Abstract
Forty common swifts (Apus apus), synanthropic birds living in an urban environment closely with humans and other animals, were hospitalized in the public veterinary hospital of the Regional Reference Center of Urban Veterinary Hygiene located in Naples, Campania Region, Italy. Each bird was sampled for bacteriological analyses. Out of 40 common swifts examined, eight were found positive for Salmonella enterica serovar Infantis although no sign of salmonellosis (e.g. diarrhoea) was shown. This is believed to be the first report of Salmonella spp. infection in common swifts. Our results suggest this avian species as a novel potential reservoir for one of most important Salmonella serovars.
Introduction
The common swift (Apus apus, order Apodiformes) could be currently considered as a bird breeding in colonies, preferentially in town and village buildings, and spending most of its active time flying. The swift's diet is based exclusively on airborne arthropods, ranging from approximately ground level to 100 m above open ground. This avian species is sexually monomorphic and long-distance migratory, whose western Palearctic populations move regularly from sub-Saharan Africa over to Europe for breeding (Miniero et al., Citation2008). At our latitudes (Naples, Italy, 40°50′0″ N, 14°15′0″ E), common swifts arrive in early spring to spend approximately 4 months (April to July) from colony establishment to fledging of the offspring. Although some studies were conducted to evaluate the role of the common swift as a bioindicator of persistent organic microcontaminants, there are no studies on detection of zoonotic agents in this avian species. However, a study conducted in South Africa by Van Vuuren & Brown (Citation1990) reported the isolation of Erysipelothrix rhusiopathiae as the causal organism of high mortality in a colony of little swifts (Apus affinis), a bird species similar to the common swift.
Herein we report the detection of Salmonella enterica serovar Infantis from common swifts hospitalized in the public veterinary hospital of the Regional Reference Center of Urban Veterinary Hygiene (in Italian, Centro di Riferimento Regionale per l'Igiene Urbana Veterinaria [CRIUV]) located in Naples, Campania Region, Italy.
Materials and Methods
Birds
During the period April/August 2011, a total of 40 common swifts were hospitalized at the public veterinary hospital of the CRIUV (Naples, Italy). They consisted of 24 adult (>60 days of age) and 16 young (<60 days of age) individuals. The age was established considering the wingspan, which is 38 to 40 cm in the adult common swift, as well as the plumage, which is totally completed in the adults. All grounded birds, showing flight inability, were found by people in different locations in the city of Naples. Flight inability was related to traumatic injuries, debilitation, and starvation. Fifteen adult and four young birds showed evidence of traumatic injuries, whereas the remaining nine adult and 12 young birds were found grounded with debilitation and starvation.
Sampling and isolation procedure
Before proceeding with diagnosis and therapy, each bird was sampled by cloacal swab for microbiological analysis. All samples were analysed for isolation of Salmonella strains using the International Organization for Standardization procedure (ISO, Citation2002). In particular, cloacal swabs were inoculated in buffered peptone water (Oxoid Ltd, Basingstoke, UK) as pre-enrichment medium, and incubated at 37°C for 18 h. After incubation, samples were inoculated into Rappaport–Vassiliadis Broth (Oxoid Ltd) as enrichment medium and were incubated at 42°C for 18 h. The cultures obtained were plated onto Xylose-Lysine-Deoxycholate Agar (Oxoid Ltd), incubated at 37°C and examined after 24 h. Suspect colonies were inoculated onto a second selective agar (Brilliant Green Agar; Oxoid Ltd) and incubated at 37°C for 24 h. All isolates were identified biochemically using the API20-E system (bioMérieux, Marcy l'Etoile, France). Salmonella isolates were serotyped according to the Kauffman–White scheme in collaboration with the National Reference Laboratory for Salmonella (IZSVe, Legnaro, Italy) and were then submitted to pulsed-field gel electrophoresis (PFGE).
Pulsed-field gel electrophoresis
Agarose plugs and PFGE conditions were performed according to the PulseNet standardized protocol (Ribot et al., Citation2006). The generated fragments were separated using the CHEF MAPPER XA Pulsed Field Electrophoresis System (BioRad, Hercules, California, USA). XbaI-digested DNA from Salmonella enterica serovar Braenderup strain H9812 was used as a molecular reference marker. Dendrogram and cluster analysis were obtained using InfoQuest™ FP version 4.5 software package (BioRad). The Dice coefficient of similarity was calculated and the dendrogram determined using the unweighted pair group method with arithmetic means.
