Abstract
Chicken astroviruses (CAstVs) have been characterized recently. Due to their relatively poor growth in cell culture, virus-specific antigens are not readily available for the development of diagnostic reagents and vaccines. For this purpose two capsid protein antigens, specified by the 11672 isolate of CAstV, were produced in insect cells following infection with recombinant baculoviruses. The GST-11672 capsid protein, a fusion protein comprising the capsid protein and glutathione-S-transferase (GST) as an N-terminal affinity tag, and the 11672 capsid protein alone were detected by western blotting as proteins of ~100 and 70 kDa, respectively. Immunization with the affinity-purified GST-11672 capsid protein produced a polyclonal rabbit antiserum, which reacted by indirect immunofluorescence with Group B CAstVs but which showed no reactivity with the Group A CAstV isolate, 612. When used as part of an immunoperoxidase-based immunohistochemical procedure, this rabbit antiserum facilitated the detection of CAstV antigen in formalin-fixed, paraffin-embedded kidney tissue at the sites of histopathology characteristic of nephritis. Although further evaluation with sera from commercial chickens is required, a prototype indirect antibody-detecting enzyme-linked immunosorbent assay (ELISA) based on affinity-purified GST-11672 capsid protein as coating antigen demonstrated considerable potential with low ELISA absorbance values being generated with sera from specific pathogen free (SPF) chickens, and high absorbance values being generated with serum samples from experimentally infected chickens. Immunization experiments of SPF chickens showed that, when administered as mixtures with oil adjuvant, crude cell lysates containing the GST-11672 capsid protein or the 11672 capsid protein elicited virus-specific antibody responses that were detectable by indirect immunofluorescence and by virus neutralization assays.
Introduction
Two avian astrovirus species are known to infect chickens, avian nephritis virus (ANV) and chicken astrovirus (CAstV). CAstV was characterized as a novel astrovirus by Baxendale & Mebatsion (Citation2004), who reported the isolation of three antigenically similar viruses from outbreaks of runting–stunting syndrome that occurred in the Netherlands in the 1980s. Since then CAstVs have been detected by reverse transcriptase-polymerase chain reaction (RT-PCR) in intestinal samples from growth-retarded and normal broilers reared in the USA, the UK and other European countries (Day et al., Citation2007; Smyth et al., Citation2009, Citation2010). The existence of two antigenically different CAstVs, namely isolate 11672 and isolate 612, which share low levels of cross-reactivity as determined by indirect immunofluorescence (IF), has been reported by Todd et al. (Citation2009a). These isolates are representative of two major CAstV groups, designated A and B, which display very low levels (38 to 40%) of capsid protein identity (Smyth et al., Citation2012). Seroprevalence investigations involving separate indirect IF tests have shown that infections with CAstVs from both groups are common in UK broiler and broiler breeder flocks, and are widespread in poultry organizations from throughout Europe (Todd et al., Citation2009b).
The detection by real-time RT-PCR of high levels of CAstV RNA in growth-retarded broilers and the capacities of CAstVs to cause varying degrees of growth depression following experimental infection of 1-day-old chicks (McNeilly et al., Citation1994; Smyth et al., Citation2007) have prompted speculation that CAstV may be contributing to growth retardation problems such as runting–stunting syndrome. In addition, the detection of CAstV in dead-in-shell chicks provided evidence of vertical transmission (Spackman et al., Citation1984) and raised the possibility that this virus may be causing hatchability problems (Todd, unpublished results). However, the extent and nature of CAstV-related disease problems remain largely unknown due to the absence of virus-specific reagents and convenient diagnostic tests. A major reason for this absence is the poor growth of CAstV in cell culture, which has made it difficult to prepare sufficiently pure virus antigen in the amounts required for the development of antibody-detecting enzyme-linked immunosorbent assays (ELISAs) and for immunization to produce virus-specific antibodies for use in antigen-detecting diagnostic methods such as IF and immunohistochemistry (IHC). In addition, poor cell culture yields will limit the development of both live and inactivated CAstV vaccines, should such be required.
In this paper we describe the production of CAstV capsid protein antigens in insect cells using recombinant baculoviruses and investigations into their use to produce a virus-specific rabbit polyclonal antiserum that can be applied in IF-based and IHC-based diagnostic tests, to serve as an ELISA antigen for the detection of antibodies in chicken sera, and to induce virus-specific antibodies in chickens in a vaccine feasibility study.
Materials and Methods
Viruses and virus growth
The origin and growth in primary chick embryo liver (CEL) cells of the 11672, FP3 and 612 isolates of CAstV were described previously (Todd et al., Citation2009a). The isolation of the CAstV VF08-29 isolate and its growth in primary CEL and LMH cells was described by Smyth et al. (Citation2012).
Sera
Antisera specific to the 11672, FP3 and 612 isolates of CAstV were prepared by experimental inoculation of specific pathogen free (SPF) chickens as described by Todd et al. (Citation2009b). Sets of serum samples were also obtained from SPF chickens (<6 weeks old) and from commercial grandparent chickens (12 to 16 weeks old).
