Abstract
An enzyme-linked immunosorbent assay (ELISA) was developed to estimate levels of IgY antibody against the bacterium Erysipelothrix rhusiopathiae in serum samples collected from the critically endangered kakapo (Strigops habroptilus, Psittaciformes, Aves) before and after vaccination against this bacterium. Relative IgY antibody titres in pre-vaccination serum samples (n = 71 individual kakapo) were normally distributed with the exception of four outliers which displayed low IgY levels. Notably all four low IgY samples were collected from fledglings 3 – 6 months old. Pre-vaccination serum samples from nine nestlings <3 months old, seven of which were hatched in incubators and had no contact with either adult kakapo or their natural environment (e.g. soil), were found to have relatively high IgY levels, suggesting transfer of maternal IgY molecules to fledglings via the yolk. IgY levels in pre-vaccination serum samples from seven kakapo aged 25 – 30 months were also relatively high, suggesting that most kakapo naturally acquire anti- E.rhusiopathiae IgYs within their first 2 years. There was no evidence that vaccination increased the kakapo population's mean anti-E.rhusiopathiae IgY levels. However, there was a significant negative relationship between an individual bird's pre-vaccination IgY level and any subsequent increase following vaccination, suggesting that vaccination may only raise the IgY levels of birds with relatively low pre-vaccination IgY levels. A statistical model of the relationship between ‘death from erysipelas’ and sex, age and transfer from one to island sanctuary to another found that only transfer was significantly associated with death from erysipelas.
Introduction
The bacterium Erysipelothrix rhusiopathiae causes a disease known as erysipelas in non-human vertebrates (Bricker & Saif, Citation1997). Although a well-documented, and frequently fatal, disease of domesticated, intensively-housed birds there are few well-documented cases of erysipelas in wild bird populations (Rosenwald & Dickinson, Citation1941; Bisgaard & Olsen, Citation1975; Swan & Lindsey, Citation1998). Indeed, to the best of our knowledge, the only well-documented case of mass mortality from erysipelas in a wild bird population occurred in 1975 when an estimated 5000 migrating eared (black-necked) grebes (Podiceps nigricolis) died of erysipelas following a heavy snowfall at Great Salt Lake in Northern Utah (Jensen & Cotter, Citation1976).
The kakapo (Strigops habroptilus) is a critically endangered, flightless parrot endemic to New Zealand (Powlesland et al., Citation2006). Prior to human colonisation kakapo were both widespread and abundant on the three main islands of the New Zealand archipelago. By 1995 a combination of forest clearance and predation by introduced mammalian predators had reduced the kakapo population to just 51 known birds (Powlesland et al., Citation2006). However, over the past two decades intensive management of kakapo transferred to predator-free islands has increased the population to 124 (R.J. Moorhouse, unpublished observations).
In July (austral mid-winter) 2004, three juvenile (∼2 years old) female kakapo died within days of being transferred to a new island. Necropsy and bacterial culture results confirmed that erysipelas was the cause of death of all three birds (Gartrell et al., Citation2005). These birds were among a group of 19 kakapo, 23% of the then world population of 83 birds, that had been transferred from one island sanctuary to another in July 2004.
These deaths precipitated the vaccination of almost the entire kakapo species with a commercial, adjuvanted vaccine containing heat-killed Erysipelothrix rhusiopathiae (Suvaxyn®E, Fort Dodge, Iowa, U.S.A). However, at the time this decision was made neither the natural prevalence of anti-E.rhusiopathiae antibodies within the kakapo population, nor the degree to which vaccination increased such antibody levels in individual kakapo, were known. Furthermore, although vaccination with dead E. rhusiopathiae vaccines has generally proven to be safe in a range of bird species, such vaccination is not entirely without risk (Blyde & Woods, Citation1999; Bricker & Saif, Citation1997; Swan & Lindsey, Citation1998). Vaccination against erysipelas may cause some individuals to experience anaphylactic shock (Bricker & Saif, Citation1997) and may predispose individuals to develop the chronic form of the disease (Gerlach, Citation1994), which can cause arthritis, reduced male fertility and endocarditis (Bickford et al., Citation1978; Bricker & Saif, Citation1997). The absence of adverse effects from E. rhusiopathiae vaccines in relatively short-lived, domesticated avian species such as chickens (Gallus domesticus) and turkeys (Meleagris gallopavo) does not guarantee that there will be no adverse effects in the much longer lived kakapo.
