Abstract
In a prospective longitudinal study, a broiler breeder flock and its progeny were monitored for the presence of avian hepatitis E virus (HEV) RNA and antibodies. The flock was part of a multiple-age farm where the presence of avian HEV with clinical signs (increased mortality and decreased egg production) was demonstrated in several previous production cycles. Samples were taken twice at the rearing site and several times at the production site from broiler breeders including cockerels and day-old chicks. The samples were investigated by conventional and real-time reverse transcriptase-polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA) and histological methods. At all time points, samples from the hens were positive for avian HEV RNA. The birds did not show any clinical signs, even though histopathological lesions of non-specific aetiology in the liver and spleen could be demonstrated. A significant increase in the number of positive birds and viral load was seen in week 45, in accordance with an increase in antibody titres. In comparison, cockerels investigated in week 62 tested negative by RT-PCR and ELISA. Avian HEV RNA was also detected in day-old chicks hatched from eggs laid in week 25, indicating vertical transmission. All partial helicase and capsid sequences retrieved within this study clustered together and were identical to previous sequences obtained from the same multiple-age farm. In conclusion, avian HEV persisted on the farm over years and circulated between the rearing and the production sites without causing any clinical signs although high viral loads in the adult hens were observed.
Introduction
Big liver and spleen disease (BLSD), first described in broiler breeders in Australia, and hepatitis splenomegaly syndrome (HSS), first described in layers and broiler breeders in North America, are both caused by avian hepatitis E virus (HEV) (Payne et al., Citation1999; Haqshenas et al., Citation2001). Data about the prevalence of avian HEV in chicken flocks are still very limited and, if present at all, are documented mainly in layers (Huang et al., Citation2002; Peralta et al., Citation2009). A few epidemiological studies were reported from Australian broiler breeder flocks affected by BLSD, but the infectious agent had not been identified because molecular methods were not available at that time (Handlinger & Williams, Citation1988; Crerar & Cross, Citation1994a). Although the diagnostic methods for avian HEV have improved in recent years, the mechanisms of virus transmission and its persistence on a farm are still largely unknown.
Three genotypes of avian HEV, according to their geographic origin – Australia, North America or Europe – have been identified (Bilic et al., Citation2009; Marek et al., Citation2010). Recently, avian HEV infections have also been described in chicken flocks from Russia, Korea and China (Zhao et al., Citation2010; Kwon et al., Citation2012; Sprygin et al., Citation2012). In Hungary and Taiwan, an apparently new genotype has been found (Bányai et al., Citation2012; Hsu & Tsai, Citation2014).
Predominantly adult layers and broiler breeders are affected by a clinical disease caused by avian HEV (Meng & Shivaprasad, Citation2013). Egg production decreases up to 20% per week and the mortality increases up to 1%. Enlarged livers and spleens, subcapsular liver haemorrhages and blood-stained fluid in the visceral cavity are the typical pathological lesions caused by avian HEV infection. However, avian HEV is also detected in apparently healthy chicken flocks (Sun et al., Citation2004a; Peralta et al., Citation2009). So far, it is not possible to definitely link differences in the viral genomes with pathogenicity (Billam et al., Citation2007, Citation2009) and the pathogenesis of avian HEV remains unclear.
Avian HEV is shed in faeces and the faecal–oral transmission route has been demonstrated (Billam et al., Citation2005). Also, it has been shown that egg whites from experimentally infected hens contain infectious virus particles but complete vertical transmission could not be established (Guo et al., Citation2007).
To further understand the complex epidemiology of avian HEV, a specific broiler breeder flock located on a multiple-age farm with a history of avian HEV was monitored from 6 weeks of age to 62 weeks of age. Broiler breeders and day-old chicks from this flock were tested for the presence of avian HEV RNA by conventional and quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and for avian HEV antibodies by enzyme-linked immunosorbent assay (ELISA). The aims of this study were to investigate the circulation of avian HEV on a multiple-age farm in a specific broiler breeder flock and to find evidence for natural vertical transmission.
