Abstract
Salmonella enterica serovar Heidelberg is one of the top three serovars implicated in human infections in Canada. In 2003, the Canadian Integrated Program for Antimicrobial Resistance Surveillance reported antimicrobial resistance (AMR) in S. Heidelberg in Canada. The study objective was to investigate the AMR of S. Heidelberg isolated from poultry in Alberta. We examined 951 S. Heidelberg poultry isolates obtained during 1996 to 2010 and tested against 18 antibiotics using the Sensititre AVIAN1F system. Temporal resistance patterns were analysed using single-level logistic regression models. Continuous variables were included in the multivariable models. Multivariable models were built and variables and interactions were included in these final models. Data were analysed using Stata 11 Intercooled. Ceftiofur resistance ranged annually from 0 to 10.5% and gentamicin resistance ranged annually from 0 to 33.3%; no isolates were enrofloxacin resistant. Resistance to amoxicillin (annual range 0 to 42.6%) varied significantly by time and interaction with commodity type. Meat turkey S. Heidelberg isolates had higher ceftiofur resistance compared with chickens: layers plus layer breeders (odds ratio = 22.6, P < 0.01) and broiler breeders (odds ratio = 9.1, P < 0.01). Gentamicin resistance decreased significantly over the study period (odds ratio = 0.72 per year, P < 0.01). Tetracycline (TET) resistance changed significantly over time (annual range 0 to 39.6%), interacting with poultry commodity type. Meat turkey isolate TET resistance, higher overall than that of chicken, increased throughout the study. All turkey breeder isolates were resistant to TET. In conclusion, this study provides AMR data for S. Heidelberg isolates from the Alberta poultry industry and demonstrated significant trends in resistance, both temporal and between poultry commodities.
Introduction
Poultry are known carriers of Salmonella enterica serovar Heidelberg and infection may cause clinical disease in young birds. Mature poultry often exhibit no clinical signs of infection. S. Heidelberg can be transmitted to meat products via contamination at the processing plant. Alternatively, as a result of systemic infection, S. Heidelberg can invade the muscle of meat birds or infect eggs through the reproductive tissue of laying hens (Gast et al., Citation2004, Citation2007). Contaminated poultry carcasses and eggs may provide a source of infection to humans when they are handled improperly (Hennessy et al., Citation2004; MacDougall et al., Citation2004). Case studies suggest that table eggs (Currie et al., Citation2005; Chittick et al., Citation2006) and chicken meat (Currie et al., Citation2005) are the main sources of S. Heidelberg infections for humans in Canada and the USA.
Salmonella species are one of the most common causes of food-borne illness in humans. S. Heidelberg infection in humans can cause mild diarrhoea to severe systemic illness (Burt et al., Citation1990; Vugia et al., Citation2004). Infections with S. Heidelberg have a higher potential (11% and 13.8%) of causing systemic illness compared with other Salmonella serovars commonly isolated from poultry, including Salmonella enterica Enteritidis (6% and 6%) and Salmonella enterica Typhimurium (6% and 5.1%) (Vugia et al., Citation2004; Crump et al., Citation2011). Crump et al. (Citation2011) found that S. Heidelberg could be isolated from blood in 13.8% of human cases and that blood isolates were more likely to be resistant to one or more antimicrobials compared with isolates obtained from stool samples.
Severe, invasive forms of S. Heidelberg infection require antimicrobial therapy. Unfortunately, there are reports of increasing antimicrobial resistance (AMR) in S. Heidelberg (Cook et al., Citation2009; European Food Safety Authority, Citation2009; Dutil et al., Citation2010). These reports cause concern because of the reduced effectiveness of therapeutic antimicrobials important to human health. The profile of S. Heidelberg often includes resistance to third-generation cephalosporins, such as ceftiofur (TIO) and ceftriaxone (European Food Safety Authority, Citation2009; Dutil et al., Citation2010; Crump et al., Citation2011). TIO resistance is important because TIO-resistant S. Heidelberg isolates are also ceftriaxone resistant. Ceftriaxone is important for treating these invasive S. Heidelberg infections in humans, especially in children and pregnant women where fluoroquinolones are not indicated and other options are limited (Dunne et al., Citation2000; Fey et al., Citation2000; Dutil et al., Citation2010).
In Alberta, S. Heidelberg remains in the top three to four serovars isolated from human cases and has increased in 2010 compared with recent years (Enteric Diseases Program, Citation2007; National Enteric Surveillance Program, Citation2011, Citation2012). Alberta Agriculture and Rural Development (AARD) monitor Salmonella spp. isolation rates from animal and poultry submissions. The objective of this paper was to describe AMR in S. Heidelberg isolated from Alberta poultry and to identify temporal trends and poultry commodity differences in AMR.
