315 – 319 . doi: 10.1637/8122-100207-ResNote.1
- Pitcovski , J. , Di-Castro , D. , Shaaltiel , Y. , Azriel , A. , Gutter , B. , Yarkoni , E. , Michael , A. , Krispel , S. and Levi , B.Z. 1996 . Insect cell-derived VP2 of infectious bursal disease virus confers protection against the disease in chickens . Avian Diseases , 40 : 753 – 761 . doi: 10.2307/1592294
- Pitcovski , J. , Gutter , B. , Gallili , G. , Goldway , M. , Perelman , B. , Gross , G. , Krispel , S. , Barbakov , M. and Michael , A. 2003 . Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens . Vaccine , 21 : 4736 – 4743 . doi: 10.1016/S0264-410X(03)00525-5
- Rémond , M. , Da Costa , B. , Riffault , S. , Parida , S. , Breard , E. , Lebreton , F. , Zientara , S. and Delmas , B. 2009 . Infectious bursal disease subviral particles displaying the foot-and-mouth disease virus major antigenic site . Vaccine , 27 : 93 – 98 . doi: 10.1016/j.vaccine.2008.10.036
- Rong , J. , Cheng , T. , Liu , X. , Jiang , T. , Gu , H. and Zou , G. 2005 . Development of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens . Vaccine , 23 : 4844 – 4851 . doi: 10.1016/j.vaccine.2005.05.015
- Rong , J. , Jiang , T. , Cheng , T. , Shen , M. , Du , Y. , Li , S. , Wang , S. , Xu , B. and Fan , G. 2007 . Large-scale manufacture and use of recombinant VP2 vaccine against infectious bursal disease in chickens . Vaccine , 25 : 7900 – 7908 . doi: 10.1016/j.vaccine.2007.09.006
- Saito , T. , Lim , W. , Suzuki , T. , Suzuki , Y. , Kida , H. , Nishimura , S.I. and Tashiro , M. 2001 . Characterization of a human H9N2 influenza virus isolated in Hong Kong . Vaccine , 20 : 125 – 133 . doi: 10.1016/S0264-410X(01)00279-1
- Sun , Y. , Pu , J. , Jiang , Z. , Guan , T. , Xia , Y. , Xu , Q. , Liu , L. , Ma , B. , Tian , F. , Brown , E.G. and Liu , J. 2010 . Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 2008 . Veterinary Microbiology , 146 : 215 – 225 . doi: 10.1016/j.vetmic.2010.05.010
- Tang , Y. , Wu , P. , Peng , D. , Wang , X. , Wan , H. , Zhang , P. , Long , J. , Zhang , W. , Li , Y. , Wang , W. , Zhang , X. and Liu , X. 2009 . Characterization of duck H5N1 influenza viruses with differing pathogenicity in mallard (Anas platyrhynchos) ducks . Avian Pathology , 38 : 457 – 467 . doi: 10.1080/03079450903349147
- Vakharia , V.N. , Snyder , D.B. , Lütticken , D. , Mengel-Whereat , S.A. , Savage , P.K. , Edwards , G.H. and Goodwin , R.A. 1994 . Active and passive protection against variant and classic infectious bursal disease virus strains induced by baculovirus-expressed structural proteins . Vaccine , 12 : 452 – 456 . doi: 10.1016/0264-410X(94)90124-4
- Wu , Z.Q. , Ji , J. , Zuo , K.J. , Xie , Q.M. , Li , H.M. , Liu , J. , Chen , F. , Xue , C.Y. , Ma , J.Y. and Bi , Y.Z. 2010 . Cloning and phylogenetic analysis of hemagglutinin gene of H9N2 subtype avian influenza virus from different isolates in China during 2002–2009 . Poultry Science , 89 : 1136 – 1143 . doi: 10.3382/ps.2010-00695
- Wyeth , P.J. and Cullen , G.A. 1976 . Maternally derived antibody–effect on susceptibility of chicks to infectious bursal disease . Avian Pathology , 5 : 253 – 260 . doi: 10.1080/03079457608418194
- Xu , K.M. , Smith , G.J. , Bahl , J. , Duan , L. , Tai , H. , Vijaykrishna , D. , Wang , J. , Zhang , J.X. , Li , K.S. , Fan , X.H. , Webster , R.G. , Chen , H. , Peiris , J.S.M. and Guan , Y. 2007 . The genesis and evolution of H9N2 influenza viruses in poultry from southern China, 2000–2005 . Journal of Virology , 81 : 10389 – 10401 . doi: 10.1128/JVI.00979-07
- Zhang , P. , Tang , Y. , Liu , X. , Liu , W. , Zhang , X. , Liu , H. , Peng , D. , Gao , S. , Wu , Y. , Zhang , L. , Lu , S. and Liu , X. 2009 . A novel genotype H9N2 influenza virus possessing human H5N1 internal genomes has been circulating in poultry in eastern China since1998 . Journal of Virology , 83 : 8428 – 8438 . doi: 10.1128/JVI.00659-09
- Zhang , P. , Tang , Y. , Liu , X. , Peng , D. , Liu , W. , Liu , H. , Lu , S. and Liu , X. 2008 . Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998–2002) . Journal of General Virology , 89 : 3102 – 3112 . doi: 10.1099/vir.0.2008/005652-0
- Zou , P. , Liu , W. and Chen , Y.-H. 2005 . The epitope recognized by a monoclonal antibody in influenza A virus M2 protein is immunogenic and confers immune protection . International Immunopharmacology , 5 : 631 – 635 . doi: 10.1016/j.intimp.2004.12.005