In December 2010 a vaccinated, adult male kakapo ‘Richard Henry’ who was of unknown, but probably great, age was also determined to have died of erysipelas (R. Jakob-Hoff, personal communication). In light of this, and the concerns described above, it was important to establish if the ongoing vaccination of this critically endangered species against E. rhusiopathiae is both necessary and effective. Here we report the development and application of an enzyme-linked immunosorbent assay (ELISA) test to investigate: (i) pre-vaccination levels of anti-E. rhusiopathiae IgYs in the kakapo population and (ii) changes in anti-E. rhusiopathiae IgY levels of individual kakapo following vaccination with the commercial E. rhusiopathiae vaccine.
Materials and Methods
Serum collection
Kakapo blood samples were collected from the medial tarsal vein using a 25 gauge sterile syringe, coagulated at ambient temperature and then centrifuged to separate the serum fraction which was frozen within hours of collection; initially at −14°C (for up to 2 weeks) then at −70°C for long-term storage.
Pre-vaccination serum sampling
Pre-vaccination serum samples (n = 71) were collected between 2002 and 2008. These samples were collected from 51 adults (> 5 years of age), seven sub-adults (between 1 and 5 years of age), four fledglings (between 3 and 6 months of age) and nine nestlings (< 3 months of age). Seven of the nine nestlings had been hatched in an incubator and subsequently hand-reared and therefore had had no contact with adult kakapo, or the natural nest environment, before their serum samples were collected.
Thirty-four of the 71 pre-vaccination serum samples were collected 1 to 3 years before the three deaths from erysipelas in 2004 and therefore may not be indicative of the antibody status of individual birds at the time of this outbreak. The remaining 37 samples were collected after the 2004 outbreak; 20 within one month, eight within three months and nine from nestlings which hatched one to three years after the outbreak.
Vaccination regime and post-vaccination serum sampling
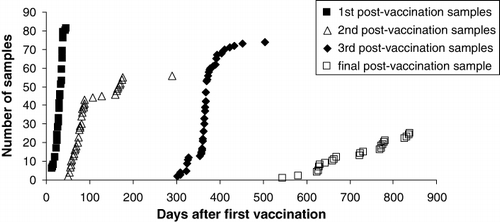
Seventy-four of the 83 kakapo alive in 2004 were vaccinated against E. rhusiopathiae between 16 July and 19 September 2004. Post-vaccination serum samples were collected during four periods; (i) 14–46 days, (ii) 50–289 days, (iii) 297–503 days and (iv) 545–839 days, after birds had received their first vaccination. Booster vaccinations were administered on the same day that post-vaccination samples were collected during periods (i) and (ii) ().
Development of an anti-E.rhusiopathiae IgY ELISA assay
Well-characterised, enzyme-conjugated, anti-parrot IgY, secondary antibodies were not available when we began this project. Consequently we developed a three-tier ELISA to detect kakapo anti-E.rhusiopathiae antibodies. The antigen coated on the plates was a suspension of the commercial vaccine used to vaccinate kakapo (Suvaxyn®E, Fort Dodge, IA, USA). The first antiserum tier was diluted kakapo serum which was then followed by rabbit anti-cockatiel IgY antisera known to cross-react with IgYs from a wide range of psittacine species (Baghian et al., Citation1999). Finally a third, horse radish peroxidase (HRP) conjugated anti-rabbit IgG was added to allow enzymatic detection. A blank, in which kakapo serum was replaced by 1% (w/v) BSA /1xPBS was included to evaluate substrate reactions within the assay, and ensure a low background OD490 (<0.1) was present. Repeatability of the assay could not be measured because of the very limited amounts of antisera available and a lack of bona fide positive and negative controls. It is noteworthy that since we developed the ELISA described herein at least two commercially available enzyme conjugated secondary antibodies that bind to parrot IgYs have been reported (de Kloet & Dorrestein, Citation2009; Cray, Citation2011). Consequently, any future ELISAs developed for the detection of parrot IgYs can have a two tier structure rather than the more complex three tier assay we used in this work.