Materials and Methods
Isolate retrieval
Isolates were previously cultured by AARD and serotyped by the Alberta Provincial Laboratory for Public Health (ProvLab) as S. Heidelberg. These isolates were retrieved from –80°C storage at the Food Safety and Animal Health Division. A portion of the isolate was cultured aseptically onto a non-selective medium (blood agar plate) and incubated at 35°C for 18 to 24 h. Prior to antimicrobial susceptibility testing, the isolate was subcultured to another blood agar plate and tested within 24 h.
Control strains
Escherichia coli ATCC 25922 was the control strain used for each run of testing. All quality control results were within the acceptable control ranges. For monthly quality control testing, four control strains were tested: E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213. All quality control results were within the acceptable control ranges.
Serotyping
Isolates were serotyped by ProvLab as S. Heidelberg. ProvLab used conventional methods (Ewing, Citation1986), based on the White–Kauffmann–Le Minor scheme (Grimont & Weill, Citation2007), to determine the O (somatic) and H (flagellar) antigens. Slide agglutination was used with Statens Serum Institut antisera (Statens Serum Institut, Copenhagen, Denmark) to determine O (somatic) antigens while tube agglutination was used with Difco™ antisera (Difco, BD Diagnostic Systems, Sparks, Maryland, USA) to determine H (flagellar) antigens. S. Heidelberg isolates were fully identified at ProvLab, but any discrepant or difficult-to-type isolates were sent to the National Microbiology Laboratory for confirmation (NML, Winnipeg, Manitoba, Canada).
Sensititre system protocol
A broth microdilution technique was used for antimicrobial susceptibility testing of the isolates to determine the minimum inhibitory concentration (MIC) according to procedures set by TREK Diagnostic Systems (Westlake, Ohio, USA). The Sensititre AVIAN1F (TREK Diagnostic Systems) plate containing 18 antimicrobials was used because of its application to avian therapeutics. The panel and concentration ranges included: amoxicillin (AMX; 0.25 to 16 µg/ml), TIO (0.25 to 4 µg/ml), clindamycin (0.5 to 4 µg/ml), enrofloxacin (ENR; 0.12 to 2 µg/ml), erythromycin (0.12 to 4 µg/ml), florfenicol (1 to 8 µg/ml), gentamicin (GEN; 0.5 to 8 µg/ml), neomycin (2 to 32 µg/ml), novobiocin (0.5 to 4 µg/ml), oxytetracycline (0.25 to 8 µg/ml), penicillin (0.06 to 8 µg/ml), spectinomycin (8 to 64 µg/ml), streptomycin (STR; 8 to 1024 µg/ml), sulfadimethoxine (32 to 256 µg/ml), sulfathiazole (32 to 256 µg/ml), tetracycline (TET; 0.25 to 8 µg/ml), trimethoprim/sulfamethoxazole (SXT; 0.5 to 9.5/2 to 38 µg/ml), and tylosin (2.5 to 20 µg/ml). Sensititre plates were read by the Sensititre Automated Reading and Incubation System (TREK Diagnostic Systems, Inc., Cleveland, OH, USA). All results were generated using the Sensititre for Windows software (TREK Diagnostic Systems, Inc).
Interpretation of results
Interpretation of the MIC values for GEN, TET, and SXT were based on the Clinical and Laboratory Standards Institute (Citation2011) guidelines. Interpretations for TIO and STR were based on the National Antimicrobial Resistance Monitoring System guidelines (CDC, Citation2010) and were the same as those used by the Canadian Integrated Program for Antimicrobial Resistance Surveillance (Government of Canada, Citation2012). The interpretation of the MIC values for the remaining antimicrobials was based on the Sensititre database information supplied by TREK Diagnostic Systems. The available MIC breakpoints for resistance/susceptibility were: AMX (≥32/ ≤ 8 µg/ml), TIO (≥8/ ≤ 2 µg/ml), clindamycin (≥4/ ≤ 0.5 µg/ml), ENR (≥2/ ≤ 0.5 µg/ml), erythromycin (≥8/ ≤ 0.5 µg/ml), florfenicol (≥8/ ≤ 2 µg/ml), GEN (≥16/ ≤ 4 µg/ml), neomycin (≥16/ ≤ 8 µg/ml), novobiocin (≥8/ ≤ 4 µg/ml), oxytetracycline (≥16/ ≤ 4 µg/ml), penicillin (≥0.06/ ≤ 0.03 µg/ml), spectinomycin (≥64/ ≤ 8 µg/ml), STR (≥64/ ≤ 32 µg/ml), sulfadimethoxine (≥512/ ≤ 256 µg/ml), sulfathiazole (≥128/ ≤ 16 µg/ml), TET (≥16/ ≤ 4 µg/ml), SXT (trimethoprim ≥4/ ≤ 2 µg/ml), sulfamethoxazole (≥76/ ≤ 38 µg/ml) and tylosin (not interpretable). S. Heidelberg displays intrinsic resistance to five of these 18 antimicrobials (penicillin, erythromycin, clindamycin, tylosin, novobiocin). For the remaining 13 antimicrobials, multidrug resistance was determined using these breakpoints and was defined as resistance to three or more drug classes. These classes and drugs include: β-lactams (AMX, TIO), aminoglycosides (GEN, neomycin, spectinomycin, STR), tetracyclines (oxytetracycline, TET), sulfas (sulfadimethoxine, sulfathiazole), potentiated sulfas (SXT) and fluoroquinolones (ENR), and florfenicol.