Materials and Methods
Sample collection and investigations on the farm
A future broiler breeder flock coming from a multiple-age farm in Poland, where clinical outbreaks (increased mortality, decreased egg production) of avian HEV with characteristic pathological lesions (enlarged livers and spleens, tender consistency of livers, subcapsular haemorrhages in livers, serosanguinous fluid in body cavity) and presence of virus confirmed by RT-PCR appeared in the preceding 2 years (Troxler, S. & Hess, M. (2011, 2012), unpublished data), was selected for this longitudinal field study. Until the start of production, the flock was vaccinated with live vaccines against Marek's disease, Newcastle disease, coccidiosis, infectious bronchitis, infectious bursal disease, chicken anaemia, Salmonella, avian encephalomyelitis, Mycoplasma gallisepticum and avian rhinotracheitis. Inactivated vaccines against infectious bronchitis, infectious bursal disease, avian rhinotracheitis, Newcastle disease, Salmonella and Escherichia coli were applied. The investigations started at 6 weeks of age and lasted until 1 week prior to slaughter (62 weeks of age) (). Live birds were randomly selected for sampling and killed by head dislocation. From each tested chicken an FTA® Classic Card (Whatman International Ltd, Maidstone, UK) with smears of the liver, spleen, bile and caecal tonsils and serum were collected. Samples were taken twice at the rearing site (weeks 6 and 10) and twice at the production site (weeks 25 and 45). The same types of samples were also collected from day-old chicks hatched from eggs of this flock, which had been laid in the same weeks (weeks 25 and 45). In addition, liver and spleen samples were collected from broiler breeder hens in weeks 50 and 52 for comparative histological and virological investigations, of which the latter was carried out by RT-PCR. Finally, liver and spleen samples (frozen and in formalin) and sera were collected at the end of production in week 62 from 10 female birds, 10 male birds and 10 day-old chicks. All samples were labelled in such a way that it was possible to link the results of serology, RT-PCR and histology, respectively, to individual birds. Furthermore, two pooled faecal samples of the broiler breeder flock were collected in week 50 for RT-PCR investigation.
Table 1. Time points of sampling of broiler breeders and day-old chicks.
The flock was observed for any clinical signs and monitored as a matter of routine. Detailed production data of the flock were collected starting from week 24 of life. These data included actual production per week (%), eggs per hen and week, hatching eggs per hen, number of hens at the beginning of the week, hen mortality, culled hens, number of cockerels at the beginning of the week, cockerel mortality, culled cockerels, percent of cockerels in the flock, hen and cockerel feed consumption per week, weight of hatching eggs, and weight of hens and cockerels. The following data were collected in the hatchery: egg weight, fertilization rate, number of healthy day-old chicks, number of day-old chicks culled, weight of day-old chicks and hatchability of eggs set (%).
RNA isolation and reverse transcriptase-polymerase chain reaction
RNA was extracted from FTA® cards by a slightly modified Solution D method (Chomczynski & Sacchi, Citation1987; Bilic et al., Citation2009) and from frozen tissues and faeces by the cador Pathogen kit (Qiagen, Hildesheim, Germany). For both methods an internal control RNA was included (Troxler et al., Citation2011) and the resulting total RNA was eluted in 50 µl ultra-purified water or the elution buffer included in the kit. In conventional and real-time RT-PCR reactions, respectively, 5 µl RNA were added, resulting in a total reaction volume of 25 µl.
Conventional RT-PCR was applied using the OneStep RT-PCR kit (Qiagen) according to manufacturer's instructions. Primer pairs “helicase” HelicaseF/R (Huang et al., Citation2002), annealing within open reading frame 1 (ORF1), and “capsid” Forw/Rev1_C-BLSV (Bilic et al., Citation2009), annealing within ORF2, respectively, were used in a final concentration of 1 µM. Real-time RT-PCR was carried out to quantify the amount of avian HEV RNA in the samples (Troxler et al., Citation2011). Negative extraction and PCR controls were included in all RT-PCR reactions. The viral load was determined by comparing the threshold cycle values of samples with a standard curve generated from in vitro-transcribed avian HEV RNA.
Histopathology
Liver and spleen samples were fixed in 4% formalin, paraffin embedded, cut at 3 µm and stained with haematoxylin and eosin. From the same birds and organs, frozen tissue samples were also collected in weeks 52 and 62 to detect and quantify the virus by RT-PCR.