Generation of the recombinant baculoviruses expressing the CAstV capsid genes
The recombinant baculoviruses were generated using the flashBACGOLD expression system (Oxford Expression Technologies, Oxford, UK). The isolation and the cloning of the 11672 CAstV capsid gene into the pCR2.1®TOPO vector (Life Technologies, Paisley, UK) was described in Smyth et al. (Citation2012). The 11672 capsid gene fragment was amplified from this Topo vector construct by PCR using the oligonucleotide primers: 5′-gctggat1ccaccatggccgataaggct and 3′-tcggaattcctcggcgtggccgcg (Life Technologies), which contained the Kozak sequence (underlined) and the BamHI (boldface type) in the 5′ primer, and the EcoRI site in the 3′ primer (boldface type). The PCR involved an initial denaturation at 94°C for 5 min, followed by 40 thermocycles each comprising denaturation at 94°C for 15 sec, annealing at 60°C for 30 sec and extension at 72°C for 2.5 min followed by a final extension step at 68°C for 7 min. The amplified fragment ~2.3 kb was digested with both BamHI and the EcoRI restriction enzymes (Life Technologies), and following gel purification using the Qiaquick gel extraction kit (Qiagen, Crawley, UK) the restricted fragment was ligated to one of two appropriately restricted vectors. These were the pAcG2T vector (BD Bioscience, Oxford, UK), which contained glutathione-S-transferase (GST) and the thrombin cleavage site located upstream of the ligation site, thereby facilitating the production of a fusion protein (GST-11672 capsid) in which the GST protein was fused in frame with the N-terminus of the 11672 capsid protein fusion protein; and the pVL1392 vector (Life Technologies), which allowed the production of the 11672 capsid protein alone. The two vector constructs were separately transformed into competent TOP 10 cells (Life Technologies). Bacterial colonies from each transformation were investigated for the presence of suitably sized inserts using restriction digest (BamHI and EcoRI) analyses of purified, small-scale plasmid DNA preparations (Qiagen). DNA sequencing, performed with Big Dye (Life Technologies) chemistry, was used to confirm that the sequences of 11672 capsid gene contained by both vectors were correct.
The pAcG2T and the pVL1392 clones, containing the 11672 capsid fragments, were designated pAcG2T-11672 and pVL1392-11672, respectively. Both clones were then transfected with linearized baculovirus DNA (flashBACGOLD kit) into Sf9 cells (Life Technologies) in Sf-900 III serum-free medium (Life Technologies) using Lipofectin (Life Technologies) according to the manufacturer's protocol. In this way the recombinant baculoviruses AcNPV-gst11672, containing the GST-11672 fusion sequence, and AcNPV-11672, containing the 11672 sequence alone, were generated. At 24 h post transfection, 1 ml of the Sf-900 III medium with 10% foetal calf serum (Life Technologies) was added to the Sf9 cells. Cells were then incubated for another 4 days at 27°C and the medium was harvested as a P1 stock. For the virus amplification, 0.5 ml of the P1 stock was added to a 100 ml culture of Sf9 cells (~1×106 cells/ml) contained within a spinner flask. After 5 days incubation at 27°C, the infected cells were harvested by centrifugation at 1000×g for 10 min at 4°C. The supernatants constituting the P2 stock pools of the recombinant baculoviruses were stored at 4°C. The P2 pools were used similarly to produce P3 working pools by infecting Sf9 cells.
Production of the recombinant CAstV capsid proteins
The GST-11672 capsid protein was produced by infecting 100 ml cultures of Hi-5 cells (1×108) in 10% foetal calf serum or Sf9 cells (1×108) in serum-free medium contained in spinner flasks with the P3 working pool of the AcNPV-gst11672 recombinant baculovirus, while the non-tagged 11672 capsid was similarly produced using Hi-5 cells only, infected with the P3 working pool of the AcNPV-11672 recombinant baculovirus. Multiplicities of infection (MOI) of ~5 were used in all cases. At 96 h post infection (p.i.), cells were centrifuged at 1000×g for 10 min. The GST-11672 capsid protein recombinant protein was purified using the BD BaculoGold GST-purification kit (BD Biosciences). The principle of GST affinity purification is that the GST moiety present in the fusion protein binds to its substrate, glutathione, which is immobilized on the beads, whereas non-GST-containing proteins do not bind. After washing, the bound GST-tagged fusion protein is displaced from the beads by an excess of soluble glutathione present in the elution buffer. In practice, cells were collected by centrifugation at 16,000×g for 10 min, resuspended in 5 ml of the BD BaculoGold lysis buffer containing protease inhibitor and incubated on ice for 45 min. The cell lysate was centrifuged at 40,000×g for 45 min, and the supernate incubated with the glutathione agarose beads for 45 min. After washing the beads twice with phosphate-buffered saline (PBS; 0.01 M, pH 7.2), the GST-11672 capsid protein was eluted in 5 ml of the BD BaculoGold GST-purification elution buffer. The cell lysate pellets obtained after ultracentrifugation, representing the insoluble fraction, were resuspended in 5 ml PBS using 3×30 sec bursts of sonication and retained for investigation. For the production of the non-fused 11672 capsid protein, Sf9 cells, infected with the AcNPV-11672 recombinant baculovirus, were collected at 96 h p.i. and resuspended in 5 ml PBS before being lysed by sonication (three bursts of 30 sec). The supernate and resuspended cell debris pellet were collected after the sonicated cell lysate was centrifuged at 16,000×g for 10 min.
