Abstract
Several outbreaks of gout were reported in commercial broilers in India during 2011 and 2012, causing up to 40% mortality in the birds. Gross and histopathological observations confirmed gout. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis from kidney samples of gout-affected birds indicated the presence of chicken astrovirus (CAstV) in 41.7% of cases and a mixed infection of CAstV and avian nephritis virus (ANV) in 36.4% of cases. CAstV isolated from gout-affected kidneys by inoculating embryonated specific pathogen free (SPF) eggs showed dwarfing in embryos and a cytopathic effect in chicken embryo kidney cells. Inoculation of 1-day-old SPF and broiler chicks with CAstVs caused gout and mortality between 4 and 10 days post inoculation. Virus isolation and qRT-PCR analysis showed the presence of only CAstV in inoculated chicks. Sequence analysis of capsid genes indicated a major group of Indian CAstVs that displayed 92.0 to 99.2% intergroup amino acid identity and 83.9 to 90.4% identity with subgroup Bi CAstVs of UK origin. We designated this group Indian Bi. Analysis of the partial polymerase amino acid sequences of our isolates indicated two groups of CAstVs (Indian 1 and 2) that displayed 90.2 to 95.5% amino acid identity between them. We thus report for the first time that, in addition to infectious bronchitis virus and ANV, CAstVs are a causative agent of gout.
Introduction
Gout is multifactorial in origin, caused by infectious bronchitis virus (IBV), avian nephritis virus (ANV) or because of poor management and nutritional factors. In India, IBV has previously been isolated from kidney samples of gout cases (Bayry et al., Citation2005). In 2011 and 2012, the Indian broiler chicken industry experienced severe outbreaks of gout that caused major financial losses from high mortality with concomitant high cost of treatments. During these outbreaks we observed gout in commercial broilers during the first week of life, causing up to 40% mortality. Affected chicks showed distended ureters with uric acid deposition, and visceral and articular gout. In these gout cases, management and nutritional factors were not involved; IBV was also ruled out, but ANV was thought to be associated with these outbreaks of gout in broilers.
ANV, originally regarded as a picornavirus, was characterized as the first astrovirus of chickens on the basis of its nucleotide sequence (Imada et al., Citation2000). ANVs are known to cause diarrhoea, tubulonephrosis, interstitial nephritis, uricosis (gout) and, finally, death (Shirai et al., Citation1991). To date, two different astrovirus species have been recognized in chickens: ANV and chicken astrovirus (CAstV). Avian astroviruses that were originally classified as enterovirus-like viruses (McNulty et al., Citation1990; McNeilly et al., Citation1994; Todd et al., Citation2009) have been linked to “runting–stunting syndrome” and “growth depression” (McNulty et al., Citation1984; Spackman et al., Citation1984; Decaesstecker et al., 1986; Smyth et al., Citation2007). Astroviruses have also been isolated from broilers with poor weight gain and enteric and kidney diseases (Reynolds & Schultz-Cherry, Citation2008). Astroviruses have also been reported in many mammalian species including humans, and have been linked to diarrhoea in many instances (Moser & Schultz-Cherry, Citation2005).
Astroviruses are small, non-enveloped, single-stranded, positive-sense RNA viruses belonging to the family Astroviridae (Mendez & Arias, Citation2007). The genome of astroviruses is 6.8 to 7.9 kb in length and consists of a 5′-untranslated region followed by three open reading frames (ORFs), a 3′-untranslated region and a poly-A tail. The genome encodes for three proteins, the non-structural polyprotein, the RNA-dependent RNA polymerase and the capsid protein (Monroe et al., Citation1993). The non-structural polyprotein and the capsid protein are encoded by individual ORFs, ORF1a and ORF2 respectively, while RNA-dependent RNA polymerase (ORF1b) is expressed via ribosomal shift mechanism (Bass & Qiu, Citation2000).
The isolation and characterization of CAstVs have been reported from broilers exhibiting runting syndrome and dead-in-shell chicks having hatchability problems (Spackman et al., Citation1984; Baxendale & Mebatsion, Citation2004; Smyth et al., Citation2009). These CAstVs are antigenically and serologically distinct from ANV, although their genomes share some sequence identity with ANV and other astroviruses. CAstV can be detected using reverse transcriptase polymerase chain reaction (RT-PCR) and quantitative (q)RT-PCR targeting ORF1b and ORF2 genes (Tang et al., Citation2005; Pantin-Jackwood et al., Citation2006, Citation2011; Todd et al., Citation2009; Smyth et al., Citation2010).
Smyth et al. (Citation2007) detected virus-specific antigen and histological lesions in the intestine, kidney and pancreas after experimental infection of specific pathogen free (SPF) chicks with the CAstV isolate FP3. This finding shows that, like ANV, CAstVs have the ability to infect internal organs. Smyth et al. (Citation2010) reported that CAstVs were detected in large amounts in kidneys and intestinal contents obtained in longitudinal surveys of four broiler flocks displaying below-average performance. More recently, de Wit et al. (Citation2011) characterized a new group of avian astroviruses associated with enteric disease and locomotion problems in chickens and turkeys.