Aliquots (1.0 ml) of the commercial vaccine used to vaccinate kakapo (Suvaxyn®) were centrifuged (13000 × g, 3 min, room temperature) and the pellet re-suspended in 10.0 ml of 0.1M NaHCO3 buffer (pH 9.5). Ninety-six well ELISA plates (Nunc-Immuno™ ELISA microtiter plates, Nunc, Roskilde, Denmark) were coated with the vaccine suspension (50.0 µl / well) and incubated (37°C, 4–5 hr). Non-specific binding sites were blocked by the addition to each well of 200.0 µl of 1.0% (w/v) bovine serum albumin (BSA) dissolved in non-sterile phosphate-buffered saline (PBS) (10 mM phosphate buffer, 2.7 mM potassium chloride, 0.137 M sodium chloride) (pH 7.2) for 2 hours at 37°C. Kakapo serum samples were diluted 1/500 in 1.0% (w/v) BSA / 1 x PBS and added to antigen-coated wells in triplicate. A 1.0% (w/v) BSA / 1x PBS blank was included in triplicate on each plate to control for background substrate reactions. Plates were then incubated overnight at 4°C.
Plate wells were washed five times with 0.5% (v/v) Tween 20 (Serva, Heidelberg, Germany) / 1 x PBS then incubated for one hour at 37°C with 100.0 µl of rabbit anti-cockatiel IgY antisera (Baghian et al. Citation1999) diluted 1/5000 in 1% (w/v) BSA / 1 x PBS. Plate wells were again washed five times before incubation with 100.0 µl of horse radish peroxidase (HRP) conjugated goat anti-rabbit IgG (Sigma-Aldridge, St Louis, MO, USA) diluted 1/1000 in 1% (w/v) BSA / 1 x PBS (incubation 45 min / 37°C).
Wells were then washed five times before addition of 100.0 µl of substrate mix (0.4 mg/ml o-phenylenediamine (Sigma), 0.1 M citric acid, 0.5 M Na2HPO4, 0.05% hydrogen peroxide), and incubated in the dark for 16 min at room temperature. Assay product development was stopped with 100.0 µl of 1 N H2SO4. Immediately after this addition, the resulting serum optical densities (OD) were determined at 490 nm (Microplate Reader, Model 550; Bio-Rad Laboratories Inc, Hercules, CA, USA) and recorded using Microplate Manager® software (Version 5.2.1; Bio-Rad).
All serum samples from a single bird were assayed on the same plate in order to optimise the comparability of samples taken from single individuals at different time points. In the absence of a bona fide negative control (i.e. kakapo serum known to be devoid of IgY molecules directed against E. rhusiopathiae) and positive control (i.e. kakapo serum containing a known titre of anti-E. rhusiopathiae IgY molecules) absolute specific antibody concentrations could not be determined. Therefore normalised OD490 values were used as a relative quantitative measure of anti-E. rhusiopathiae IgY levels. An internal control was included with each assay to adjust for changes in OD490 due to inter-assay variation. This internal control was a pre-vaccination serum sample, from a bird named ‘Aroha’, that consistently returned the lowest OD490 value. Thus, serum from Aroha was included, in triplicate, on each plate as a reference sample for normalising the OD490 values. The lowest average OD490 for the Aroha sample from all plates assayed was 0.365. The OD490 values of other sera assayed were normalised to this value by subtracting the difference in OD490 of the Aroha average (three wells) between the two plates from the OD490 of each individual well on that plate. Data were recorded as the average OD490 of triplicate wells for each sample after such normalisation to the lowest OD490 reading for the reference sample. The resulting corrected OD490 data used in the analyses were denoted cOD490. In summary cOD490 sample-x = mean OD490 sample-x - (mean OD490 control sample - 0.365).