Polyacrylamide gel electrophoresis and western blotting immunoblotting
Samples were boiled in NuPage LDS buffer (Life Technologies) for 10 min. Twenty microlitres of the solubilized samples were loaded into Bis–Tris polyacrylamide 4 to 12% gel (Life Technologies) using the XCell SureLock Mini-Cell system (Life Technologies) and the fractionated proteins were subsequently transferred to the nitrocellulose membrane (Life Technologies). The membrane was blocked with 5% skimmed milk in PBS for 1 h, and then incubated with mouse monoclonal antibody (mAb) 17 (diluted 1 in 200) in 5% skimmed milk in PBS. This mAb was produced in this laboratory by immunizing mice with semi-purified preparations of the cell-culture grown 11672 isolate of CAstV (Todd, unpublished results). The membrane was washed three times in PBS and then incubated in rabbit anti-mouse immunoglobulin conjugated with alkaline phosphatase for 1 h (Life Technologies). Bands were visualized using Novex AP Chromogenic Substrate, nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (Life Technologies). For the Coomassie blue staining, the Bis–Tris polyacrylamide 4 to 12% gel was washed three times with PBS for 5 min each. The gel was then stained with SimplyBlue (Life Technologies) for 3 h, followed by washing twice with PBS.
Production of rabbit polyclonal antiserum specific to 11672 capsid protein
Following the collection of pre-immune bleeds, rabbits were immunized on three occasions at intervals of approximately 4 weeks with preparations of affinity-purified GST-11672 capsid protein (1 to 2 µg/ml in elution buffer), which had been thoroughly mixed with equal volumes of Quil A adjuvant (1 mg/ml) (Brenntag Biosector, Frederikssund, Denmark). On each immunization occasion, a total volume of 250 µl antigen/adjuvant mixture was inoculated subcutaneously into each rabbit spread over four sites. Serum samples were collected ~2 weeks after the third immunization.
Immunization of chickens
The immunogenicities of the two recombinant CAstV capsid proteins were investigated by immunizing SPF chickens with oil-adjuvanted mixtures of infected cell culture lysates. For the preparation of GST-11672 capsid protein lysate, spinner flasks containing 100 ml cultures Hi-5 cells (~1×108) in medium containing 10% foetal calf serum were infected with the P3 pool of AcNPV-gst11672 recombinant baculovirus at an MOI of ~5. After 120 h of incubation, the cells were collected at 1000×g for 10 min and then resuspended in 50 ml PBS. For the preparation of the recombinant 11672 capsid protein lysate, spinner cultures (100 ml) of Sf9 cells (~1×108) in Sf-900 III serum-free medium were infected with the P3 pool of AcNPV-11672 recombinant baculovirus at MOI of ~5. The use of Sf9 cells grown in serum-free medium for the production of the non-tagged 11672 capsid as opposed to Hi-5 cells enriched the proportion of virus protein present by eliminating the presence of serum proteins from the immunogen. After 120 h, the infected cells were then centrifuged at 1000×g for 10 min and the pellets were resuspended in 50 ml PBS. In each of the two preparations, the cells were fully lysed by repeated 30 sec bursts of sonication. Similar cultures of uninfected Hi-5 and Sf9 cells were similarly prepared for vaccine control purposes. For the emulsification, 50 ml cell lysate containing 6.5% surfactant (Croda France, Trappes, France) was thoroughly blended with three volumes of oil (ESSO France, Paris La Défense, France) for 15 min using an Ultraturax blender, taking care to prevent over-heating.
In the first immunization experiment, groups of 4-week-old SPF chicks (Valo, Cuxhaven, Germany), which had been reared from hatch in negative-pressure isolators, were inoculated at 4 and 8 weeks old with either the emulsified recombinant GST-11672 capsid protein lysate or with the emulsified lysate prepared with uninfected Hi-5 cells. In the second experiment, similar groups of 4-week-old SPF chickens, reared in isolators, were similarly inoculated with emulsified recombinant 11672-capsid protein lysate and emulsified uninfected Sf9 cell lysate preparations. For additional control purposes, three non-inoculated birds were reared within each of the isolators containing birds vaccinated with virus antigen. Blood samples were collected when the inoculated and non-inoculated chickens were killed at 4 (pre-immune), 7, 8, 10 and 11 weeks old and serum samples were produced for serological testing.
Indirect immunofluorescence
Indirect IF tests, performed with primary CEL cells or LMH cells that were infected with either the 11672 CAstV isolate, the VF08-29 CAstV isolate or the 612 CAstV isolate, were undertaken as described previously (Todd et al., Citation2009b). A similar method was used with Sf9 cells that were infected with the recombinant baculoviruses. Briefly, adherent Sf9 cells (~1×106/ml) were seeded in six-well plates (Corning Costar; Sigma-Aldrich Co., Dorset, UK) containing sterile glass coverslips (11 mm diameter). Cells were then infected with either the AcNPV-gst11672 or AcNPV-11672 recombinant baculoviruses at an MOI of ~5, and at 96 h p.i. the coverslip cultures were washed twice in PBS and then fixed with acetone for 10 min. The fixed cells on coverslips were then incubated at 37°C for 1 h with mAb 17 (dilution 1 in 800 in PBS), after which the coverslips were washed three times with PBS and then incubated for 1 h with the rabbit anti-mouse Ig antibodies conjugated with fluorescein isothyocyanate (FITC; DAKO, Ely, UK), which had been diluted 1:80 in PBS. Following additional washing, the coverslips were mounted with a drop of citifluor (Citifluor, London, UK) and viewed with the Leica fluorescent microscope at a magnification of×400.