This led us to the hypothesis that the kidney lesions, gout and early broiler mortality during the first week of life could be caused by CAstVs. The aim of the present study was to investigate the role of CAstV as the causative agent associated with gout in commercial broilers in India.
Materials and Methods
Case history and collection of samples
In 2011 and 2012, many flocks in the northern, western, southern and eastern zones of India experienced severe mortality during the first week after hatching. Post-mortem examination of affected chicks revealed lesions of gout. We collected 894 kidney samples and submitted them to the Poultry Diagnostic and Research Center (PDRC), Division of Venkateshwara Hatcheries Private Limited, Pune, India for diagnosis and further studies.
Sample processing and nucleic acid extraction
Inocula were prepared as 10% weight/volume suspensions in phosphate buffered saline (pH 7.2) by trituration followed by three freeze–thaw cycles, then centrifugation at 1600 × g for 30 min at 4°C and filtration through 0.20 µm filters. Supernatants were stored at −80°C until further use.
RNA was extracted using TRIzoL LS reagent (LifeTechnologies, Grand Island, NY, USA), and DNA was extracted from the filtrates using the DNeasy blood and tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Nucleic acid extracted from allantoic fluids from uninoculated embryonated SPF chicken eggs was used as a negative control. Extracted nucleic acid samples were stored at −80°C until further use.
Quantitative RT-PCR and quantitative PCR assays
The kidney samples were screened for CAstV, ANV, IBV and infectious bursal disease virus (IBDV) using qRT-PCR (TaqMan® One-Step RT-PCR Master Mix Reagents Kit; Applied Biosystems, Hammonton, NJ, USA). The samples were also screened for chicken infectious anaemia virus (CIAV) using qPCR (TaqMan® Fast Universal PCR Master Mix; Applied Biosystems). The primers and probes used in this study are listed in .
Table 1. Primers and probes used for qRT-PCR and qPCR assays.
The qRT-PCR reactions were set up in duplicate for each sample in a total volume of 12.5 µl per replicate reaction. Each reaction comprised 6.25 µl TaqMan® One-Step RT-PCR 2X Master Mix, 0.3 µl 40X MultiScribe and RNase inhibitor mix (Applied Biosystems), primers to a final concentration of 400 nM, probe to a final concentration of 120 nM, 2 µl sample RNA and nuclease-free water to 12.5 µl. The qPCR reactions were also set up in duplicate for each sample with a total volume of 20 µl per replicate reaction. Each reaction consisted of 10 µl TaqMan® Fast Universal PCR 2X Master Mix, primers to a final concentration of 400 nM, probe to a final concentration of 120 nM, 4 µl sample DNA and nuclease-free water to 20 µl.
The qRT-PCR and qPCR reactions were conducted using the StepOne™ Real-Time PCR System (Applied Biosystems). qRT-PCR started with a reverse transcription stage at 50°C for 30 min, an initial denaturation stage at 95°C for 10 min, followed by 40 cycles of denaturation at 94°C for 15 sec, primer annealing and template amplification at 60°C for 45 sec. qPCR reactions started with an initial denaturation stage at 94°C for 20 sec, followed by 40 cycles of denaturation at 94°C for 10 sec, primer annealing and template amplification at 60°C for 30 sec.
Reverse transcriptase polymerase chain reaction
RT-PCR was carried out to amplify part of ORF1b as described by Tang et al. (Citation2005) with minor modifications. For the complete amplification of ORF2, primers were designed based on GenBank accession numbers: HQ185564, JF832365 and JF414802. The list of primers used is presented in . One-step RT-PCR was performed using the Thermo Scientific Verso 1-Step RT-PCR Kit (Thermo Fisher Scientific, Somerset, New Jersey, USA) according to the manufacturer's instructions.
Table 2. Primers used for RT-PCR amplification and sequencing of astroviruses.
Sequencing and sequence analysis
Amplicons were gel purified using a PureLink™ Quick Gel extraction kit (Invitrogen, Carlsbad, California, USA). The purified PCR products were sequenced in both directions using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Nucleotide sequences were subjected to BLAST analysis (http://blast.ncbi.nlm.nih.gov/) to confirm that the sequences represented CAstV. The sequences were compiled and aligned, and phylogenetic trees of the nucleic acid and putative amino acid sequences were established using MEGA version 5 (Tamura et al., Citation2011).
Cell culture
Chicken embryo kidney cells (CEKCs) were prepared from 17-day-old SPF embryos following standard procedures (Schat & Sellers, Citation2008). Cells were resuspended in Eagle's modified essential medium containing 10% foetal bovine serum (Sigma-Aldrich, St Louis, Missouri, USA), seeded at 1.3 × 106 cells per well in six-well plates (Becton Dickinson, Franklin Lakes, New Jersey, USA) and incubated at 37°C in a humidified atmosphere of 5% CO2 in air.