Serology
To determine the presence of antibodies against avian HEV in the sera, the BLS ELISA antibody detection kit (BioChek, Reeuwijk, the Netherlands) was applied according to the manufacturer's instructions. After measuring the absorbance at 405 nm, the titres and the cut-off value were calculated with the help of BioChek software V.2010.
Phylogenetic analyses
DNA from selected positive samples (broiler breeders aged 6, 10, 45, 50, 52 and 62 weeks and day-old chicks of week 25) was eluted from 2% agarose gels applying the Gel Extraction Kit (Qiagen) and directly sequenced in both directions using the PCR primers (LCG Genomics, Berlin, Germany). If insufficient DNA was obtained for direct sequencing, the PCR products were cloned into the pCR®4-TOPO® vector by using the TOPO TA Cloning® Kit for Sequencing (Life Technologies, Carlsbad, CA, USA) and sequenced in both directions using the M13 primers (LCG Genomics).
Alignments and phylogenetic analyses of partial helicase and capsid sequences were performed with the Accelrys Gene software, version 2.5 (Accelrys, San Diego, CA, USA) and MegAlign application of Lasergene software, version 10 (DNASTAR Inc., Madison, WI, USA) with default settings. The sequences obtained within this study were compared with sequences either published in GenBank or obtained from routine samples in our laboratory. Unique sequences were deposited into EMBL database and have the accession numbers HG917393 to HG917399.
Results
Investigations on the farm
The investigated Ross 308 broiler breeder flock consisted of 61,200 female and 8720 male chicks at the day of placement on the rearing site. The birds were transferred from the rearing site to the production site with divisions in terms of weight at the age of 10 to 12 weeks. The rearing and production sites of the farm are at a distance of approximately 1.5 km. Production started in week 23 of life and at this time the investigated flock consisted of 11,285 hens and 1112 cockerels.
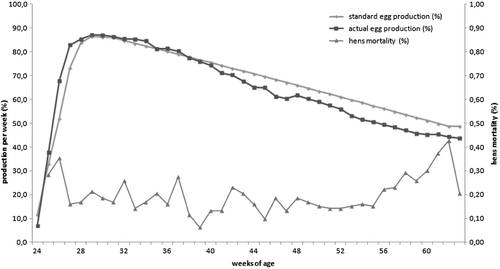
No significant changes in any of the production data were noticed. There was a slight loss in egg production starting in week 38. The maximum decrease compared with the standard was 6% and the average decrease from week 38 to week 62 was 4.9% (). Nevertheless, the overall performance of the flock was satisfactory and especially the mortality was not affected ().
Reverse transcriptase-polymerase chain reaction results
At all times, samples from broiler breeder hens were positive for avian HEV RNA either by conventional RT-PCR (helicase or capsid primers) or real-time RT-PCR (). Positive results were received in week 6 from five out of 20 birds, in week 10 from three out of 20 birds, in week 25 from four out of 16 birds, in week 45 from nine out of 10 birds, in week 52 from four out of five birds and in week 62 from two out of 10 birds. The 10 cockerels tested in week 62 were negative for avian HEV in conventional and real-time RT-PCR.
Table 2. Results for individual birds obtained by conventional RT-PCR, real-time RT-PCR and ELISA.
Avian HEV RNA was detected from six out of 12 day-old chicks investigated in week 25. All samples from day-old chicks obtained in weeks 45 and 62 were negative by RT-PCR.
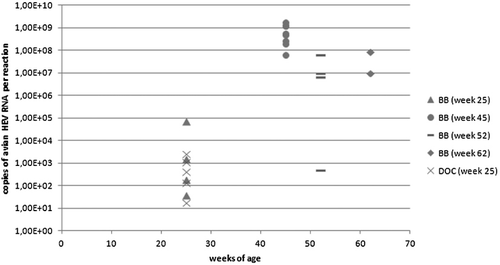
Quantitative RT-PCR revealed a significant increase in the viral loads between week 25 and 45 ( and ). Four birds were positive by real-time RT-PCR in week 25 with a mean viral load of 1.72 × 104 copies per reaction. The mean viral load of the eight positive birds in week 45 was 7.41 × 108 copies per reaction. The four positive birds in week 52 had a mean viral load of 2.02 × 107. Finally, the two positive birds in week 62 had a mean viral load of 4.81 × 107 copies per reaction. The faecal samples collected in week 50 were positive by conventional RT-PCR with both primer pairs. The viral load was 2.8 × 108 and 1.64 × 108 copies per reaction, respectively.