Immunohistochemistry
Samples of kidney tissues from commercially reared broilers were submitted for diagnostic investigation of kidney disease problems including visceral gout. The consignment included samples of frozen kidney from four broilers (1 to 16 days of age) collected from four affected flocks. Testing by real-time RT-PCR tests, developed in this laboratory (Smyth et al., Citation2010), showed the presence of very high levels of CAstV RNA (>107 virus RNA copies) and medium levels of ANV RNA (103 to 106 virus RNA copies) in the four kidney samples. Additional samples of two of the kidney tissues from 9-day-old chickens, which had tested positive for CAstV when frozen kidney tissues had been tested by RT-PCR, were received already fixed in 10% buffered formalin and these were further processed for histology and then for immunohistochemistry, with which the detection of CAstV antigen was investigated. The formalin-fixed tissues were in paraffin wax and sections were cut at 4 µm. Negative kidney tissues from age-matched, SPF chickens known to be uninfected with CAstV were also processed. Sections were lifted onto coated microscope slides. Paraffin wax was removed from the sections using xylene and rehydrated through alcohol to water. Endogenous peroxidase was blocked using 0.5% hydrogen peroxide in methanol for 20 min. The wash buffer was Tris-buffered saline, 5 mM, pH 7.6 (TBS). The antigen retrieval was 0.1% Protease 14 (Sigma) in TBS for 5 min at room temperature. Sections were incubated overnight in the rabbit anti-GST-11672 capsid protein antiserum at a dilution of 1:350 in TBS (primary antibody) at room temperature. For control purposes, sections were also incubated with TBS alone (no primary antibody control). Primary antibody was detected by incubating in peroxidase-labelled polymer conjugated to anti-mouse and anti-rabbit immunoglobulins (REAL™ Envision™/Rab/Mouse reagent; DAKO) for 30 min at room temperature. DAB chromogen (Vector Laboratories, Peterborough, UK) was used with a haematoxylin (Harris's haematoxylin; Clin-Tech Ltd, Guildford, UK) counter-stain to display and disclose the reaction.
Virus neutralization test
A virus neutralization (VN) test for detecting antibodies to the 11672 CAstV isolate was developed and applied to samples of chicken and rabbit serum, which had been heat-treated at 56°C for 30 min. Serial two-fold dilutions of these serum samples in 250 µl volumes of cell culture maintenance medium containing 2% foetal calf serum (2%MM), were mixed with 100 median tissue culture infectious dose virus, also contained in 250 µl of 2%MM. After incubation at 37°C for 1 h, 100 µl volumes of the virus:serum mixtures were added to monolayer cultures of LMH cells, which had been grown in 24-well Costar plates, and which were present in 1 ml of 2%MM when the virus mixtures were added. After incubation at 37°C for 5 days the cells were washed three times with PBS containing Tween 80, air-dried for ~1 h and then fixed by adding 500 µl of 4% paraformaldehyde for 10 min at room temperature. After washing, 500 µl methanol with 0.1% hydrogen peroxide was added to each well for 5 min at room temperature and, after further washing, CAstV antigen was detected by successive incubations of 1 h at 37oC of the cells in each well with 500 µl volumes of primary antibody (usually convalescent experimental chicken serum) and horseradish peroxidase (HRPO)-conjugated secondary antibody (usually rabbit anti-chicken immunoglobulins), followed by the addition of aminoethyl carbazole substrate (Life Technologies) for 15 min at room temperature in the dark.
Indirect enzyme-linked immunosorbent assay
Initially, ELISA microtitre plates (Immulon 1 B; Thermo Fisher Scientific, Hemel Hempstead, UK) were coated overnight at 4°C with the affinity-purified GST-11672 capsid protein (1 to 2 µg/ml elution buffer) at dilutions ranging from 1:100 to 1:20,000 in 0.05 M sodium carbonate–bicarbonate buffer, pH 9.6. Adsorbed antigen was detected by sequential 1 h incubations at 37 °C with mAb 17, which had been diluted to 1:800 in dilution buffer, comprising 0.05 M PBS, pH 7.2 containing 0.05% Tween 20 and 2% NaCl, and HRPO-anti-mouse Ig conjugate (DAKO) diluted to 1:500. The coating, primary antibody and conjugated antibody incubations were followed by thorough washing with washing buffer, which comprised PBS containing 0.05% Tween 20 and 0.17 M NaCl. The enzyme substrate 3,3′,5,5′-tetramethybenzidine (Millipore-Chemicon, Temecula, California, USA) was incubated at 37°C for 10 min in the dark before the reaction was stopped by the addition of 50 µl of 2 M H2SO4 to each well. The absorbance was read at 450 nm with a 620 nm correction filter using a Tecan plate reader (Tecan, Reading, UK). A similar protocol was used in subsequent work in which chicken antibodies reactive with affinity-purified GST-11672 antigen were detected. In the optimized test for detecting chicken antibody, a dilution of 1:400 was used. This involved incubating antigen-coated ELISA plates for 1 h at 37 °C with, firstly, samples of chicken serum (diluted from 1:100) and, secondly, HRPO-anti-chicken Ig conjugate (DAKO) diluted to 1:5000 followed by the addition of enzyme substrate.
Results
Detection of recombinant CAstV capsid proteins by indirect immunofluorescence
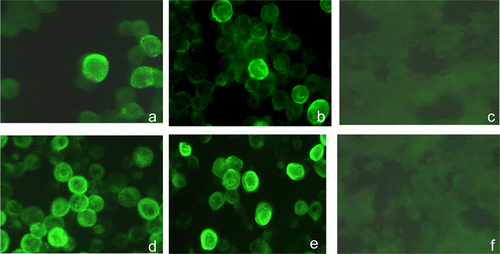
IF staining performed with the CAstV-specific mAb 17 was used initially to investigate the production of recombinant CAstV capsid proteins in insect cells. Following infections of Sf9 cells at a MOI of ~5 with their respective recombinant baculoviruses, both the recombinant GST-11672 capsid and 11672 capsid proteins were shown to be expressed in the cytoplasm at 24 h p.i. The highest staining intensities for both recombinant proteins were observed at 96 h p.i. (). Some infected cells showed fluorescently staining inclusion bodies, but uninfected cells showed no specific staining.