Virus isolation
Inocula prepared from 18 different kidney samples that were positive only for CAstV in the qRT-PCR assays were used for virus isolation by inoculation of five 9-day-old embryonated eggs per sample by the allantoic route. Samples of allantoic fluid were collected 48 h post inoculation (p.i.) and tested for CAstV, ANV and IBV by qRT-PCR. The same eggs were incubated for an additional 72 h, chilled and the embryos examined for lesions.
Five randomly selected original kidney tissue homogenate samples were also used for virus isolation in CEKC cultures. Samples (0.2 ml) were inoculated in duplicate wells and cells were observed for 5 days for cytopathic effect. At day 5 p.i. the cells were scraped from the surface, followed by three freeze–thaw cycles and centrifugation, after which the supernatants were inoculated onto 24 h CEKC cultures. The cell suspensions were screened by qRT-PCR for the presence of CAstV, ANV and IBV. The CAstV isolate (PDRC/526/North Zone) was titrated in embryonated chicken eggs and CEKCs, and titres were calculated according to the method of Reed & Muench (Citation1938).
Experimental reproduction of gout
Three experiments were conducted. In Experiment 1, 40 1-day-old SPF chicks (Venkys India Ltd, Pune, India) were placed in an isolator and inoculated by the oculonasal route with 0.2 ml inoculum prepared from kidney samples collected from gout-affected commercial broilers from a flock in the north zone (PDRC/526/North Zone) that was positive for CAstV as confirmed by sequencing (JX945857). Twenty chicks were kept as uninoculated controls in a separate isolator. Isolators were maintained under negative pressure in an experimental animal house at PDRC, Pune.
In Experiment 2, 40 1-day-old Vencobb commercial broiler chicks were placed in an isolator and inoculated oculonasally with 0.2 ml inoculum prepared from kidney samples of Experiment 1. Ten 1-day-old uninoculated SPF chicks were placed in the same isolator as the contact control group. Ten Vencobb commercial broiler chicks and 10 SPF chicks were placed in a separate isolator as the negative control group.
In Experiment 3, 40 1-day-old SPF chicks were inoculated oculonasally with 0.2 ml allantoic fluid from CAstV-infected embryos that had been inoculated with kidney samples from experimentally infected chicks in Experiment 1. Twenty SPF chicks were kept as uninoculated controls in a separate isolator. This experiment was repeated (Experiments 4, 5 and 6) using three of the CAstV isolates obtained in this study (PDRC/574/North Zone [JX945862], PDRC/542/South Zone [JX945860], and PDRC/1804/South Zone [KC618324]). In each case the inoculum was allantoic fluid collected from eggs inoculated with the original clinical material. In all of the above experiments, mortality was checked and kidney samples were collected for virus isolation, histopathological examination and screening for CAstV, ANV and IBV by qRT-PCR. At 14 days p.i. all surviving birds were euthanized and examined for lesions.
All experimental work was performed in accordance with institutional ethical guidelines.
Histopathology
Samples of the kidney, liver and heart from field clinical cases and experimentally infected birds were placed in 10% buffered formaldehyde and processed for histopathological examination. Sections were stained with haematoxylin and eosin.
GenBank accession numbers for the reported sequences
The gene sequences for the CAstV isolates for ORF2 and ORF1b have been submitted to GenBank and the accession numbers are provided later in and .
Results
Case history, histopathology and virus detection
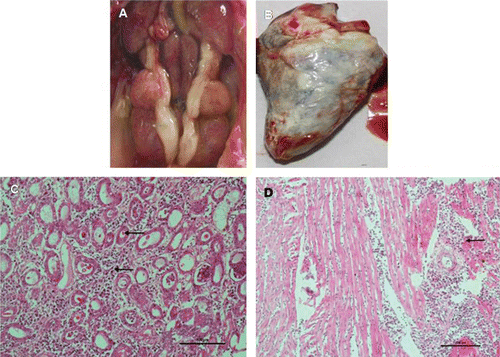
One of the commercial broiler flocks containing 100,000 chicks experienced sudden severe mortality (35%) between 6 and 9 days of age. The major post-mortem findings were swollen kidneys, prominent ureters and visceral gout (). In some chicks, articular gout and deposition of urates on the liver were observed (data not shown). Histopathological studies revealed necrosis and degeneration of the epithelial cells of the proximal convoluted tubules with infiltration of granulocytes and interstitial lymphocytes in kidneys (). Hyaline granular degeneration and infiltration of inflammatory cells in the myocardium of the heart were also observed (), as was acute cellular swelling in the liver. Thickening of the artery in the spleen and congestion in spleens and lungs were also observed (data not shown). Water deprivation, nutritional factors and other management errors were ruled out as being the causes of this outbreak of gout. The kidney samples from this particular flock were positive for CAstV and negative for IBV, ANV, chicken anaemia virus (CAV) and IBDV, as detected by qRT-PCR and qPCR and confirmed by nucleotide sequencing (data not shown) .
We then collected 894 kidney samples from gout-affected chicks from different regions of India and tested them for the presence of CAstV, ANV, IBV, IBDV and CAV. It was observed that 373 (41.7%) samples were positive for CAstV alone, while 326 (36.4%) were positive for both CAstV and ANV and 18 (2.0%) were positive for CAstV, ANV and IBV (). Overall 717 (80.2%) samples were positive for CAstV. All samples were negative for IBDV and CAV.