Statistical analyses
All statistical analyses were conducted using R version 2.15.3 (R Core Team Citation2013). cOD490 values were visually checked for normality and the relationship between cOD490 and chick age assessed using linear regression. The significance of any change in mean population cOD490 values following vaccination was assessed using ANOVA and the relationship between post-vaccination change in the cOD490 values of individual birds and their pre-vaccination cOD490 values was assessed using linear regression. Logistic regression was used to assess the significance of any association between death from erysipelas and the following three factors: sex, age and being among the group of birds transferred to Chalky Island in 2004.
Results and Discussion
Anti-E. rhusiopathiae IgY levels of unvaccinated kakapo as indicated by serum cOD490values
With the exception of four clear low value outliers, cOD490 values were approximately normally distributed with a median cOD490 value of 0.872 (). In the absence of a bona fide negative control (i.e. kakapo serum definitely lacking anti-E.rhusiopathiae antibodies) it is unclear what cOD490 value would correspond to the total absence of anti-E.rhusiopathiae IgY antibodies in a serum sample. It is noteworthy that two of the four outlying cOD490 values were from two of the three young kakapo that died of erysipelas in 2004 (i.e. Aurora and Aroha, ). An archived, pre-transfer blood sample had not been collected from the third deceased kakapo so no pre-vaccination cOD490 value is available for it. However, it should be remembered that because the Aurora and Aroha serum samples were collected >2 years before the erysipelas outbreak they may not be indicative of cOD490 values at the time these individuals became sick and died.
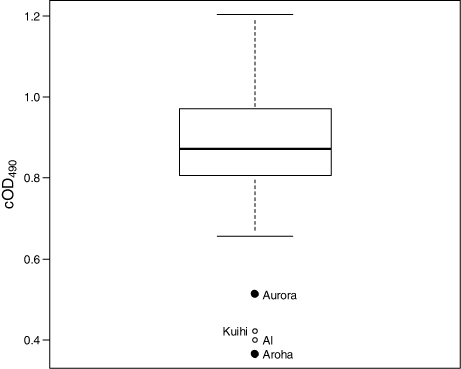
Indeed, comparison of pre-vaccination cOD490 values of sera from young kakapo 0 – 30 months post-hatching ( a, b) suggests that kakapo 5 – 15 months old have relatively low anti-E.rhusiopathiae IgY titres compared either to those <3 months old (a) or >25 months old (b). Therefore, the low pre-vaccination cOD490 values of the four ‘outliers’ in could simply reflect the age of these birds when these samples were collected rather than being indicative of greater susceptibility to erysipelas at the time of the 2004 outbreak.
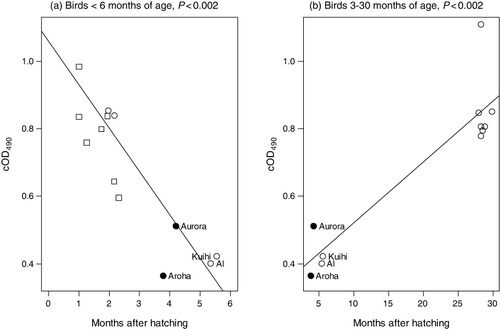
The cOD490 values of serum samples from nine kakapo <3 months old were higher than those of birds between 3 and 6 months of age (a). This is consistent with transfer of maternal antibodies to chicks, via the yolk, as has been documented in many bird species (Boulinier & Staszewski Citation2008; Hasselquist & Nilsson Citation2009; Garnier et al. Citation2012). Indeed, as seven of the serum samples assayed were from birds hatched in an incubator, and subsequently hand-reared by humans, there is no other apparent explanation as these birds were very unlikely to have been exposed to E. rhusiopathiae. The cOD490 values of kakapo >25 months of age were also higher than those of birds between 3 and 6 months of age, which suggests that most juvenile kakapo have increased anti-E. rhusiopathiae IgY antibodies by the time they reach 25 months of age (b).