Statistical analysis
Temporal patterns in resistance for AMX, TIO, GEN and TET were analysed using single-level logistic regression models for each antimicrobial. Isolates were categorized as resistant or not; intermediate isolates were classified as non-resistant. Other indicator variables considered in these models were the poultry commodity type on the farm where the isolate was obtained (broiler chicken, broiler breeder, layer plus layer breeder chicken, meat turkey, or turkey breeder) and the source of the isolate (farm environment, bird fluff, barn litter or other samples, including animal organs, chick pads or unidentified source). Continuous variables were assessed for linearity with the outcome and all variables were considered for inclusion in the final, multivariable models if likelihood ratio test two-sided P values were ≤0.20 (Dohoo et al., Citation2009). Final, multivariable models were built using manual, backwards, stepwise selection and variables and interactions were included in final models if two-sided P values were ≤0.05. Variables not significant to each model were included as confounders if they altered the log-odds of any model coefficient by >25%. All data were analysed using Stata 11 Intercooled (StataCorp, College Station, Texas, USA).
Results
The Food Safety and Animal Health Division tested the antimicrobial susceptibilities of 951 S. Heidelberg isolates that were obtained from poultry sources in Alberta from 1996 to 2010. Of the 951 isolates, resistance to at least one of the 13 antimicrobials was found in 63.3% (95% confidence interval = 60.2 to 66.4%). Multidrug resistance was present in 88 isolates (9.3%, 95% confidence interval = 7.5 to 11.3%). The annual and overall prevalences of resistance to AMX, TET, TIO, GEN, STR and SXT are presented in . The majority of isolates (94.8%) were of chicken origin and almost one-half of all isolates (42.2%) were from the farm environment (). The isolate source (e.g. environment, fluff, litter or other) was not significant and did not act as a confounder in any model. The source was therefore not included in any multivariable model (results not shown).
Table 1. Annual antimicrobial susceptibilities of S. Heidelberg isolates from poultry obtained by the Food Safety and Animal Health Division of AARD from 1996 to 2010.
Table 2. Descriptive statistics for the sources of S. Heidelberg isolates from poultry.
AMX resistance changed significantly over time and was modelled using a quadratic (squared term) for year of sample submission (). There was a significant interaction between the year quadratic and commodity type. The predicted probabilities from the final logistic regression model for AMX resistance are shown in . Resistance was higher in isolates from chickens (broiler breeder, broilers, layers and layer breeders) at the beginning and end of the study period compared with those from turkeys (meat turkeys and turkey breeders). However, in the middle of the study, the opposite relationship was true.
Figure 1. Predicted probabilities of AMX resistance from the final logistic regression model of S. Heidelberg isolates from poultry in Alberta.
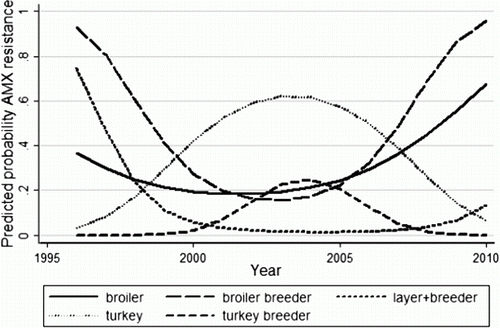
Table 3. Odds ratios and 95% confidence intervals for the logistic regression model for AMX resistance in S. Heidelberg isolates from poultry.