Table 3. Quantitative RT-PCR and qPCR detection of CAstV, ANV and IBV in kidney samples collected from commercial broiler flocks suffering from gout.
Virus isolation
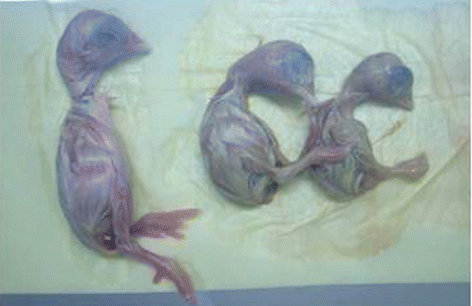
Kidney samples that were only positive for CAstV were randomly selected for virus isolation and molecular characterization. Eighteen samples were inoculated into SPF embryonated chicken eggs via the allantoic route. At 5 days p.i., all samples caused stunting of embryos with yellowish discolouration, necrosis of the livers, and pale and swollen kidneys (). A significant difference in weight was observed between infected and control embryos. The average weight for embryonated eggs that had been inoculated with the 18 CAstV isolates was 11.25 ± 1.0 g compared with 19.4 ± 0.4 g for the controls (P < 0.001, Student's t-test). Allantoic fluids collected 48 h p.i. were positive only for CAstV.
Five of these 18 samples were inoculated onto CEKCs and showed marked cytopathic effect including rounding, clumping and detachment of cells from the surface after three passages; the supernatants were positive for CAstV (data not shown). A high titre was observed in the embryonated chicken eggs (log10 5.2 median embryo infectious doses/ml) as compared with the CAstV adapted in CEKCs, which had a titre of log10 4.3 median tissue culture infectious doses/ml.
Experimental reproduction of gout in specific pathogen free and broiler chicks
To investigate the reproducibility of experimental gout in chicks we conducted three different experiments as described in Materials and Methods. In Experiment 1, we observed that some inoculated birds were dull and drowsy at day 4 p.i. and experienced up to 80% mortality by day 10 p.i. (). In Experiment 2, at day 6 p.i. some birds were found dull and drowsy, with 67.5% mortality in commercial broiler chicks and 100% mortality in contact SPF chicks at day 10 p.i. In Experiment 3, clinical signs were very similar to those observed in Experiment 1 with 90% mortality at day 10 p.i. (). Experiments 4, 5 and 6, which were replicates of Experiment 3, showed 95%, 90% and 100% mortality respectively at day 10 p.i. (). In all of the experiments, mortality in the SPF chicks started at day 5 p.i. and most of the chicks had died by day 10 p.i. In Experiment 2, mortality in the commercial broilers started at day 7 p.i. and continued up to day 10. In all of the above six experiments, the post-mortem findings consisted of swollen kidneys with urate deposition, and visceral and articular gout in both inoculated and contact chicks (). Kidney samples were positive for CAstVs and negative for ANV and IBV. These CAstVs were re-isolated by inoculating 9-day-old SPF embryos (). None of the uninoculated chicks in any experiment showed clinical signs and kidney samples were negative for CAstV, ANV and IBV.
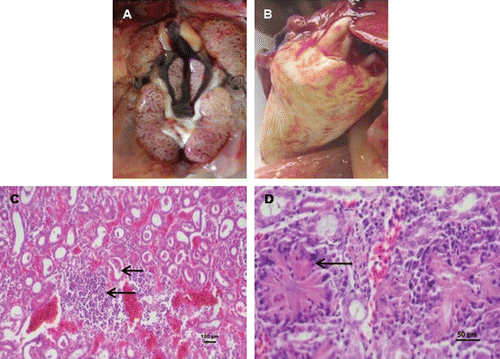
Table 4. Experimental reproduction of gout by oculonasal inoculation of 1-day-old SPF and commercial broiler chicks with CAstV.
Table 5. CAstV in vivo pathogenicity study in 1-day-old SPF chicks.
On histopathological examination, the kidneys of the inoculated chicks from all six experiments showed tubular congestion and parenchymatous interstitial nephritis with deposition of urates () and crystal formation and lymphocyte infiltration in the kidneys (). All tissues from uninoculated control birds appeared histologically normal.