Serology
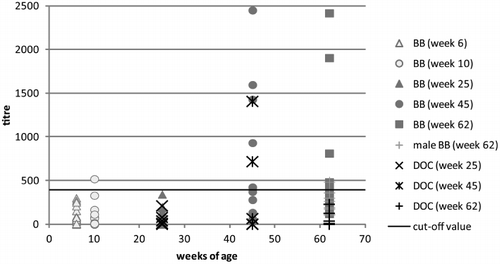
All serum samples of broiler breeders tested with the BLS ELISA at the age of 6, 19 and 25 weeks had titres below the cut-off value with the exception of one sample in week 10, which was slightly above the cut-off value ( and ). The sera from day-old chicks in week 25 were also negative for avian HEV antibodies. In week 45, HEV antibodies in sera were measured in five out of 10 broiler breeders as well as in two out of 10 sera from day-old chicks. Broiler breeders' titres persisted at this level until week 62 but no antibodies were detected in sera of day-old chicks at this time. Sera from 62-week-old cockerels had low antibody titres below or slightly above the cut-off value.
Histopathology
In total, livers and spleens from 30 adult broiler breeders (including 10 cockerels) were available for histological examination. Additionally, 25 of those samples were also available for RT-PCR, of which tissues from six birds were positive for avian HEV.
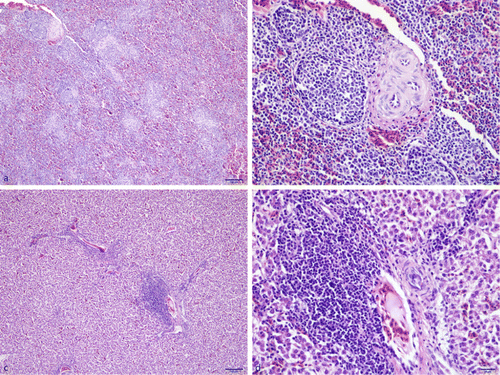
Spleens that were found positive by RT-PCR showed lymphoid hyperplasia and increased numbers of lymphoid nodules (). The livers of the same birds were infiltrated by mononuclear inflammatory cells around portal areas. In most of the livers, vacuolation of hepatocytes was seen ().
Phylogenetic analyses
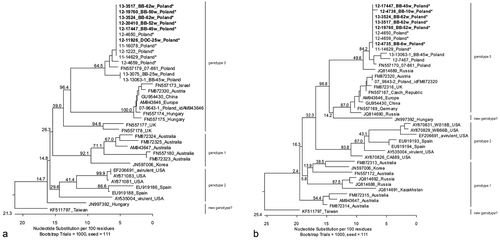
Positive RT-PCR samples from a total of six samplings (collected between week 6 and week 62 of broiler breeders' age) were analysed, using a 130-base-pair helicase or 124-base-pair capsid gene sequence. Sequences grouped together and were classified within genotype 3 of avian HEV (). In the helicase region, the obtained sequences were 100% identical to each other and 98.5 to 100% identical to sequences obtained from samples of the same farm but from five previous flocks kept in the preceding 2 years. The analysed helicase sequence obtained from a day-old chick in week 25 grouped within the sequences of the broiler breeders. The second nearest similarity (94.6 to 97.7%) was observed for samples obtained between 2007 and 2013 from another broiler breeder farm in Poland. Similar to helicase, the capsid sequences obtained within this study were 100% identical not only between each other but also in comparison with those obtained from three previous flocks of the same farm kept in the preceding 2 years. The only exception was a sequence from a 10-week-old broiler breeder that had only 99.2% sequence identity with the others. The sequences from the investigated farm were 95.2 to 98.4% identical to the sequences from the other Polish broiler breeder farm mentioned above.