Analyses of recombinant proteins by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and western blotting
Following infection of Sf9 or Hi-5 cells with the AcNPV-gst11672 recombinant baculovirus, western blotting performed with the CAstV-specific mAb 17 demonstrated the presence of the GST-11672 capsid fusion protein, which was estimated to be ~100 kDa in size when compared with the protein size markers (). Additional bands corresponding to proteins of higher and lower molecular weights and including a strongly-staining band of ~200 kDa were also often detected by this western blotting approach (). Since analysis of infected cells harvested at different times p.i. showed that maximal amounts of the recombinant GST-11672 capsid protein were detected 72 to 96 hr p.i. (data not shown), a 96 h incubation period was chosen for subsequent recombinant protein production work. At this harvesting time, the GST-11672 capsid protein was not detectable by western blotting when small volumes (10 to 20 µl) of cell culture supernate were analysed (). This GST fusion protein was released from the infected cells using lysis buffer (BD BaculoGold) or by sonication, although, in both cases, substantial amounts of this protein were retained in the insoluble cellular material collected by centrifugation. Affinity purification using GST-binding affinity beads (BD BaculoGold) was used to purify the 100 kDa GST-11672 capsid protein from the majority of cellular proteins (). A smaller cellular protein of ~40 kDa was also detected by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) in the affinity-purified preparations, but this did not react by western blotting (). SDS-PAGE analysis showed that thrombin treatment of the affinity-purified GST-11672 capsid protein generated a protein of ~70 kDa. However, western blotting showed that thrombin digestion was incomplete and a number of protein bands of smaller size were detected by immunostaining ().
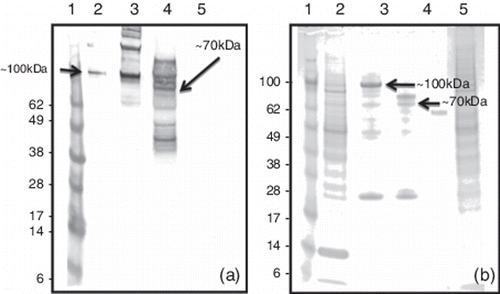
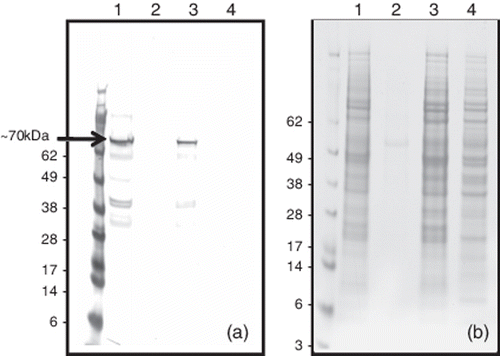
Infecting Sf9 cells with the AcNPV-11672 recombinant baculovirus resulted in the production of the 11672 capsid protein without the GST affinity tag. This was detected in lysates of infected cells by western blotting using mAb 17 as a protein band of ~70 kDa (), and maximal amounts were detected at 72 and 96 h p.i. Little or no recombinant protein was detected in the cell culture supernatant using western blotting. Even at 120 h p.i., when cell morphology had deteriorated, the vast bulk of the recombinant protein was detected in the cellular pellet collected by low-speed centrifugation. The recombinant 11672 capsid protein was released into the soluble fraction using lysis buffer or by repeated sonication treatments.
Production and characterization of polyconal antiserum specific to recombinant GST-11672 capsid
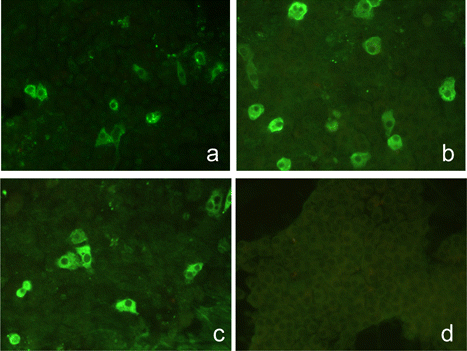
The antiserum raised by immunizing rabbits with the affinity-purified GST-11672 protein was firstly characterized in terms of its ability to react with CAstV-specific antigen using indirect IF performed with primary CEL cells infected with the 11672 CAstV isolate. When used in combination with the FITC-conjugated anti-rabbit Ig, the rabbit polyclonal antiserum produced virus-specific fluorescent staining at dilutions up to 1:2048. The fluorescent staining patterns observed resembled those obtained using convalescent chicken antisera and the CAstV-specific mAb 17 (). The rabbit antiserum also produced virus-specific staining with CEL cells infected with the VF08-29 CAstV isolate at dilutions up to 1:4096 but no virus-specific staining was obtained with cells infected with the 612 CAstV isolate (data not shown), even when low dilutions (1:32) of the rabbit antiserum were used.
The rabbit antiserum to GST-11672 capsid also contained virus-neutralizing antibodies, with a titre of 1:2000 being determined using the VN test based on the 11672 CAstV isolate.