Sequence analysis
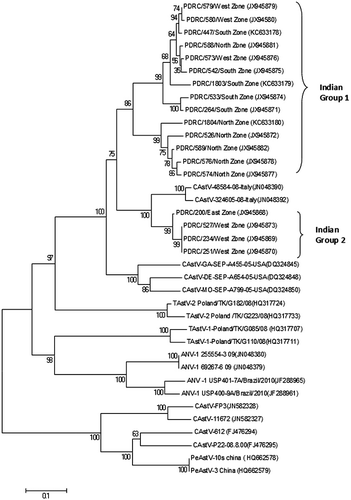
The complete capsid and partial polymerase gene sequences of CAstV were identified by sequencing of RT-PCR amplified products. Comparison of the capsid nucleotide sequences of 18 isolates showed that the capsid gene consists of 2214 nucleotides encoding a protein of 738 amino acid residues. A phylogenetic tree analysis and pairwise comparison of the capsid protein revealed a major group of isolates that we designated Indian subgroup Bi with amino acid identity of 92.0 to 99.2% (). When compared with other CAstVs, it was observed that the PDRC/526/north zone isolate of Indian subgroup Bi shared amino acid identity of 83.9 to 90.4% with group B and 39.4 to 41.2% with group A UK isolates (Smyth et al., Citation2012), and of 82.9% with GA 2011 USA isolate (JF414802). Comparison of this isolate with other avian astroviruses showed 16.1% identity with ANV-1 (HM029238), 27.8% with ANV-2 (HQ330493), 35.6% with TAstV-1 (Y15936) and 17.5% with TAstV-2 (NC005790). Pairwise comparison showed that the N-terminal region (1 to 415 residues) among the Indian subgroup Bi isolates was conserved and showed 97.6 to 99.9% amino acid identity, whereas the C-terminal region (416 to 738 residues) showed 84.2 to 97.8% identity. When we compared the capsid N-terminal region of Indian CAstV isolates with Bi and Bii UK CAstVs, 90.8 to 92.8% and 90.6 to 92.3% amino acid identity respectively was observed, whereas the C-terminal region shared 83.9 to 93.2% and 69.9 to 78.0% amino acid identity respectively.
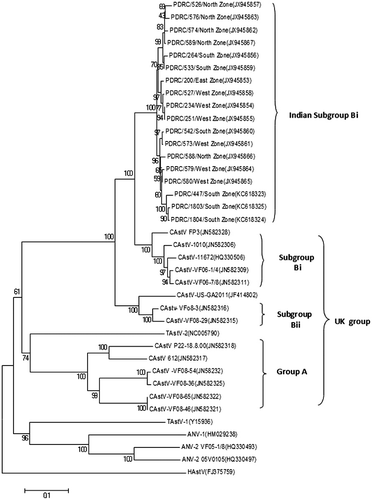
The partial polymerase gene nucleotide sequence, approximately 601 nucleotides in length, was identified by sequencing, which corresponded to genome nucleotide positions from 3574 to 4175 in the G4260 strain of ANV (AB033998) and from 3550 to 4151 in the genome of the CAstV (NC003790). A phylogenetic tree and pairwise comparison of the partial polymerase amino acid sequences from 18 CAstVs indicated two groups, which we designated Indian group 1 and Indian group 2; Indian group 1 consisting of 14 isolates, and Indian group 2 consisting of four isolates (). Indian groups 1 and 2 shared 90.2 to 95.5% amino acid identity. The amino acid identity shared within Indian group 1 was 97.0 to 98.5%, and within Indian group 2 was 99.5%. When compared with other CAstVs, it was observed that the PDRC/447/south zone isolate of Indian group 1 shared amino acid identity of 95.2% with the CAstV-48584-08 isolate from Italy (JN048390) and 95.3% with the CAstV-GA-SEP-A455-05-USA isolate (DQ324845). The PDRC/527/West zone isolate of Indian group 2 shared amino acid identity of 97.6% with the CAstV-48584-08 isolate from Italy (JN048390) and of 95.9% with the CAstV-GA-SEP-A455-05-USA isolate (DQ324845). Comparison of our isolates with the UK subgroup Bi CAstV isolates shows very low percentage identity (8 to 11%). Comparisons with other avian astroviruses indicated amino acid identities of 54.8 to 55.5% with ANV-1 (JN048380), 58.6 to 60.7% with TAstV-1 (HQ317733) and 71.6 to 73.1% with TAstV-2 (NC005790).
Discussion
In the present study, we report the isolation, identification and molecular characterization of CAstVs from kidneys of gout-affected broiler chickens in India. All flocks showed severe mortality during the first week of life due to visceral and articular gout. The histopathological examination revealed parenchymatous interstitial nephritis with deposition of urates. These observations suggest that the CAstVs isolated from these samples might be affecting kidney function, leading to gout in the chicks. The majority of the field samples (80.2%) from gout-affected broilers were positive for CAstVs, although mixed infection (ANV + CAstV) were also detected in 36.4% of the samples. In the mixed infection, the level of detection of CAstV was higher than for ANV, and ANV alone was not detected in these samples. ANV infection has been reported to cause subclinical infections in broiler flocks resulting from enteritis, and growth problems including infectious runting and stunting syndrome (McNulty et al., Citation1984; Shirai et al., Citation1992). Therefore, it seems that the CAstV is the major viral agent responsible for the gout problems. The pathogenic effects of ANV and CAstV in mixed infection could be additive or synergistic; something that needs to be investigated.
We observed that CAstV replicated better in developing chicken embryos than in cell cultures such as CEKCs. A higher virus titre was observed in the embryonated chicken eggs as compared with the CEKC adapted CAstV. Baxendale & Mebatsion (Citation2004) reported the isolation and propagation of CAstV in chicken embryo liver cells and LMH cells. Embryonated chicken eggs thus appear better than CEKC for the isolation and propagation of Indian CAstVs.