Antimicrobial susceptibility tests
All isolates were submitted to antimicrobial susceptibility testing using the disk diffusion method where the susceptible and resistance breakpoint levels of the antimicrobials were based on those specified by the Clinical Laboratory Standards Institute, formerly the National Committee for Clinical Laboratory Standards (NCCLS), performance standard documents: (NCCLS) M31-A2 (NCCLS, Citation2002), and M100-S15 (NCCLS, Citation2005). The antimicrobials tested were ampicillin (10 µg), chloramphenicol (30 µg), streptomycin (10 µg), sulfamethoxazole (300 µg), tetracycline (30 µg), cefotaxime (30 µg), gentamicin (10 µg), kanamycin (30 µg), sulfamethoxazole-trimethoprim (23.75/1.25 µg), nalidixic acid (30 µg), and ciprofloxacin (5 µg). Escherichia coli ATCC 25922 was used as a control strain in each experiment. The inhibition zones were measured and scored as sensitive, intermediate susceptibility and resistant according to the NCCLS guidelines (NCCLS, 2002, 2005).
Results
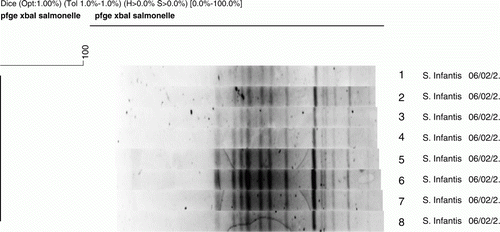
Salmonella spp. were isolated from 8/40 (20.0%; 95% confidence interval = 9.6 to 36.1%) samples collected. Five positive birds came from the city centre, whereas the remaining positive birds came from suburban areas of the city of Naples. All strains were serotyped as S. enterica serovar Infantis. All positive samples were from adult common swifts, among which six were clinically healthy showing only flight debilitation, and two showed traumatic injuries, with anaemia and cachexia. Diarrhoea was not seen in any bird. As shown by PFGE (), the eight S. Infantis strains investigated shared an indistinguishable XbaI-PFGE pattern (Dice coefficient = 100%).
Figure 1. Unweighted pair group method with averages dendrogram of PFGE profiles identified in eight S. Infantis strains belonging to common swifts (A. apus) after restriction with XbaI. The strains share the same pulse type.
With respect to antimicrobial susceptibility testing, the S. Infantis strains showed multiple drug sensitivity. In particular, all the isolates were susceptible to streptomycin, sulphonamide, tetracycline, cefotaxime, gentamycin, kanamycin, sulfamethoxazole-trimethoprim, nalidixic acid, ciprofloxacin and enrofloxacin. In contrast, the S. Infantis strains were exclusively resistant to ampicillin and sulphamethoxazole trimethoprim.
Discussion
Salmonella pathogens are one of the main causes of human food-borne illness worldwide, and wild bird infections usually reflect contamination of the environment by humans or livestock (Cízek et al., Citation1994). In fact, some bird-borne zoonotic pathogens may have increased prevalence in urban areas owing to human-driven mechanisms. For example, supplemental wild bird feeding, which occurs commonly in the urban environment, has been linked with increases in the prevalence of some bacterial species within wild birds. Supplemental feeding promotes disease emergence by creating high densities of birds, high concentrations of faeces and stress owing to social interactions (Hamer et al., Citation2011).
There are no data about the isolation of S. Infantis from the common swift, although this Salmonella serotype is consistently isolated from broiler chickens, pigs, and humans worldwide (Hauser et al., Citation2012). While most bird infections are subclinical, salmonellosis is a cause of sporadic mortality particularly among young birds in large breeding colonies and birds around feeders, as reported by Daoust & Prescott (Citation2007). Among wild birds the most commonly isolated serotype is Salmonella Typhimurium, which appears to be adapting to some avian species such as songbirds that frequent bird feeders (Hamer et al., Citation2011).