Discussion
Avian HEV is the causative agent of HSS and BLSD (Meng & Shivaprasad, Citation2013) but it is also found in apparently healthy chicken flocks (Sun et al., Citation2004a; Peralta et al., Citation2009; Sprygin et al., Citation2012). HSS is typically described in adult layers in North America (Ritchie & Riddell, Citation1991; Haqshenas et al., Citation2001; Agunos et al., Citation2006), whereas BLSD was mainly reported in broiler breeders in Australia and Europe (Handlinger & Williams, Citation1988; Crerar & Cross, Citation1994a; Massi et al., Citation2005; Morrow et al., Citation2008). So far, the only prospective field study investigating avian HEV infections in chickens has been conducted in birds aged between 12 and 30 weeks, without further specifying the type of chickens (Sun et al., Citation2004a). With this limitation in mind, the aim of the present study was to investigate the progress of an avian HEV infection in a broiler breeder flock, mainly breeder hens, and its progeny.
In the present study, conventional RT-PCR was applied for phylogenetic analyses and real-time RT-PCR was used in order to determine the viral load. Several conventional RT-PCR methods for avian HEV exist (Payne et al., Citation1999; Huang et al., Citation2002; Sun et al., Citation2004a, Citationb; Bilic et al., Citation2009) but their specificities and sensitivities are not known. Equal sensitivity of the applied conventional RT-PCR and real-time RT-PCR was reported (Troxler et al., Citation2011), which was confirmed in the present study. However, some samples were only positive by one of those methods. This might happen if the concentration of target is low and the methods reach their detection limit. In this case a sample will be negative although target RNA is present with an average frequency of 37% (Ke et al., Citation2006). Another explanation is that the three ORFs of avian HEV were expressed in different amounts. A shift of positive samples obtained with capsid primers (weeks 6 and 10) to samples positive with helicase primers (week 25) was observed. The ORF1 polyprotein is translated directly from the viral RNA; later the ORF2 and ORF3 proteins are translated from a 2.2 kb subgenomic RNA (Ahmad et al., Citation2011). This implies that the parts of viral RNA coding for ORF1 (helicase) and ORF2 (capsid) are replicated in different amounts, depending on the stage of infection. In samples with high viral loads (weeks 45, 50, 52 and 62) positive results were found independent of primer location, either targeting the helicase or the capsid gene.
Nucleic acid of avian HEV was also demonstrated in day-old chicks hatched from eggs laid in week 25 of broiler breeders' age. This is in agreement with experimental data demonstrating that chicken embryos can be infected intravenously with avian HEV (Payne et al., Citation1993) and infectious avian HEV is present in egg whites coming from intravenously infected hens (Guo et al., Citation2007). Interestingly, day-old chicks hatched from eggs laid in weeks 45 and 62 were negative for avian HEV. A possible explanation for this could be the rise in HEV antibodies noticed in the hens at that time. This theory is supported by Crerar & Cross (Citation1994a), who already assumed that vertical transmission occurs before the hens develop antibodies.
It is established in the literature that mainly adult chickens between 30 and 72 weeks suffer from an HEV infection (Meng & Shivaprasad, Citation2013) and prevalence of antibodies increases with age (Huang et al., Citation2002; Peralta et al., Citation2009). Furthermore, seroconversion in subclinically infected chickens was reported between weeks 17 and 19 of life (Sun et al., Citation2004a). Interestingly, Zhao et al. (Citation2013) reported a decline of seropositive birds with age in birds from flocks with an incidence of BLSD. This could not be observed in the actual study as birds stayed negative until 25 weeks of life and positive birds were detected at 45 weeks until slaughtering. This might reflect a more typical clinical situation characterized by the presence of avian HEV in low amounts already on the rearing site and multiplication in the chickens coinciding with production. Surprisingly, day-old chicks hatched from eggs laid in week 62 had no antibodies against avian HEV. In this context, one needs to mention that at this time point only 50% of the hens were positive and the majority with low antibody titres, obviously insufficient to measure maternal antibodies in progenies. However, differences between the applied ELISA systems in the aforementioned studies with unknown sensitivity and specificity limit such comparisons. In general the measured antibody titres were low except for some hens in weeks 45 and 62 and day-old chicks in week 45. The cockerels investigated in week 62 were negative by ELISA (except for one, which was slightly above the cut-off value) and were also negative by RT-PCR, indicating lower susceptibility, if at all, of male birds.