Isolation of CAstV from clinical cases and its subsequent use for experimental reproduction of gout in SPF and commercial broiler chicks satisfies Koch's postulates (Falkow, Citation1988). The CAstV isolated from experimental gout was further confirmed by nucleotide sequencing and BLASTN analysis. These results indicate that CAstV is a major aetiological agent in outbreaks of gout in India. Baxendale & Mebatsion (Citation2004) reported that CAstV may vary in virulence and that co-infection with other agents, as well as age of the birds, may result in variable clinical manifestations. Moser et al. (Citation2007) reported that mammalian astroviruses cause increased permeability of epithelial cells even in the absence of virus replication. Increased permeability of kidney epithelial cells caused by astrovirus certainly could explain the development of gout. Thus, our findings substantiate the previous reports. The pathogenesis of CAstV may also depend upon the isolate, viral load, age of chicks, interaction with other viruses and level of maternal antibodies (Todd et al., Citation2009a). Interestingly, in the experimental study carried out in commercial broilers we observed a lower incidence of mortality as compared with the SPF chicks. This could be because of the presence maternal antibodies against the CAstVs in commercial broilers, which might indicate that vaccination of broiler parents against CAstV could provide protection of chicks against the infection during the first week of life and thus prevention of gout. In our study the large amount of CAstV detected might be due to the circulation of divergent CAstVs in broiler flocks throughout India. The high level of resistance of these viruses to commonly used disinfectants might have contributed to horizontal transmission in young chicks (Guy et al., Citation2009; Todd et al., Citation2009a).
Molecular characterization of avian astroviruses has been done by nucleotide sequencing of the ORF1a and ORF1b regions (Pantin-Jackwood et al., Citation2006; Todd et al., Citation2011).The capsid precursor protein gene (ORF2) is the most variable astrovirus gene, which is responsible for the variation in antigenicity and pathogenicity (Smyth et al., Citation2009; Pantin-Jackwood et al., Citation2011;Smyth et al., Citation2012). We analysed the complete capsid protein sequence of 18 CAstV isolates, which consisted of 738 amino acids, and found a major group that we designated “Indian Bi” based on their close relationship to CAstVs from the Bi subgroup identified by Smyth et al. (Citation2012). Smyth et al. (Citation2012) reported the existence of two capsid groups with different length of the amino acids, 718 to 721, and 738 and 743 respectively. Our CAstV isolates are similar to group B of the UK isolates (Smyth et al., Citation2012) since the length of amino acids was 738. Indian CAstV isolates were closely related to isolates of subgroup Bi of the UK isolates and to the GA 2011 USA isolate.
Evidence from human astrovirus studies suggest that the C-terminal region contains neutralizing epitopes, thereby facilitating its interaction with the host cell receptors and the host's immune response (Bass & Upadhyapuda, Citation1997; Krishna, Citation2005). Detailed examination of the Indian CAstV isolate's capsid protein N-terminal capsid region (residues 1 to 415) revealed that this region was conserved, with 97.6 to 99.3% amino acid identity, while the C-terminal region (416 to the end) was found to be variable, sharing 84.2 to 97.8% identity.
In our study, the phylogenetic tree based on polymerase gene sequences suggests the presence of two groups of CAstVs in India, designated Indian groups 1 and 2. Isolates belonging to Indian group 1 were mostly from the north, south and west zones, while Indian group 2 isolates were mostly from the east and west zones. These two groups are clearly distant from the CAstV UK group, pigeon CAstV from China, and other astroviruses (ANV, TAstV-1 and TAstV-2). But on the basis of capsid gene sequence our Indian CAstVs are closely related to the subgroup Bi of the UK isolates. Although the ORF1b gene is highly conserved when compared with the ORF2 gene, a number of genetic variations in the ORF1b gene have been described by Pantin-Jackwood et al. (Citation2006) and in TAstV-2 by Cattoli et al. (Citation2007). However, to truly confirm the existence of these two Indian CAstV groups, the complete polymerase gene sequence will be helpful.
Recently, Smyth et al. (Citation2012) compared capsid-based groups of CAstVs with the polymerase-based groups of CAstV and found that they do not correspond to each other. Similarly, our isolates could be differentiated into a major group (Bi) based on the capsid sequences, and two different groups (1 and 2) based on the partial polymerase sequence. Also, the classification based on the capsid sequences does not correspond with the classification based on the polymerase sequences. Determination of the complete genome therefore needs to be considered for genotyping of CAstVs.
The in vivo pathogenicity of different CAstV isolates from the Indian subgroup Bi was examined to ascertain their virulence. Their ability to reproduce gout in SPF chicks proves that they are pathogenic. To our knowledge this is the first study reporting the isolation of CAstVs from kidneys of gout-affected chicks, together with their molecular characterization and use to reproduce gout experimentally. Based on these investigations, CAstV infection has been implicated as a major aetiological agent in gout-affected commercial broilers in India. CAstV should therefore be included in the differential diagnosis of gout or nephritis problems in addition to ANV and IBV. Determination of the complete genome sequence of CAstV will be helpful for further molecular genotyping. Capsid protein sequence diversity and antigenic variation exhibited by CAstVs has to be considered for the development of diagnostic reagents and for vaccine development in the future.