In the present study, however, all Salmonella-positive common swifts appeared healthy without diarrhoea. This finding is noteworthy as the potential zoonotic aspect of this Salmonella serovar, although it was not possible to speculate on their role as transient or longer term carriers of S. Infantis.
S. Infantis, in fact, is a cause of human salmonellosis in several countries and represents the third most frequent serotype isolated in Europe (1.1%) after Salmonella Enteritidis and S. Typhimurium (Dionisi et al., Citation2011). Moreover, in recent years S. Infantis has represented the third Salmonella serovar amongst environmental samples in Italy (Dionisi et al., Citation2011). In the European Union, an international surveillance network, designated Enter-net, has been active since 1994. Italy participates in the Enter-net surveillance system, and information on approximately 10,000 Salmonella isolates from different sources is collected every year. S. Typhimurium, S. Enteritidis and S. Infantis usually account for more than 70% of the strains responsible for human infections in Italy (Busani et al., Citation2004).
It is not possible to speculate regarding the source of Salmonella infections in the present study because the common swift populations move regularly from sub-Saharan Africa over to Europe for breeding and very little is known of those periods when swifts are presumably in central and southern Africa. The possible cause of infection could be represented by insects. These birds usually eat airborne arthropods such as house flies (Musca domestica) or mosquitos (Culicidae) and, as reported by Olsen et al. (Citation2000), S. Infantis was isolated from houseflies and from dump flies. In addition, sampling was carried out in the city of Naples in a period of waste crisis that involved the whole Campania region. This problem was also reported by Barba et al. (Citation2011), who underlined how three decades of illegal practices of waste dumping and consequent environmental abuse have made the Campania region a unique case in the context of waste-related health outcomes. The waste crisis may therefore have caused an increase in environmental contamination by different pathogens such as S. Infantis. As reported by Hamer et al. (Citation2011), the key features of the urban environment that promote the transmission of pathogens include increased host contact rates and susceptibility to infection, high rates of pathogen introductions, pollution and stress that reduce host immune function, and warmer microclimates and reduced seasonality that allow the environmental persistence of some pathogens.
With respect to antimicrobial resistance, S. enterica antibiotic multiresistant strains have been isolated with increasing frequency from human infections, farm animals, and foodstuffs (Scuderi et al., Citation2000). S. Infantis has shown an increase in antimicrobial resistance since 2005. Our results show that antimicrobial resistance does not appear to be an important issue because the S. Infantis strains isolated have shown a low rate of antimicrobial resistance in line with that reported before 2005 (Dionisi et al., Citation2011). Furthermore, the eight S. Infantis strains investigated shared an indistinguishable XbaI-PFGE pattern, suggesting that they belong to the same clone. Other studies reported a similar high genetic relationship between S. Infantis strains sampled over a longer period in several countries throughout the world (Hauser et al., Citation2012), indicating that the serovar is disseminated worldwide and possesses a highly clonal population structure. As hypothesized by Hauser et al. (Citation2012), S. Infantis has possibly developed mechanisms protecting the serovar from major genetic rearrangements or horizontal genetic transfers. Another explanation also reported by Hauser et al. (Citation2012) is that the serovar has a recent ancestor and was yet not able to accumulate major evolutionary changes and their broad dissemination.
In conclusion, this study demonstrates the presence of S. Infantis in common swifts across a gradient of urbanization and suggests a potential public health risk to the high-density human populations within an area already critically affected by the waste crisis. Synanthropic birds such as common swifts therefore play important roles in the maintenance and movement of zoonotic pathogens, and their influence on the urban environment should receive more attention.
Acknowledgements
The authors are grateful to the OIE, National Reference Laboratory for Salmonella (IZSVe, Legnaro, Italy) for Salmonella serotyping, and to all veterinarians of CRIUV, in particular to Dr Vincenzo Caputo, Dr Marina Pompameo, Dr Guido Rosato and Dr Valerio Toscano for their precious collaboration.