Resistance to TIO also changed significantly over time (). The prevalence of resistance in each year was compared with 1% resistance in 2001. A number of years were omitted from the model as they had no resistant isolates (1996 to 1998, 2003, 2008 and 2010). Conversely, 2000 (odds ratio [OR] = 12.8) and 2009 (OR = 24.8) had significantly higher resistance than 2001; the remaining years were not significantly different from 2001. Isolates from meat turkeys had significantly more TIO resistance compared with those from layers and layer breeder chickens (OR = 22.6) and broiler breeder chickens (OR = 9.1); the comparison of isolates from meat turkeys with those from broiler chickens was not significant.
Table 4. Odds ratios and 95% confidence intervals for the logistic regression model for TIO resistance in S. Heidelberg isolates from poultry.
The results from the final logistic regression model for GEN resistance are shown in . GEN resistance decreased significantly from the beginning to the end of study period. For each 1-year increase in time, the OR for GEN resistance is 0.72. Conversely, this represents a 40% decrease in risk of GEN resistance for a 1-year period. Broilers (OR = 27.3), broiler breeders (OR = 22.9), meat turkeys (OR = 26.6) and turkey breeders (OR = 62.4) had significantly higher GEN resistance compared with layers and layer breeders. No other commodity comparisons were significantly different.
Table 5. Odds ratios and 95% confidence intervals for the logistic regression model for GEN resistance in S. Heidelberg isolates from poultry.
TET resistance changed significantly over time and was modelled using a quadratic for year of submission (). It also had a significant interaction with commodity type. The predicted probabilities from the final logistic regression model for TET resistance are shown in . Resistance in isolates from meat turkeys decreased from 1996 to 2000 and then increased dramatically to the end of the study period. Conversely, resistance in isolates from all chicken commodities (broilers, broiler breeders, layers and layer breeders) increased slightly from 1996 to 2002, then decreased to 2010. With the exception of 1998 to 2002, resistance was higher in isolates from meat turkeys compared with those from all chicken commodities. Turkey breeders were not included in the model because all turkey breeder isolates were resistant to TET.
Figure 2. Predicted probabilities of TET resistance from the final logistic regression model of S. Heidelberg isolates from poultry in Alberta. All turkey breeder isolates were resistant to TET.
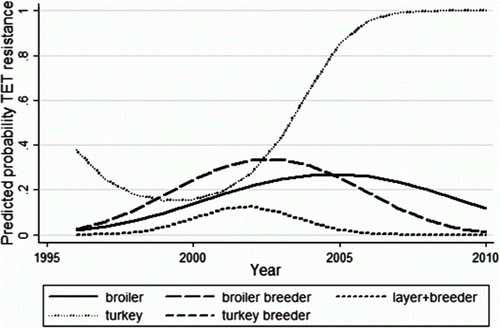
Table 6. Odds ratios and 95% confidence intervals for the logistic regression model for TET resistance in S. Heidelberg isolates from poultry.
Discussion
This study describes the AMR of S. Heidelberg isolates from poultry in Alberta from 1996 to 2010. As this serovar has been implicated in human septicaemia (Vugia et al., Citation2004), resistance in this organism is particularly important. Few data have been published in Alberta with respect to AMR in S. Heidelberg from poultry. Johnson et al. (Citation2005) published AMR patterns in Salmonella spp. isolated from food animals from 1996 to 1999 in Alberta. However, their study reported resistance in 44 S. Heidelberg isolates in total from multiple animal sources, compared with 951 isolates examined in this study from poultry. The current study provides resistance data on a larger bank of isolates that are specifically from poultry, which expands our knowledge of this subject and provides baseline data for the future.