Histopathological changes in clinical BLSD were summarized as a lymphoproliferative phase followed by lymphoid destruction in the liver and spleen (Handlinger & Williams, Citation1988). Multifocal, coalescing coagulative necrosis, perivasculitis in livers and spleens, and generalized lymphoid depletion in spleens are typical findings in HSS and BLSD (Billam et al., Citation2005; Agunos et al., Citation2006; Morrow et al., Citation2008). The livers and spleens investigated in the current study showed less severe lesions. This is in accordance with previous work (Billam et al., Citation2009), which showed milder changes (lymphocytic and heterophilic periphlebitis and fibrinoid necrosis in the liver) in chickens infected with an avian HEV strain recovered from a healthy chicken compared with chickens infected with an avian HEV strain obtained from a diseased chicken.
Phylogenetic analysis of helicase and capsid nucleic acid sequences allows the separation of avian HEV into three genotypes, corresponding to their geographic origin (Marek et al., Citation2010). Accordingly, the isolates from broiler breeders and day-old chicks investigated in this study grouped within genotype 3, which contains predominantly European isolates. Recently, based on phylogenetic analysis of the whole genome, a new genotype of avian HEV, consisting of a Hungarian and a Taiwanese strain, has been proposed (Bányai et al., Citation2012; Hsu & Tsai, Citation2014). The close phylogenetic relationship between those two viruses was supported in this study by analysing helicase nucleic acid sequences but not by capsid nucleic acid sequences, arguing for further analyses. Remarkably, the nucleic acid sequences for the virus detected in the broiler breeder flock were nearly identical to those sequences from avian HEVs present on the farm during previous years. To our knowledge this is the first phylogenetic analysis using samples from the same location but from different years and disease conditions. Consequently, persistence of the virus on the same farm over years could be demonstrated, independent of clinical signs. Beside other factors (e.g. immune status of birds), genetic differences between HEV isolates and the infectious dose might be the reason for different grades of pathogenicity of avian HEV (Sun et al., Citation2004a; Agunos et al., Citation2006; Billam et al., Citation2007). Part of this hypothesis was not supported by our study because we did not find any sequence differences between samples from healthy birds of this study and samples from diseased birds obtained earlier from the farm. Also the viral load did not seem to have an effect on pathogenicity. Neither the birds with relatively low viral loads (week 25) nor the birds with high viral loads (weeks 45 and 62) showed any clinical signs. Experimentally, one could demonstrate that chickens of different age, breed and sex can be infected with avian HEV (Clarke et al., Citation1990; Payne et al., Citation1993; Crerar & Cross, Citation1994b; Sun et al., Citation2004b; Billam et al., Citation2005, Citation2009; Huang et al., Citation2005). Remarkably, BLSD affects only sexually mature broiler breeder hens (Crerar & Cross, Citation1994a; Handlinger & Williams, Citation1988; Morrow et al., Citation2008). This study successfully characterized the progress of subclinical HEV infection in female broiler breeders and their progeny. However, additional host factors such as stress, a certain hormone or immune status, or other disease conditions might be responsible for disease outbreaks that remain unclear at present.
Acknowledgements
The authors thank Beatrice Grafl and Claudia Hess for their contribution to initial sample processing. Patricia Wernsdorf contributed with excellent technical assistance in histology. In addition, the authors thank Ana Marek for her contribution to process of sequencing data.
Funding
This work was partly done within the Centre of Excellence for Poultry (CEPO) project, funded by the European Regional Development Fund, Cross-border Cooperation Programme Austria–Hungary 2007–2013.
Additional information
Funding
References
- Agunos, A.C., Yoo, D., Youssef, S.A., Ran, D., Binnington, B. & Hunter, D.B. (2006). Avian hepatitis E virus in an outbreak of hepatitis–splenomegaly syndrome and fatty liver haemorrhage syndrome in two flaxseed-fed layer flocks in Ontario. Avian Pathology, 35, 404–412. 10.1080/03079450600920976
- Ahmad, I., Holla, R.P. & Jameel, S. (2011). Molecular virology of hepatitis E virus. Virus Research, 161, 47–58. 10.1016/j.virusres.2011.02.011
- Bányai, K., Tóth, Á.G., Ivanics, E., Glávits, R., Szentpáli-Gavallér, K. & Dán, Á. (2012). Putative novel genotype of avian hepatitis E virus, Hungary, 2010. Emerging Infectious Diseases, 18, 1365–1368.