Acknowledgements
The authors thank Venkateshwara Hatcheries Pvt. Ltd, Pune for funding this project and for permission to publish the results. They also thank Dr Mohan R. Wani of the National Centre of Cell Science, Pune, India for kind help in preparing the manuscript.
References
- Bass, D.M. & Qiu, S. (2000). Proteolytic processing of the astrovirus capsid. Journal of Virology, 74, 1810–1814. 10.1128/JVI.74.4.1810-1814.2000
- Bass, D.M. & Upadhyapuda, U. (1997). Characterization of human serotype 1 astrovirus-neutralizing epitopes. Journal of Virology, 71, 8666–8671.
- Baxendale, W. & Mebatsion, T. (2004). The isolation and characterization of astroviruses from chickens. Avian Pathology, 33, 364–370. 10.1080/0307945042000220426
- Bayry, J., Goudar, M., Nighot, P.K., Kshirsagar, S.G., Ladman, B.S., Gelb, J., Ghalsasi, G.R. & Kolte, G.N. (2005). Emergence of a nephropathogenic avian infectious bronchitis virus with a novel genotype in India. Journal of Clinical Microbiology, 43, 916–918. 10.1128/JCM.43.2.916-918.2005
- Callison, S.A., Hilt, D.A., Boynton, T.O., Sample, B.F., Robison, R., Swayne, D.E. & Jackwood, M.W. (2006). Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods, 138, 60–65. 10.1016/j.jviromet.2006.07.018
- Cattoli, G., DeBattisti, C., Toffan, A., Salviato, A., Lavazza, A., Cerioli, M. & Capua, I. (2007). Co-circulation of distinct genetic lineages of astroviruses in turkeys and guinea fowl. Archives of Virology, 152, 595–602. 10.1007/s00705-006-0862-4
- Decaesstecker, M., Charlier, G. & Meulemans, G. (1986). Significance of parvoviruses, enterolike viruses and reoviruses in the aetiology of the chicken malabsorption syndrome. Avian Pathology, 15, 769–782. 10.1080/03079458608436339
- De Wit, J.J., Dam, G.B., de Laar, J.M., Biermann, Y., Verstegen, I., Edens, F. & Schrier, C.C. (2011). Detection and characterization of a new astrovirus in chicken and turkeys with enteric and locomotion disorders. Avian Pathology, 40, 453–461. 10.1080/03079457.2011.596813
- Falkow, S. (1988). Molecular Koch’s postulates applied to microbial pathogenicity. Clinical Infectious Diseases, 10, s274–s276. 10.1093/cid/10.Supplement_2.S274
- Guy, J.S., McNulty, M.S. & Hayhow, C.S. (2009). Avian enterovirus-like viruses. In Y.M. Saif, A.M. Fadly, L.K. Glisson, L.R. McDougald, L.K. Nolan & D.E. Swayne. (Eds.). Diseases of Poultry 12th edn (pp. 326–331). Oxford: Blackwell Publishing.
- Imada, T., Yamaguchi, S., Mase, M., Tsukamoto, K., Kubo, M. & Morooka, A. (2000). Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. Journal of Virology, 74, 8487–8493. 10.1128/JVI.74.18.8487-8493.2000
- Krishna, N. K. (2005). Identification of structural domains involved in astrovirus capsid biology. Viral Immunology, 18, 17–26. 10.1089/vim.2005.18.17
- Markowski-Grimsrud, C.J., Miller, M.M. & Schat, K.A. (2002). Development of strain-specific real-time PCR and RT-PCR assays for quantitation of chicken anemia virus. Journal of Virological Methods, 101, 135–147. 10.1016/S0166-0934(01)00430-X
- McNeilly, F., Connor, T.J., Calvert, V.M., Smyth, J.A., Curran, W.L., Morley, A.J., Thompson, D., Singh, S., McFerran, J.B., Adair, B.M. & McNulty, M.S. (1994). Studies on a new enterovirus-like virus isolated from chickens. Avian Pathology, 23, 313–327. 10.1080/03079459408418999
- McNulty, M.S., Allan, G.M., Connor, T.J., McFerran, J.B. & McCracken, R.M. (1984) An entero-like virus associated with the runting syndrome in broiler chickens. Avian Pathology, 13, 429–439. 10.1080/03079458408418545
- McNulty, M.S., Connor, T.J., McNeilly, F. & McFerran, J.B. (1990). Biological characterisation of avian enteroviruses and enterovirus-like viruses. Avian Pathology, 19, 75–87. 10.1080/03079459008418658
- Mendez, E. & Arias, C.F. (2007). Astroviruses. In D.M. Knipe, P.M. Howley, D.E. Griffin, R.A. Lamb, S.E. Straus, M.A. Martin, & B. Roizman (Eds), Fields Virology Vol.1, 5th edn (pp. 981–1000). Philadelphia: Lippincott-Williams and Wilkins.