What factors are associated with death of kakapo from erysipelas?
Because pre-vaccination serum samples from birds that died of erysipelas were collected 2 – 4 years before their death, the cOD490 values of these samples may not be indicative of those at the time of death. For this reason pre-vaccination serum cOD490 values were not included as a potential explanatory factor in a statistical model of factors contributing to mortality from erysipelas. Of the three factors that were included; sex, age and being transferred to Chalky island in 2004, only the latter was significantly associated with increased risk of death from erysipelas (Logistic regression; P = 0.007).
Outbreaks of erysipelas in both wild and captive birds typically occur after periods of cold, wet weather (Jensen & Cotter, Citation1976; Bricker & Saif, Citation1997; Swan & Lindsey, Citation1998; Blyde & Woods, Citation1999), and the 2004 transfer of kakapo to Chalky Island took place in just such conditions. With the exception of Richard Henry, who died 6 years after the 2004 outbreak, there were no subsequent deaths from erysipelas among kakapo that were not transferred to Chalky Island in 2004, even though these birds were neither treated, nor vaccinated, until at least 3 weeks after the three deaths on Chalky Island.
In contrast to the 2004 episode, Richard Henry's death was an isolated event. Furthermore, unlike the birds that died in 2004 Richard Henry had been vaccinated against E. rhusiopathiae. Richard Henry was the sole survivor of a relict, all male population from which breeding was last recorded in the late 19th century and was showing signs of very advanced age (e.g. cataracts, wrinkled skin) when he died. In addition he was the only kakapo known to have a heavy infestation of the gut parasite Tetratrichomonas spp., which suggests that his immune system was not functioning adequately in the year before his death. Collectively, these factors suggest that Richard Henry's death was an isolated case of a senescent bird succumbing to an opportunistic infection by E. rhusiopathiae.
Changes in kakapo serum cOD490 values following vaccination
We investigated the effect of vaccination on the mean serum cOD490 values of the kakapo population. Note that because some pre-vaccination samples were collected years before birds were vaccinated they may not be indicative of birds’ anti-E.rhusiopathiae IgY levels at the time of vaccination. Because of this, and to achieve a balanced ANOVA design, we compared mean pre- and post-vaccination cOD490 values of a subsample of 11 kakapo from which pre-vaccination samples were collected on the same day as their first vaccination, and from which all four post-vaccination serum samples had been collected (). None of the four mean post-vaccination cOD490 levels differ significantly (P < 0.05) from the mean pre-vaccination level.
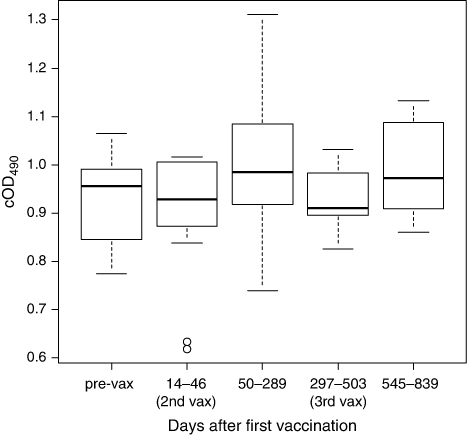
We also investigated the effect of vaccination on serum cOD490 levels of individual birds. To reduce the possibility that serum cOD490 values could have changed for reasons other than vaccination, we restricted this analysis to a subsample of 31 kakapo from which pre-vaccination serum samples were collected on the same day as their first vaccination and for which at least one post-vaccination sample was available for comparison. A significant, negative, linear relationship between pre-vaccination cOD490 values and change in serum cOD490 after vaccination is apparent 297 to 839 days after the first vaccination (c, d). Birds with relatively high pre-vaccination serum cOD490 levels displayed little or no change in serum cOD490 following vaccination. This suggests that vaccination may only have increased the IgY levels of kakapo that had relatively low pre-vaccination anti-E.rhusiopathiae IgY levels compared to the population median.