This study found that 63.3% of S. Heidelberg isolates from Alberta poultry harboured resistance to at least one antimicrobial and that 9.3% of isolates displayed multidrug resistance. In comparison, when Lynne et al.(Citation2009) examined S. Heidelberg isolates using the antimicrobial susceptibility testing method described by the Clinical and Laboratory Standards Institute, they found 72% of isolates from various food animals (cattle, swine, chicken and turkey) were resistant to at least one antimicrobial. They also found that the prevalence of resistance in S. Heidelberg was highest in isolates from cattle and swine and lowest in chicken. The previous Alberta study by Johnson et al. (Citation2005) found that 65.9% of the 44 S. Heidelberg isolates from all livestock had resistance to one or more antimicrobials. They also found that AMR was more prevalent in Salmonella spp. isolated from cattle compared with poultry. However, the US Department of Agriculture (Citation2011) report on isolates of Salmonella spp. from various livestock species found that the prevalence of S. Heidelberg recovery was very low from cattle carcasses (no isolates in 1998, 2000, 2004, 2005, and 2007 to 2010 to a maximum three isolates [6.4%] in 2001) compared with broiler carcasses (16 [3.5%] isolates in 2010 to a maximum 262 [24.9%] isolates in 2002). Taken together, although fewer isolates are obtained from non-poultry livestock, the AMR profile is typically more extensive. A review of our isolate bank showed that very few S. Heidelberg isolates originated from animals (23/974) other than poultry (951/974); we therefore did not include comparison analysis from other livestock in this study. If there is more resistance in isolates from non-poultry species, as suggested by Johnson et al. (Citation2005), there may be a need to explore a potential association between antimicrobial use and selection pressure in mammalian food animals compared with poultry. This varied resistance pattern could suggest differences in S. Heidelberg genes or in gene regulation with respect to invasiveness, survivability and/or resistance in this bacterium isolated from poultry or other animals. When examining S. Heidelberg isolates from different sources, Han et al. (Citation2011) found that in 78 of the isolates obtained from human sources and subsequently tested, 47% were resistant to antimicrobials. Han et al. (Citation2011) further went on to compare their own work with four other studies where the overall results suggest that AMR is less prevalent in S. Heidelberg isolated from humans when compared with S. Heidelberg isolated from other animals.
S. Heidelberg demonstrated moderate levels of resistance to AMX in this study (annual levels as high as 42.6%) (). This level of resistance to AMX is interesting because AMX is not registered for use in poultry. It is possible that AMX resistance is co-selected by use of another antimicrobial class. β-Lactamase resistance genes are located on mobile genetic elements, for example plasmids, that harbour resistance genes for other classes of antimicrobials such as tetracyclines (van der Bij & Pitout, Citation2012). This would allow for co-selection of β-lactam resistance through the use of tetracyclines (chlortetracycline and oxytetracycline), which are approved for use by poultry producers in Canada. As seen in , S. Heidelberg TET resistance ranged annually from 0 to 39.6%. Molecular plasmid isolation and profiling would be warranted to further explain the presence of AMX resistance in S. Heidelberg from poultry in Alberta.
Of particular interest in this study was resistance to TIO (ranging annually from 0 to 10.5%) and GEN (ranging annually from 0 to 33.3%) (). In contrast, 100% of the isolates were susceptible to ENR (data not shown). Other studies have also determined that there is very low resistance identified in S. Heidelberg to fluoroquinolones such as ciprofloxacin (Johnson et al., Citation2005; Musgrove et al., Citation2006; Patchanee et al., Citation2008; Borsoi et al., Citation2009; Cook et al., Citation2009; Lynne et al., Citation2009; Barnhill et al., Citation2010; Crump et al., Citation2011; Han et al., Citation2011). These antibiotics are categorized to be of very high (TIO, fluoroquinolones) or high (GEN) importance for human medicine by Health Canada's (2002) Veterinary Drugs Directorate. Even though TIO, GEN and ENR may not specifically be used in human medicine, resistance to them may convey resistance to human drugs in the same classes that are important to treat human bacterial infections (McDermott et al., Citation2002; Zhao et al., Citation2008; Dutil et al., Citation2010).
While resistance to TIO was not significantly different between S. Heidelberg from broilers and table egg layers (), overall resistance to AMX, GEN and TET was higher in the isolates from broiler and broiler breeder chickens compared with table egg layers for the study period (Tables 3, 5 and 6). It is possible that this difference corresponds with the practice of limited antimicrobial use in table egg layer flocks because of the withdrawal times required for approved antimicrobials in this commodity (Health Canada, Citation2002). TET is labelled to provide support for table egg layer birds following vaccination; however, most vaccination protocols are completed while the flock is still in the pullet barn. In Alberta, to the best of our knowledge, commercial table egg producers do not readily use antimicrobials for disease treatment or prophylaxis. This information parallels the lack of requests by commercial table egg producers to feed companies for this (or any other) medication in that commodity. TET is used for treatment of diseases in other poultry commodities, and for disease control and growth promotion in other livestock species, but data on its total use in Alberta's poultry industry are unknown.
S. Heidelberg AMR to GEN is significantly more prevalent in floor-raised birds (broilers, broiler breeders, meat turkeys and turkey breeders) compared with birds raised in cages (layers and layer breeders) (). This supports the hypothesis that management can play a role in AMR development if floor-raised birds are treated more frequently with antimicrobials. GEN is registered for use in chickens (excluding table egg laying chickens) and turkeys (Health Canada, Citation2002). Due to the intensive labour requirement to treat a flock, the application in a barn setting is probably not taking place. However, automation provides a mechanism to administer this antibiotic at hatcheries. Present use in Alberta is unknown.