Application of polyconal antiserum specific to recombinant GST-11672 capsid in an immunohistochemistry test
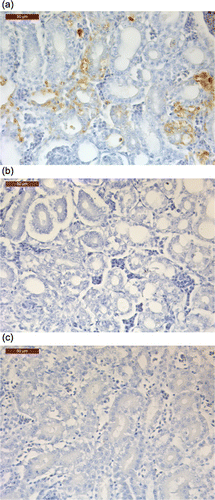
Histopathological examination of kidneys from a field case of broilers with kidney disease revealed a mainly mild urate nephrosis. Changes included tubular distension, degenerate tubular epithelia with cellular material and granulocytes in tubular lumen and occasional urate tophi. IHC revealed positive granular staining that was most evident in degenerate tubule epithelial cells (). Staining was absent in sections where the primary antibody was not applied and in kidneys from age-matched, uninfected SPF chickens.
Evaluation of GST-11672 capsid protein as an ELISA antigen
The ability of the affinity-purified GST-11672 capsid protein to serve as a coating antigen in an antibody-detecting indirect ELISA test was investigated. Antigen eluted from the affinity beads was estimated to be present at a concentration of ~1 to 2 µg/ml. Initial investigation demonstrated that, following overnight adsorption at 4°C, coating dilutions of up to 1:10,000 of eluted antigen could be detected (absorbance value >0.5) when relatively high concentrations of CAstV-specific mAb 17 were used for detection in conjunction with HRPO-anti-mouse Ig conjugate. However, in subsequent work in which antibodies present in convalescent chicken antisera were being assayed, the eluted antigen was used at a dilution of 1:200 to 1:400. Using these levels of coating antigen and the described protocol (see Materials and Methods), 20 serum samples from uninfected SPF chickens (<6 weeks old) produced a mean ELISA absorbance value of 0.021 (standard deviation = 0.012), while absorbance values ranging from 0.706 to 3.334 were obtained for 1:100 dilutions of selected CAstV experimental antisera, which were raised to the 11672 and FP3 CAstV isolates (). When dilutions of the CAstV experimental antisera were tested, indirect ELISA titres ranging from 1:1000 to 1:64,000 were determined. The indirect IF titres obtained with these experimental antisera were 1:3200 or 1:6400. ELISA testing showed that 60 serum samples, which were obtained from three commercial chicken flocks (12 to 16 weeks old) and which tested negative by the indirect IF test, had a mean ELISA absorbance of 0.085 (standard deviation = 0.031) and a range of 0.042 to 0.182. When 28 serum samples that were positive by the indirect IF assay were tested, ELISA absorbances ranging from 0.308 to 2.368 (mean = 0.829) were recorded. Eight of the 28 samples had absorbances greater than 1.000, 20 samples had absorbances greater than 0.500, and all 28 samples had values above the provisional absorbance cut-off value of 0.200, which was selected following consideration of the mean (0.085) and standard deviation (0.031) values obtained with the 60 serum samples from negative flocks.
Table 1. Indirect ELISA and immunofluorescence assay results obtained with experimental chicken antisera.
Immunogenicities of recombinant CAstV capsid proteins
Two chicken immunization experiments were undertaken, the first using the GST-11672 capsid and the second using the 11672 capsid. In both cases, crude lysates containing approximately similar levels (~0.5 µg/dose) of recombinant protein as well as cell-derived proteins were administered after blending with oil adjuvant. In the first experiment, antibody to CAstV 11672 was detected by indirect IF (titres 1:256 and 1:512) in all six birds that were killed and tested 4 weeks after the first immunization (). The indirect IF titres determined for serum samples taken from the six birds that were tested at 2 weeks after the second immunization ranged from 1:100 to 1:1000. Four of these six sera were found to have virus-neutralizing antibodies with titres ranging from 1:16 to 1:64. All serum samples taken from similarly treated chickens, which had been immunized with clarified sonicated lysate prepared from uninfected Hi5 cells tested negative by indirect IF for antibody reactive with CAstV 11672.
Table 2. Immunization of 4-week-old SPF chickens with GST-11672 capsid.
The second experiment performed with the non-fused 11672 capsid protein involved sampling birds 3 and 4 weeks after the first and second immunizations (). With the exception of one sample (Bird #8), CAstV 11672-specific antibody was undetectable (<1:32) in samples collected after the first immunization at either 3 or 4 weeks post immunization. After the second immunization, virus-specific antibody was detected in three out of four samples at 3 weeks post immunization (indirect IF titres 1:128 to 1:516) and in three out of four samples at 4 weeks post immunization (indirect IF titres 1:1000 or 1:2000). Six of the eight birds sampled after the second immunization were also positive for virus-neutralizing antibodies, with titres of 1:64 to 1:256 being detected. Post-immunization serum samples taken from birds immunized with sonicated lysates prepared from uninfected Sf9 cells tested negative by indirect IF, as did samples from non-immunized birds that were reared in the isolators for control purposes.
Table 3. Immunization of 4-week-old SPF chickens with 11672 capsid.
Discussion
This paper describes the production of CAstV capsid proteins using recombinant baculoviruses and investigations to assess their usefulness for developing diagnostic tests and vaccines.
Because earlier studies with human astroviruses had indicated that recombinant baculoviruses could be used to produce astrovirus capsid protein as virus-like particles (Caballero et al., Citation2004), there were strong grounds for believing that the capsid proteins of avian astroviruses would also be produced in forms resembling those found in the native virus. Although CAstV virus-like particles were not visualized in this study, the abilities of baculovirus-derived CAstV capsid proteins to induce virus-specific antibodies that neutralize infectious virus and that produce IF staining patterns similar to those generated using convalescent chicken antisera support the view that the recombinant capsid proteins were immunogenically similar to the native proteins.