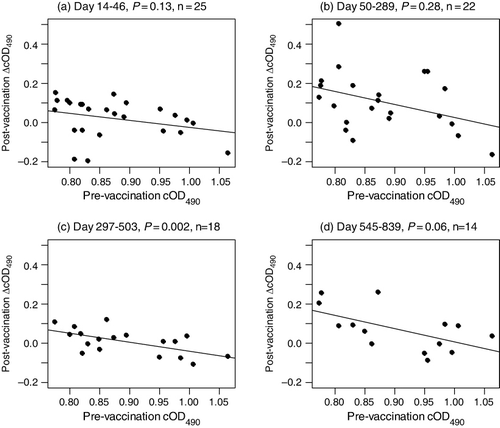
Because it is not appropriate to perform a challenge experiment on a critically endangered species like the kakapo we cannot determine what serum cOD490 value is indicative of an anti-E.rhusiopathiae IgY level sufficient to provide immunity against erysipelas. However, the fact that just four kakapo have died of erysipelas in the 25 years during which we have been able to reliably monitor kakapo survival suggests that most birds have acquired natural immunity to this disease or that kakapo generally have low susceptibility to it.
Tests of mottled petrel (Pterodroma inexpectata) and sooty shearwater (Puffinus griseus) carcasses collected on Codfish Island indicated that 10 of 15 contained live E. rhusiopathiae bacillus (Gartrell et al. Citation2005). Adults and chicks of these species were common on the island immediately prior to the 2004 transfer of kakapo to Chalky Island. Furthermore, all kakapo have, at some stage in their lives, lived in close proximity to seabirds and consequently are likely to have been exposed to E. rhusiopathiae. An exact mechanism of potential transmission from seabird to kakapo is unknown but it is noteworthy that ectoparasites are potential vectors of E. rhusiopathiae (Bricker & Saif, Citation1997; Chirico et al., Citation2003).
The fact that the four kakapo known to have died of erysipelas were either juveniles or, probably, very old, suggests that these age groups may be more vulnerable than others to erysipelas. Greater susceptibility among juveniles has been reported in ducks (Graham et al., Citation1939) and emus (Swan & Lindsey, Citation1998). Although we are not aware of any reports of increased susceptibility with age, kakapo are much longer lived than the intensively farmed species on which current perceptions are based. The kakapo population's surviving founders, which were captured as adults on Stewart Island in the early 1980s, must now be over 30 years old. Statistical estimates of kakapo longevity based on the survival of radio-tagged birds estimate an average longevity of 62 years with 95% confidence limits of 44 and 96 years.
In conclusion our results suggest that kakapo nestlings obtain anti-E.rhusiopathiae IgY levels comparable to those of adult birds via maternal transfer, and that these appear to decline between 3 and 6 months of age, then increase after 25 months of age. A high incidence of natural immunity, or low susceptibility, is consistent with both the ubiquity of E. rhusiopathiae in seabird colonies on Codfish Island and the low incidence of kakapo mortality from erysipelas. Vaccination does not appear to have increased the average serum cOD490 levels of the kakapo sub-population examined but this could be because the sample size was too small to detect an effect. The fact that individual kakapo with pre-vaccination serum cOD490 levels higher than the population median tended to display little or no increase in serum cOD490 following vaccination suggests that vaccination may only increase the cOD490 levels of birds with lower pre-vaccination cOD490 levels. Further work is required to determine the duration of protection, if any, achieved by the vaccination of this critically endangered species.
Acknowledgements
We thank Kakapo Programme Rangers for their help with field work and kakapo blood sample collection. We are very grateful to the late Prof. Abolghasem Baghian (Louisiana State University School of Veterinary Medicine, USA) for supplying the rabbit anti-cockatiel immunoglobulin antisera and to two anonymous reviewers for their suggestions and comments on an earlier draft of this paper.