S. Heidelberg AMR to TIO in meat turkeys was significantly more prevalent compared with AMR in table egg layer birds (). This could be a result of the low number of turkey producers that had over-represented their commodity. It could also suggest that there may be less antimicrobials used in the layer commodity compared with the meat turkey system. In addition the significant difference in TIO resistance between meat turkeys and broiler breeders is interesting. The speculation that TIO could be used more in the turkey industry (hence more AMR) in Alberta during the study period cannot be ruled out. TIO has been used as a prophylactic in poults at the hatchery in various jurisdictions across Canada. Although turkeys had S. Heidelberg with higher overall levels of resistance to TIO, GEN and TET, and higher AMX resistance in the middle of the study period, these results should be considered with care because there was a relatively small number of turkey producers represented in this database compared with the number of other poultry producers. Overall, we are interpreting these data with some caution because we lack accurate antimicrobial usage data in floor-raised birds.
TIO resistance in S. Heidelberg isolated from chicken meat has been examined by others in Canada (Dutil et al., Citation2010). They found that TIO resistance in isolates from retail chicken in Québec and Ontario changed over time and correlated this change with the voluntary withdrawal of TIO usage in Québec hatcheries. Further, they were able to correlate the temporal pattern of TIO resistance in S. Heidelberg in human isolates to those in retail chicken in these regions. We are unable to draw any similar conclusions as our samples are over-represented by isolates () obtained from apparently healthy bird environments (e.g. environmental barn samples [42.2%], hatchery fluff samples [12%], and litter samples [29.3%]).
The commodity differences for resistance in S. Heidelberg isolates are interesting and varied in Alberta. Without antimicrobial use information for the poultry sector in Alberta, it is difficult to attribute these differences to varied use practices, although this is one potential explanation. Broilers, broiler breeders, turkeys and turkey breeders are floor-raised. The selective pressure in floor-raised flocks could contribute to the development of resistance genes on plasmids that could pass to S. Heidelberg. Approximately 42% of our samples (proportionally the largest commodity group) were sourced from floor-raised broiler breeder operations (). It is therefore possible that broiler breeder S. Heidelberg isolates are over-represented in our isolate bank, which could affect the prevalence of resistance we detected, but this alone may not explain the differences between the chicken commodities.
In conclusion, this study showed that there were significant temporal trends in resistance of S. Heidelberg in Alberta poultry over time, and that there were differences in resistance between poultry commodities in the province, both between chicken and turkey, and between chicken meat and chicken egg laying birds. Examination of AMR patterns in other Salmonella serotypes isolated from Alberta poultry, such as Salmonella enterica serovars Enteritidis, Typhimurium and Kentucky, would be warranted. Also, collection of antimicrobial use data from the Alberta food animal industries and the human sector in the future could help to elucidate reasons for the changing AMR patterns in S. Heidelberg seen over time.
Acknowledgements
This work was collaboratively funded by the Alberta Livestock and Meat Agency and AARD. The authors would like to thank Ms Carol Goertz (AARD) for her technical support, Dr Jagdish Patel (AARD) for his constructive and helpful comments on the manuscript, and the Enteric Department, ProvLab, for Salmonella serotyping.
References
- Barnhill , A.E. , Weeks , K.E. , Xiong , N. , Day , T.A. & Carlson , S.A. 2010 . Identification of multiresistant Salmonella isolates capable of subsisting on antimicrobials . Applied and Environmental Microbiology , 76 , 2678 – 2680 . doi: 10.1128/AEM.02516-09
- Borsoi , A. , Santin , E. , Santos , L.R. , Salle , C.T.P. , Moraes , H.L.S. & Nascimento , V.P. 2009 . Inoculation of newly hatched broiler chicks with two Brazilian isolates of Salmonella Heidelberg strains with different virulence gene profiles, antimicrobial resistance, and pulsed field gel electrophoresis patterns to intestinal changes evaluation . Poultry Science , 88 , 750 – 758 . doi: 10.3382/ps.2008-00466
- Burt C.R. , Proudfoot , J.C. , Roberts , M. & Horowitz , R.H. 1990 . Fatal myocarditis secondary to Salmonella septicemia in a young adult . Journal of Emergency Medicine , 8 , 295 – 297 . doi: 10.1016/0736-4679(90)90009-K
- Government of Canada . 2012 . Canadian Integrated Program for Antimicrobial Resistance Surveillance 2008 Report . Public Health Agency of Canada Guelph , ON . – xv, 146p.
- CDC . 2010 . National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): Human Isolates Final Report, 2008 . Atlanta , GA : US Department of Health and Human Services, CDC .