The affinity-purified GST-11672 capsid protein proved to be useful in two respects; firstly, as an immunogen; and secondly, as an antigen. As an immunogen, it facilitated the production of a polyclonal rabbit antiserum with high levels of specific antibody reactive with the 11672 isolate of CAstV and antigenically related CAstVs. To date, the production of such a reagent has not been possible, because, due to the relatively poor growth of CAstV in cell culture, we have not been able to produce virus of sufficient purity and in the required amounts for rabbit immunization. Although our earlier work using small amounts of impure virus allowed the production of a small panel of mouse mAbs, including mAb 17 that was used in this study, these mAbs have been of limited use as diagnostic reagents, and polyclonal antiserum with reactivities to a broader range of virus epitopes was considered desirable. Using indirect IF, the rabbit antiserum raised to the affinity-purified GST-11672 capsid stained virus-specific antigen present in cells infected with the 11672 and VF08-29 isolates of CAstV, but produced no staining in cells infected with the 612 CAstV isolate (data not shown). The antigenic cross-reactivity exhibited by the 11672 and VF08-29 isolates can be explained by a recent sequencing study, which showed that these isolates share ~85% capsid protein amino acid identity and are representative of subgroups i and ii of the Group B CAstVs, respectively. Furthermore, the lack of antigenic reactivity displayed by the 612 isolate can be explained by the fact that the capsid of this Group A CAstV shares a very low (38 to 40%) amino acid identity with the CAstVs from Group B (Smyth et al., Citation2012). One can therefore conclude that the rabbit antiserum raised to GST-11672 capsid will be useful for the detection of all Group B CAstVs irrespective of subgroup, and will not detect Group A CAstVs. In this study we have shown that this antiserum can serve as the primary antibody in an IHC-based test using paraffin-embedded, formalin-fixed kidney tissues, in which CAstV antigen was detected in large amounts at sites showing nephropathy-associated histopathology in kidney tissue from broilers with kidney disease (). Although other infectious and non-infectious causes of the lesions cannot be ruled out, our finding highlights the need for avian pathologists to consider CAstV when cases of nephropathy in chickens are being investigated. The rabbit anti-CAstV antiserum might also be used to develop more rapid fluorescent antibody tests performed with frozen tissue sections or kidney impression smears.
The affinity-purified GST-11672 capsid protein also demonstrated its potential as a coating antigen for use in an indirect ELISA for detecting antibodies in convalescent chicken sera. Although the use of an indirect IF test based on the 11672 isolate of CAstV has previously been described (Todd et al., Citation2009b), to date the development of an indirect ELISA using cell-culture-derived virus has not been possible due to the relatively poor growth of CAstV isolates in cell culture. Although small amounts of recombinant protein (1 to 2 µg/ml elution buffer) were produced in this study, when used as a coating antigen at dilutions of 1:200 or 1:400, substantial ELISA absorbance values (up to 3.334) and high indirect ELISA titres (up to 1:64,000) were determined with serum samples obtained by experimentally infecting SPF chickens with the 11672 and FP3 CAstV (subgroup B i) isolates. Absorbance values ranging from 0.308 to 2.368 (mean 0.829) were obtained with 28 serum samples that tested positive by the indirect IF test based on the 11672 CAstV isolate. In contrast, very low absorbance values were produced with sera from 20 SPF (values <0.100) chickens and from 60 commercial chickens (values <0.200) that tested negative by indirect IF test based on the 11672 isolate of CAstV. On the basis of the indirect IF results obtained with the rabbit antiserum raised to the GST-11672 capsid, we would predict that the ELISA based on the GST-11672 capsid antigen will allow the detection of antibodies to Group B CAstVs but not the detection of antibodies specific to Group A CAstVs. Although this indirect ELISA method demonstrates considerable potential, it is acknowledged that further evaluation work with substantial numbers of serum samples from commercial chickens will be required before it can be used diagnostically. In this connection, the possibility that chicken sera react with the GST component of the affinity-purified ELISA antigen to generate false-positive reactions needs to be investigated and ruled out. One obvious area for application is the screening of SPF flocks, particularly those producing embryonated eggs for avian vaccine production. Like ANV, CAstV is highly prevalent, has a widespread distribution and is vertically transmitted (Todd, unpublished findings). As such, CAstV may be recognized in due course as an additional “specified” pathogen. ELISAs for detecting CAstV antibodies would be useful to demonstrate that SPF flocks are free from infection. Since undertaking this study, Sellers et al. (Citation2010) described the use of an indirect ELISA test for detecting CAstV antibody. This test, which was also based on a recombinant baculovirus-derived, affinity-purified (His-tag) CAstV capsid protein, specified by the CAstV-UGA-2006 strain, was used to determine antibody induced by an experimental vaccine, but its use to detect antibody in commercial flocks has not yet been described. The recent capsid sequence comparison study by Smyth et al. (Citation2012) showed that CAstV-UGA-2006 strain differs from the 11672 CAstV isolate in that it belongs to Group B subgroup ii, whereas the 11672 isolate belongs to Group B subgroup i (Smyth et al., Citation2012).