References
- Barba , M. , Mazza , A. , Guerriero , C. , Di Maio , M. , Romeo , F. , Maranta , P. , Marino I.G. , Paggi , M.G. & Giordano , A. 2011 . Wasting lives: the effects of toxic waste exposure on health. The case of Campania, Southern Italy . Cancer Biology & Therapy , 12 , 106 – 111 . doi: 10.4161/cbt.12.2.16910
- Busani , L. , Graziani , C. , Battisti , A. , Franco , A. , Ricci , A. , Vio , D. , Digiannatale , E. , Paterlini , F. , D'Incau , M. , Owczarek , S. , Caprioli , A. & Luzzi , I. 2004 . Antibiotic resistance in Salmonella enterica serotypes Typhimurium, Enteritidis and Infantis from human infections, foodstuffs and farm animals in Italy . Epidemiology and Infection , 132 , 245 – 251 . doi: 10.1017/S0950268803001936
- Cízek , A. , Literák , I. , Hejlícek , K. , Treml , F. & Smola , J. 1994 . Salmonella contamination of the environment and its incidence in wild birds . Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health , 41 , 320 – 327 . doi: 10.1111/j.1439-0450.1994.tb00234.x
- Daoust , P.Y. & Prescott , J.F. 2007 . Salmonellosis . In N.J. Thomas , D.B. Hunter , & C.T. Atkinson . Infectious Diseases of Wild Birds (pp. 270 – 288 ). Ames : Blackwell Publishing .
- Dionisi , A.M. , Lucarelli , C. , Benedetti , I. , Owczarek , S. & Luzzi , I. 2011 . Molecular characterisation of multidrug-resistant Salmonella enterica serotype Infantis from humans, animals and the environment in Italy . International Journal of Antimicrobial Agents , 38 , 384 – 389 . doi: 10.1016/j.ijantimicag.2011.07.001
- Hamer , S.A. , Lehrer , E. & Magle , S.B. 2011 . Wild birds as sentinels for multiple zoonotic pathogens along an urban to rural gradient in greater Chicago, Illinois . Zoonoses and Public Health , 59 , 355 – 364 . doi: 10.1111/j.1863-2378.2012.01462.x
- Hauser , E. , Tietze , E. , Helmuth , R. , Junker , E. , Prager , R. , Schroeter , A. , Rabsch , W. , Fruth , A. , Toboldt , A. & Malorny , B. 2012 . Clonal dissemination of Salmonella enterica serovar Infantis in Germany . Foodborne Pathogens and Disease , 9 , 352 – 360 . doi: 10.1089/fpd.2011.1038
- ISO 2002 . Microbiology of Food and Animal Feeding Stuff-Horizontal Method for the Detection of Salmonella . ISO 6579 . Geneva : International Organization for Standardization .
- Miniero , R. , Carere , C. , De Felip , E. , Iacovella , N. , Rodriguez , F. , Alleva , E. & di Domenico , A. 2008 . The use of common swift (Apus apus), an aerial feeder bird, as a bioindicator of persistent organic microcontaminants . Annali dell'Istituto Superiore di Sanità , 44 , 187 – 194 .
- NCCLS 2002 . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard , 2nd edn . NCCLS document M31-A2 . Wayne : National Committee for Clinical Laboratory Standards .
- NCCLS 2005 . Performance Standards for Antimicrobial Susceptibility Testing; Fifteenth Informational Supplement . NCCLS document M100-S15 . Wayne : National Committee for Clinical Laboratory Standards .
- Olsen , A.R. & Hammack , T.S. 2000 . Isolation of Salmonella spp. from the housefly, Musca domestica L., and the dump fly, Hydrotaea aenescens (Wiedemann) (Diptera: Muscidae), at caged-layer houses . Journal of Food Protection , 63 , 958 – 960 .
- Ribot , E.M. , Fair , M.A. , Gautom , R. , Cameron , D.N. , Hunter , S.B. , Swaminathan , B. , & Barrett , T.J. 2006 . Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet . Foodborne Pathogens and Disease , 3 , 59 – 67 . doi: 10.1089/fpd.2006.3.59
- Scuderi , G. , Fantasia , M. & Niglio , T. 2000 . Results of the three-year surveillance by the Italian SALM-NET System: human isolates of Salmonella serotypes . European Journal of Epidemiology , 16 , 377 – 383 . doi: 10.1023/A:1007678901475
- Van Vuuren , M. & Brown , J.M. 1990 . Septicaemic Erysipelothrix rhusiopathiae infection in the Little Swift (Apus affinis) . Journal of the South African Veterinary Association , 61 , 170 – 171 .