- Chittick , P. , Sulka , A. , Tauxe , R.V. & Fry , A.M. 2006 . A summary of national reports of foodborne outbreaks of Salmonella Heidelberg infections in the United States: clues for disease prevention . Journal of Food Protection , 69 , 1150 – 1153 .
- Clinical and Laboratory Standards Institute . 2011 . Performance standards for antimicrobial susceptibility testing; Twenty-first informational supplement CLSI document M100-S21 . Wayne , PA : Clinical and Laboratory Standards Institute .
- Cook , A. , Reid-Smith , R. , Irwin , R. , McEwen , S.A. , Valdivieso-Garcia , A. & Ribble , C. 2009 . Antimicrobial resistance in Campylobacter, Salmonella and Escherichia coli isolated from retail turkey meat from Southern Ontario, Canada . Journal of Food Protection , 72 , 473 – 481 .
- Crump , J.A. , Medalla , F.M. , Joyce , K.W. , Krueger , A.L. , Hoekstra R.M. , Whichard , J.M. , Barzilay , E.J. & Emerging Infections Program NARMS Working Group . 2011 . Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007 . Antimicrobial Agents and Chemotherapy , 55 , 1148 – 1154 . doi: 10.1128/AAC.01333-10
- Currie , A. , MacDougall , L. , Aramini , J. , Gaulin , C. , Ahmed , R. & Isaacs , S. 2005 . Frozen chicken nuggets and strips and eggs are leading risk factors for Salmonella Heidelberg infections in Canada . Epidemiology and Infection , 133 , 809 – 816 . doi: 10.1017/S0950268805004383
- Dohoo , I. , Martin , W. & Stryhn , H. 2009 . Veterinary Epidemiologic Research , 2nd edn . Charlottetown : VER Inc .
- Dunne , E.F. , Fey , P.D. , Kludt , P. , Reporter , R. , Mostashari , F. , Shillam , P. , Wicklund , J. , Miller , C. , Holland , B. , Stamey , K. , Barrett , T.J. , Rasheed , J.K. , Tenover , F.C. , Ribot , E.M. & Angulo , F.J. 2000 . Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC β-lactamase . Journal of the American Medical Association , 284 , 3151 – 3156 . doi: 10.1001/jama.284.24.3151
- Dutil , L. , Irwin , R. , Finley , R. , Ng , L.K. , Avery , B. , Boerlin , P. , Bourgault , A.M. , Cole , L. , Daignault , D. , Desruisseau , A. , Demczuk , W. , Hoang , L. , Horsman , G.B. , Ismail , J. , Jamieson , F. , Maki , A. , Pacagnella , A. & Pillai , D.R. 2010 . Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada . Emerging Infectious Diseases , 16 , 48 – 54 . doi: 10.3201/eid1601.090729
- Enteric Diseases Program . 2007 . Laboratory Surveillance Data for Enteric Pathogens in Canada: Annual Summary 2006 . National Microbiology Laboratory, Public Health Agency of Canada, The Canadian Science Centre for Human and Animal Health . Winnipeg , MB , Canada .
- European Food Safety Authority . 2009 . Quantitative estimation of the impact of setting a new target for the reduction of Salmonella in breeding hens of Gallus gallus . The EFSA Journal , 1036 , 1 – 68 .
- Ewing , W.H. 1986 . Edwards and Ewing's Identification of Enterobacteriaceae , 4th edn . New York : Elsevier Science Publishing .
- Fey , P.D. , Safranek , T.J. , Rupp , M.E. , Dunne , E.F. , Ribot , E. , Iwen , P.C. , Bradford , P.A. , Angulo , F.J. & Hinrichs , S.H. 2000 . Ceftriaxone-resistant Salmonella infection acquired by a child from cattle . The New England Journal of Medicine , 342 , 1242 – 1249 . doi: 10.1056/NEJM200004273421703
- Gast , R.K. , Guard-Bouldin , J. & Holt , P.S. 2004 . Colonization of reproductive organs and internal contamination of eggs after experimental infection of laying hens with Salmonella heidelberg and Salmonella enteritidis . Avian Diseases , 48 , 863 – 869 . doi: 10.1637/7204-05050R
- Gast , R.K. , Guraya , R. , Guard-Bouldin , J. , Holt , P.S. & Moore , R.W. 2007 . Colonization of specific regions of the reproductive tract and deposition at different locations inside eggs laid by hens infected with Salmonella Enteritidis or Salmonella Heidelberg . Avian Diseases , 51 , 40 – 44 . doi: 10.1637/0005-2086(2007)051[0040:COSROT]2.0.CO;2
- Grimont , P.A.D. & Weill , F.-X. 2007 . Antigenic Formulae of the Salmonella Serovars , 9th edn . Paris : WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur .
- Han , J. , David , D.E. , Deck , J. , Lynne , A.M. , Kaldhone , P. , Nayak , R. , Stefanova , R. & Foley , S.L. 2011 . Comparison of Salmonella enterica serovar Heidelberg isolates from human patients with those from animal and food sources . Journal of Clinical Microbiology , 49 , 1130 – 1133 . doi: 10.1128/JCM.01931-10
- Health Canada . 2002 . Uses of Antimicrobials in Food Animals in Canada: Impact on Resistance and Human Health . Report of the Advisory Committee on Animal Uses of Antimicrobials and Impact on Resistance and Human Health, June . Veterinary Drugs Directorate , Guelph , ON , Canada .
- Hennessy , T.W. , Cheng , L.H. , Kassenborg , H. , Ahuja , S.D. , Mohle-Boetani , J. , Marcus , R. , Shiferaw , B. & Angulo , F.J. 2004 . Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites . Clinical Infectious Diseases , 38 , 237 – 243 . doi: 10.1086/381593
- Johnson , J.M. , Rajic , A. & McMullen , L.M. 2005 . Antimicrobial resistance of selected Salmonella isolates from food animals and food in Alberta . Canadian Veterinary Journal , 46 , 141 – 146 .
- Lynne , A.M. , Kaldhone , P. , David , D. , White , D.G. & Foley , S.L. 2009 . Characterization of antimicrobial resistance in Salmonella enterica serotype Heidelberg isolated from food animals . Foodborne Pathogens and Disease , 6 , 207 – 215 . doi: 10.1089/fpd.2008.0172
- MacDougall , L. , Fyfe , M. , McIntyre , L. , Paccagnella , A. , Cordner , K. , Kerr , A. & Aramini , J. 2004 . Frozen chicken nuggets and strips – a newly identified risk factor for Salmonella Heidelberg infection in British Columbia, Canada . Journal of Food Protection , 67 , 1111 – 1115 .
- McDermott , P.F. , Bodeis , S.M. , English , L.L. , White , D.G. , Walker , R.D. , Zhao , S. , Simjee , S. , & Wagner , D.D. 2002 . Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones . Journal of Infectious Diseases , 185 , 837 – 840 . doi: 10.1086/339195
- Musgrove , M.T. , Jones , D.R. , Northcutt , J.K. , Cox , N.A. , Harrison , M.A. , Fedorka-Cray , P.J. & Ladely , S.R. 2006 . Antimicrobial resistance in Salmonella and Escherichia coli isolated from commercial shell eggs . Poultry Science , 85 , 1665 – 1669 .
- National Enteric Surveillance Program . 2011 . Annual Summary 2009 . Winnipeg , MB : Public Health Agency of Canada .
- National Enteric Surveillance Program . 2012 . Annual Summary 2012 . Winnipeg , MB : Public Health Agency of Canada .
- Patchanee , P. , Zewde , B.M. , Tadesse , D.A. , Hoet , A. & Gebreyes , W.A. 2008 . Characterization of multidrug-resistant Salmonella enterica serovar Heidelberg isolated from humans and animals . Foodborne Pathogens and Disease , 5 , 839 – 851 . doi: 10.1089/fpd.2008.0149
- United States Department of Agriculture . 2011 . Serotypes Profile of Salmonella Isolates from Meat and Poultry Products: January 1998 through December 2010 . Food Safety and Inspection Service . Washington , DC , USA .
- van der Bij , A.K. & Pitout , J.D. 2012 . The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae . Journal of Antimicrobial Chemotherapy , 67 , 2090 – 2100 . doi: 10.1093/jac/dks214
- Vugia D.J. , Samuel , M. , Farley , M.M. , Marcus , R. , Shiferaw , B. , Shallow , S. , Smith , K. & Angulo , F.J. 2004 . Invasive Salmonella infections in the United States, FoodNet, 1996–1999: incidence, serotype distribution, and outcome . Clinical Infectious Diseases , 38 , 149 – 156 . doi: 10.1086/381581
- Zhao , S. , White , D.G. , Friedman , S.L. , Glenn , A. , Blickenstaff , K. , Ayers , S.L. , Abbott , J.W. , Hall-Robinson , E. & McDermott , P.F. 2008 . Antimicrobial resistance in Salmonella enterica Serovar Heidelberg Isolates from retail meats, including poultry, from 2002 to 2006 . Applied and Environmental Microbiology , 74 , 6656 – 6662 . doi: 10.1128/AEM.01249-08