- Bilic, I., Jaskulska, B., Basic, A., Morrow, C.J. & Hess, M. (2009). Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. Journal of General Virology, 90, 863–873. 10.1099/vir.0.007179-0
- Billam, P., Huang, F.F., Sun, Z.F., Pierson, F.W., Duncan, R.B., Elvinger, F., Guenette, D.K., Toth, T.E. & Meng, X.J. (2005). Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. Journal of Virology, 79, 3429–3437. 10.1128/JVI.79.6.3429-3437.2005
- Billam, P., LeRoith, T., Pudupakam, R.S., Pierson, F.W., Duncan, R.B. & Meng, X.J. (2009). Comparative pathogenesis in specific-pathogen-free chickens of two strains of avian hepatitis E virus recovered from a chicken with Hepatitis–Splenomegaly syndrome and from a clinically healthy chicken. Veterinary Microbiology, 139, 253–261. 10.1016/j.vetmic.2009.06.008
- Billam, P., Sun, Z.F. & Meng, X.J. (2007). Analysis of the complete genomic sequence of an apparently avirulent strain of avian hepatitis E virus (avian HEV) identified major genetic differences compared with the prototype pathogenic strain of avian HEV. Journal of General Virology, 88, 1538–1544. 10.1099/vir.0.82754-0
- Chomczynski, P. & Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry, 162, 156–159. 10.1016/0003-2697(87)90021-2
- Clarke, J.K., Allan, G.M., Bryson, D.G., Williams, W., Todd, D., Mackie, D.P. & McFerran, J.B. (1990). Big liver and spleen disease of broiler breeders. Avian Pathology, 19, 41–50. 10.1080/03079459008418654
- Crerar, S. & Cross, G. (1994a). Epidemiological and clinical investigations into big liver and spleen disease of broiler breeder hens. Australian Veterinary Journal, 71, 410–413. 10.1111/j.1751-0813.1994.tb00956.x
- Crerar, S. & Cross, G. (1994b). The experimental production of big liver and spleen disease in broiler breeder hens. Australian Veterinary Journal, 71, 414–417. 10.1111/j.1751-0813.1994.tb00958.x
- Guo, H., Zhou, E.M., Sun, Z.F. & Meng, X.-J. (2007). Egg whites from eggs of chickens infected experimentally with avian hepatitis E virus contain infectious virus, but evidence of complete vertical transmission is lacking. Journal of General Virology, 88, 1532–1537. 10.1099/vir.0.82689-0
- Handlinger, J.H. & Williams, W. (1988). An egg drop associated with splenomegaly in broiler breeders. Avian Diseases, 32, 773–778. 10.2307/1590997
- Haqshenas, G., Shivaprasad, H.L., Woolcock, P.R., Read, D.H. & Meng, X.J. (2001). Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. The Journal of General Virology, 82, 2449–2462.
- Hsu, I.W.-Y. & Tsai, H.-J. (2014). Avian hepatitis E virus in chickens, Taiwan, 2013. Emerging Infectious Diseases, 20, 149–151. 10.3201/eid2001.131224
- Huang, F.F., Haqshenas, G., Shivaprasad, H.L., Guenette, D.K., Woolcock, P.R., Larsen, C.T., Pierson, F.W., Elvinger, F., Toth, T.E. & Meng, X.J. (2002). Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States. Journal of Clinical Microbiology, 40, 4197–4202. 10.1128/JCM.40.11.4197-4202.2002
- Huang, F.F., Pierson, F.W., Toth, T.E. & Meng, X.J. (2005). Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV. Journal of General Virology, 86, 2585–2593. 10.1099/vir.0.81070-0
- Ke, G.M., Cheng, H.L., Ke, L.Y., Ji, W.T., Chulu, J.L.C., Liao, M.H., Chang, T.J. & Liu, H.J. (2006). Development of a quantitative Light Cycler real-time RT-PCR for detection of avian reovirus. Journal of Virological Methods, 133, 6–13. 10.1016/j.jviromet.2005.09.011
- Kwon, H.M., Sung, H.W. & Meng, X.-J. (2012). Serological prevalence, genetic identification, and characterization of the first strains of avian hepatitis E virus from chickens in Korea. Virus Genes, 45, 237–245. 10.1007/s11262-012-0761-6
- Marek, A., Bilic, I., Prokofieva, I. & Hess, M. (2010). Phylogenetic analysis of avian hepatitis E virus samples from European and Australian chicken flocks supports the existence of a different genus within the Hepeviridae comprising at least three different genotypes. Veterinary Microbiology, 145, 54–61. 10.1016/j.vetmic.2010.03.014
- Massi, P., Tosi, G., Bassi, D., Gelmetti, D., Lavazza, A., Lombardi, G. & Torcoli, G. (2005). Big liver and spleen disease in broiler breeders in Italy. Italian Journal of Animal Science, 4, 303–305.