References
- Baghian, A., Reyes, C.V., Mendoza, A., Tully, T.N. & Kousoulas, K.G. (1999). Production of a rabbit anti-cockatiel immunoglobulin G and characterization of its cross reactivities with immunoglobulin G of other psittacine species. Avian Diseases, 43, 48–54. 10.2307/1592761
- Bickford, A., Corstvet, R. & Rosenwald, A. (1978). Pathology of experimental erysipelas in turkeys. Avian Diseases, 22, 503–518. 10.2307/1589306
- Bisgaard, M. & Olsen, P. (1975). Erysipelas in egg-laying chickens: clinical, pathological and bacteriological investigations. Avian Pathology, 4, 59–71.
- Blyde, D.J. & Woods, R. (1999). Erysipelas in malleefowl. Australian Veterinary Journal, 77, 434–435. 10.1111/j.1751-0813.1999.tb12084.x
- Boulinier, T. & Staszewski, V. (2008) Maternal transfer of antibodies: raising immuno-ecology issues. Trends in Ecology and Evolution, 23, 282–288. 10.1016/j.tree.2007.12.006
- Bricker, J. & Saif, Y. (1997). Erysipelas. In B. Calnek, H.J. Barnes, C.W. Beard, L.R. McDougald & Y.M. Saif, (Eds.). Diseases of Poultry (pp. 302–313). Ames, Iowa: Mosby-Wolfe.
- Chirico, J., Eriksson, H., Fossum, O. & Jansson, D. (2003). The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens. Medical and Veterinary Entomology, 17, 232–234. 10.1046/j.1365-2915.2003.00428.x
- de Kloet, S.R. & Dorrestein, G.M. (2009) Presence of avian bornavirus RNA and anti-avian bornavirus antibodies in apparently healthy macaws. Avian Diseases, 53, 568–573. 10.1637/8828-040209-Reg.1
- Cray, C. (2011) Infectious and zoonotic disease testing in pet birds. Clinics in Laboratory Medicine, 31, 71–85. 10.1016/j.cll.2010.10.008
- Garnier R., Ramos R., Staszewski V., Militão T., Lobato E., González-Solís J. & Boulinier T. (2012). Maternal antibody persistence: a neglected life-history trait with implications from albatross conservation to comparative immunology. Proceedings of the Royal Society B. Biological Sciences., 279, 2033–2041. 10.1098/rspb.2011.2277
- Gartrell, B.D., Alley, M.R., Mack, H., Donald, J., McInnes, K. & Jansen, P. (2005). Erysipelas in the critically endangered kakapo (Strigops habroptilus). Avian Pathology, 34, 383–387. 10.1080/03079450500268583
- Gerlach, H. (1994). Bacteria. In B. Ritchie, G. Harrison & L. Harrison, (Eds.). Avian Medicine: principles and applications (pp. 949–983). Lake Worth, Florida: Wingers Publishing.
- Graham, R., Levine, N. & Hester, H. (1939). Erysipelothrix rhusiopathiae associated with a fatal disease in ducks. Journal of the American Veterinary Medical Association, 95, 211–216.
- Hasselquist D, &Nilsson JA. (2009) Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Philosophical Transactions of the Royal Society B Biological Sciences 364, 51–60.
- Jensen, W. & Cotter, S. (1976). An outbreak of erysipelas in eared grebes (Podiceps nigricolis). Journal of Wildlife Diseases, 12, 583–586.
- Powlesland, R.G., Merton, D.V. & Cockrem, J.F. (2006). A parrot apart: the natural history of the kakapo (Strigops habroptilus), and the context of its conservation management. Notornis, 53, 3–26.
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
- Rosenwald, A. & Dickinson, E. (1941). Swine erysipelas in turkeys. American Journal of Veterinary Research, 2, 202–213.
- Swan, R.A. & Lindsey, M.J. (1998). Treatment and control by vaccination of erysipelas in farmed emus (Dromaius novohollandiae). Australian Veterinary Journal, 76, 325–327. 10.1111/j.1751-0813.1998.tb12356.x