Immunization experiments performed with chickens showed that both the GST-11672 capsid and the non-tagged 11672 capsid proteins produced by recombinant baculoviruses were immunogenic. This was evident from their abilities to elicit antibody responses that were virus specific, as demonstrated by indirect IF and by VN tests. In both cases, the inocula comprised unpurified cell lysates, which were prepared by sonicating infected cells collected by low-speed centrifugation, followed by thorough blending with an oil adjuvant. The use of unpurified and unconcentrated cell lysates simulated the approach likely to be adopted in large-scale vaccine manufacture, where commercial considerations apply. It is acknowledged, however, that, given the exposure of commercial poultry to insects, vaccinating poultry with crude insect cell lysates may have the potential to sensitize birds to insects and induce immunopathologies. Our results with the GST-11672 capsid protein showed that virus-specific antibodies were detected 4 weeks after the first immunization, whereas with the exception of one bird (of eight investigated) the first immunization with the 11672-capsid (non-tagged) failed to induce detectable antibody responses at 3 and 4 weeks post immunization. However, after the second immunization with this non-tagged antigen, virus-specific antibodies were detected at 3 and 4 weeks post immunization in three of the four birds tested, with the antibody titres detected at 4 weeks post immunization being higher than those detected after 3 weeks. On the basis of these initial experiments with the non-tagged 11672 capsid antigen, it would appear that not all birds behave similarly and that two immunizations may be required to elicit a detectable virus-specific antibody response. A vaccine challenge model will be required to determine whether these virus-specific antibody levels are protective. Sellers et al. (Citation2010) described an investigation into the protective effect of an experimental recombinant baculovirus-derived CAstV capsid protein vaccine. Their work involved immunizing broiler parent chickens on up to three occasions with an affinity-purified capsid protein, specified by the CAstV-UGA-2006, mixed with Freund's adjuvant, and subjecting progeny chicks to a runting–stunting disease model, which was experimentally induced by placing the chicks on contaminated litter. The authors reported that some protection was conferred when the vaccinated parents received three immunizations. It must be acknowledged, however, that investigations into CAstV vaccine feasibility are at a very early stage. A better defined experimental CAstV disease model, involving the inoculation of 1-day-old progeny chicks with a pathogenic CAstV isolate, will probably be required to determine whether breeder vaccination can be successful and to determine the levels of VN antibody required for protection. We suspect that the amounts of recombinant GST-11672 capsid and 11672 capsid produced in our investigation are relatively low in comparison with those that might be produced in larger-scale, optimized insect cell expression systems. If this is the case, it is probable that higher levels of virus-specific antibodies, including VN antibodies, would be induced if larger amounts of virus antigen were inoculated. The use of unpurified and unconcentrated cell lysates simulated the approach likely to be adopted in large-scale vaccine manufacture, where commercial considerations apply. Nevertheless, our study has shown that CAstV capsid antigens produced by recombinant baculoviruses are immunogenic for chickens and, as such, display vaccine potential.
Acknowledgements
This work was funded in part by MERIAL, Biotechnology and Biological Sciences Research Council, the Department of the Environment, Food and Rural Affairs, and the Department of Agriculture and Rural Development for Northern Ireland and the Agri-Food & Biosciences Institute for Northern Ireland.
References
- Baxendale, W. & Mebatsion, T. (2004). The isolation and characterisation of astroviruses from chickens. Avian Pathology, 33, 364–370.
- Caballero, S., Guix, S., Ribes, E., Bosch, A. & Pintó, R.M. (2004). Structural requirements of astrovirus virus-like particles assembled in insect cells. Journal of Virology, 23, 13285–13292.
- Day, J.M., Spackman, E. & Pantin-Jackwood, M. (2007). A multiplex RT-PCR test for differential identification of turkey astrovirus type 1, turkey astrovirus type 2, chicken astrovirus, avian nephritis virus and avian rotavirus. Avian Diseases, 51, 681–684.
- McNeilly, F., Connor, T.J., Calvert, V.M., Smyth, J.A., Curran, W.L., Morley, A.J., Thompson, D., Singh, S., McFerran, J.B., Adair, B.M. & McNulty, M.S. (1994). Studies on a new enterovirus-like virus isolated from chickens. Avian Pathology, 23, 313–327.
- Sellers, H., Linneman, E., Icard, A.H. & Mundt, E. (2010). A purified recombinant baculovirus expressed capsid protein of a new astrovirus provides partial protection to runting–stunting syndrome in chickens. Vaccine, 28, 1253–1263.
- Smyth, J.A., Connor, T.J., McNeilly, F., Moffet, D.A., Calvert, V.M. & McNulty, M.S. (2007). Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathology, 36, 119–126.
- Smyth, V.J., Jewhurst, H.L., Adair, B.M. & Todd, D. (2009). Detection of chicken astrovirus by reverse transcription polymerase chain reaction. Avian Pathology, 38, 293–301.
- Smyth, V.J., Jewhurst, H.L., Wilkinson, D.S., Adair, B.M., Gordon, A.W. & Todd, D. (2010). Development and evaluation of real-time TaqMan® RT-PCR assays for the detection of avian nephritis virus and chicken astrovirus in chickens. Avian Pathology; 39, 467–474.
- Smyth, V.J., Todd, D., Trudgett, J., Lee, A., & Welsh, M.D. (2012). Capsid protein seqeunce diversity of chicken astrovirus. Avian Pathology, 41, 151–159.
- Spackman, D., Gough, R.E., Collins, M.S. & Lanning, D. (1984). Isolation of an enterovirus-like agent from the meconium of dead-in-shell chicken embryos. The Veterinary Record, 114, 216–218.
- Todd, D., Smyth, V.J., Ball, N.W., Donnelly, B.M., Wylie, M., Knowles, N.J. & Adair, B. (2009a). Characterisation of chicken enterovirus-like viruses, duck hepatitis virus (DHV) type 2 and DHV type 3 as astroviruses. Avian Pathology, 38, 21–29.
- Todd, D., Wilkinson, D.S., Jewhurst, H.L., Wylie, M., Gordon, A.W., Adair, B.M. (2009b). A seroprevalence investigation of chicken astrovirus infections. Avian Pathology, 38, 301–309.