- Monroe, S.S., Jiang, B., Stine, S.E., Koopmans, M. & Glass, R.I. (1993). Subgenomic RNA sequence of human astrovirus supports classification of Astroviridae as a new family of RNA viruses. Journal of Virology, 67, 3611–3614.
- Moser, L.A., Carter, M. & Schultz-Cherry, S. (2007). Astrovirus increases epithelial barrier permeability independently of viral replication. Journal of Virology, 81, 11937–11945. 10.1128/JVI.00942-07
- Moser, L.A. & Schultz-Cherry, S. (2005). Pathogenesis of astrovirus infection. Viral Immunology, 18, 4–10. 10.1089/vim.2005.18.4
- Pantin-Jackwood, M.J., Spackman, E. & Woolcock, P.R. (2006). Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: implications for diagnostics. Avian Diseases, 50, 397–404. 10.1637/7512-020606R.1
- Pantin-Jackwood, M.J., Strother, K.O., Mundt, E., Zsak, L., Day J.M. & Spackman, E. (2011). Molecular characterization of avian astroviruses. Archives of Virology, 156, 235–244. 10.1007/s00705-010-0849-z
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty percent endpoints. The American Journal of Hygiene, 27, 493–497.
- Reynolds, D.L. & Schultz-Cherry, S. (2008). Astrovirus infections. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.). Diseases of Poultry 12th edn (pp. 351–355). Ames, IA: Blackwell Publishing.
- Schat, K.A. & Sellers, H.S. (2008). Cell-Culture Methods. In L. Dufour-Zavala, D.E. Swayne, J.R. Glisson, J.E. Pearson, W.M. Reed, M.J. Jackwood & P.R. Woolcock (Eds). A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens 5th edn (pp 195–203). Athens, GA: American Association of Avian Pathologists.
- Shirai, J., Nakamura, K., Nozaki, H. & Kawamura, H. (1991). Differences in the induction of urate deposition of specific pathogen-free chicks inoculated with avian nephritis virus passaged by five different methods. Avian Pathology, 35, 269–275.
- Shirai, J., Tanimura, N., Uramoto, K., Narita, M., Nakamura, K. & Kawamura, H. (1992). Pathologically and serologically different avian nephritis virus isolates implicated in etiology of baby chick nephropathy. Avian Diseases, 36, 369–377. 10.2307/1591515
- Smyth, J.A., Connor, T.J., McNeilly, F., Moffet, D.A., Calvert, V.M. & McNulty, M.S. (2007). Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathology, 36, 119–126. 10.1080/03079450601161398
- Smyth, V.J., Jewhurst, H.L., Adair, B.M. & Todd, D. (2009). Detection of chicken astrovirus by reverse transcriptase-polymerase chain reaction. Avian Pathology, 38, 293–299. 10.1080/03079450903055397
- Smyth, V.J., Jewhurst, H.L., Wilkinson, D.S., Adair, B.M., Gordon, A.W. & Todd, D. (2010). Development and evaluation of real-time TaqMan® RT-PCR assays for the detection of avian nephritis virus and chicken astrovirus in chickens. Avian Pathology, 39, 467–474. 10.1080/03079457.2010.516387
- Smyth, V.J., Todd, D., Trudgett, J., Lee, A. & Welsh, M.D. (2012). Capsid protein sequence diversity of chicken astrovirus. Avian Pathology, 41, 151–159. 10.1080/03079457.2011.652938
- Spackman, D., Gough, R.E., Collins, M.S. & Lanning, D. (1984). Isolation of an enterovirus-like agent in the meconium of dead-in-shell chicken embryos. The Veterinary Record, 114, 216–218. 10.1136/vr.114.9.216-a
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739. 10.1093/molbev/msr121
- Tang, Y., Ismail, M.M. & Saif, Y.M. (2005). Development of antigen captures enzyme-linked immunosorbent assay and RT-PCR for detection of turkey astroviruses. Avian Diseases, 49, 182–188. 10.1637/7255-080504R
- Todd, D., Smyth V.J., Ball, N.W., Donnelly, B.M., Wylie, M., Knowles, N.J. & Adair, B.M. (2009). Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathology, 38, 21–30. 10.1080/03079450802632056
- Todd, D., Trudgett, J.S., Smyth, V.J., Donnelly, B., McBride, N. & Welsh, M.D. (2011). Capsid protein sequence diversity of avian nephritis virus. Avian Pathology, 40, 249–259. 10.1080/03079457.2011.553583
- Todd, D., Wilkinson, D.S., Jewhurst, H.L., Wylie, M., Gordon, A.W. & Adair, B.M. (2009a). A seroprevalence investigation of chicken astrovirus infections. Avian Pathology, 38, 301–309. 10.1080/03079450903055421
- Wang, Y., Qi, X., Gao, H., Gao, Y., Lin, H., Song, X., Pei, L. & Wang, X. (2009). Comparative study of the replication of infectious bursal disease virus in DF-1 cell line and chicken embryo fibroblasts evaluated by a new real-time RT-PCR. Journal of Virological Methods, 157, 205–210. 10.1016/j.jviromet.2009.01.001