- Meng, X.J. & Shivaprasad, H.L. (2013). Avian Hepatitis E Virus Infections. In D.E. Swayne (Ed.). Diseases of Poultry 13th edn (pp. 494–512). Iowa: Wiley-Blackwell.
- Morrow, C., Samu, G., Matrai, E., Klausz, A., Wood, A., Richter, S., Jaskulska, B. & Hess, M. (2008). Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathology, 37, 527–535. 10.1080/03079450802356946
- Payne, C.J., Ellis, T.M., Plant, S.L., Gregory, A.R. & Wilcox, G.E. (1999). Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Veterinary Microbiology, 68, 119–125. 10.1016/S0378-1135(99)00067-X
- Payne, C.J., Plant, S.L., Ellis, T.M., Hillier, P.W. & Hopkinson, W. (1993). The detection of the big liver and spleen agent in infected tissues via intravenous chick embryo inoculation. Avian Pathology, 22, 245–256. 10.1080/03079459308418918
- Peralta, B., Biarnés, M., Ordóñez, G., Porta, R., Martín, M., Mateu, E., Pina, S. & Meng, X.-J. (2009). Evidence of widespread infection of avian hepatitis E virus (avian HEV) in chickens from Spain. Veterinary Microbiology, 137, 31–36. 10.1016/j.vetmic.2008.12.010
- Ritchie, S.J. & Riddell, C. (1991). “Hepatitis-splenomegaly” syndrome in commercial egg laying hens. The Canadian Veterinary Journal/ La Revue Veterinaire Canadienne, 32, 500–501.
- Sprygin, A.V., Nikonova, Z.B. & Zinyakov, N.G. (2012). Avian hepatitis E virus identified in Russian chicken flocks exhibits high genetic divergence based on the ORF2 capsid gene. Avian Pathology, 41, 459–463. 10.1080/03079457.2012.711464
- Sun, Z.F., Larsen, C.T., Dunlop, A., Huang, F.F., Pierson, F.W., Toth, T.E. & Meng, X.J. (2004a). Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. Journal of General Virology, 85, 693–700. 10.1099/vir.0.19582-0
- Sun, Z.F., Larsen, C.T., Huang, F.F., Billam, P., Pierson, F.W., Toth, T.E. & Meng, X.J. (2004b). Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. Journal of Clinical Microbiology, 42, 2658–2662. 10.1128/JCM.42.6.2658-2662.2004
- Troxler, S., Marek, A., Prokofieva, I., Bilic, I. & Hess, M. (2011). TaqMan real-time reverse transcription-PCR assay for universal detection and quantification of avian hepatitis E virus from clinical samples in the presence of a heterologous internal control RNA. Journal of Clinical Microbiology, 49, 1339–1346. 10.1128/JCM.01626-10
- Zhao, Q., Sun, Y., Zhao, J., Hu, S., Zhao, F., Chen, F., Clavijo, A., Zhou, E.-M. & Xiao, Y. (2013). Development and application of an indirect ELISA for detection of antibodies against avian hepatitis E virus. Journal of Virological Methods, 187, 32–36. 10.1016/j.jviromet.2012.08.026
- Zhao, Q., Zhou, E.M., Dong, S.W., Qiu, H.K., Zhang, L., Hu, S.B., Zhao, F.F., Jiang, S.J. & Sun, Y.N. (2010). Analysis of avian hepatitis E virus from chickens, China. Emerging Infectious Diseases, 16, 1469–1472. 10.3201/eid1609.100626