Abstract
Two commercial Midwestern egg-type chicken flocks experienced significant increases in mortality rates in April 2013 with clinical signs appearing in 17-week-old pullets on Farm A and in 46-week-old hens on Farm B. Average weekly mortality was 0.44% over a 4-week period on Farm A and 0.17% over an 8-week period on Farm B. On Farm A, flocks in the affected house had a 45% decrease in daily egg production from weeks 19 to 27 when compared with standard egg production curves (P < 0.01) while no decrease in egg production was noticed on Farm B. Post-mortem examination revealed changes consistent with hepatitis-splenomegaly syndrome, including hepatomegaly with serosanguineous fluid in the coelomic cavity and hepatic subcapsular haemorrhages. Microscopic lesions were characterized by multifocal necrotizing hepatitis and intrahepatic haemorrhage. No significant bacteria were recovered from liver samples, but 72 to 100% of the liver samples from affected chickens on Farm A (8/11) and Farm B (7/7) contained detectable amounts of avian hepatitis E virus (aHEV) RNA as determined by polymerase chain reaction. Sequencing and phylogenetic analysis of a 361-base-pair fragment of the helicase gene demonstrated 98.6 to 100% nucleotide identity between the aHEV genomes from Farm A and Farm B, whereas identities ranged from 74.6 to 90.5% when compared with other representative sequences. Sequences from this study clustered within aHEV genotype 2 previously recognized in the USA. In contrast to other reported aHEV outbreaks that occurred in 30-week-old to 80-week-old chickens, in the present investigation clinical aHEV was identified in 17-week-old chickens on one of the farms.
Introduction
Hepatitis E virus (HEV) is a member of the genus Hepevirus within the family Hepeviridae, which are non-enveloped, single-stranded RNA viruses with icosahedral symmetry consisting of at least four recognized genotypes of mammalian HEV and a separate floating species consisting of four genotypes of avian hepatitis E virus (aHEV) (Bilic et al., Citation2009; Marek et al., Citation2010; Bányai et al., Citation2012; Meng et al., Citation2012; Hsu & Tsai, Citation2014). Phylogenetic analysis of the full or nearly complete genome of aHEV strains indicated the presence of genotype 1 in Australia and Korea, genotype 2 in the USA, genotype 3 in Europe and China and, more recently, a novel genotype 4 has been described in Hungary and Taiwan (Bilic et al., Citation2009; Zhao et al., Citation2010; Bányai et al., Citation2012; Kwon et al., Citation2012; Hsu & Tsai, Citation2014). Additional putative genotypes comprising isolates from North America and Europe have been identified through analysis of a 130-nucleotide fragment of the helicase gene, suggesting that diversity within aHEV could be higher (Marek et al., Citation2010).
aHEV was associated with big liver and spleen disease in Australia (Payne et al., Citation1999) and hepatitis-splenomegaly syndrome (HSS) in North America (Haqshenas et al., Citation2001). Subsequently, aHEV infection has been associated with disease outbreaks in chicken flocks worldwide (Huang et al., Citation2002; Agunos et al., Citation2006; Morrow et al., Citation2008; Bilic et al., Citation2009; Marek et al., Citation2010; Zhao et al., Citation2010; Sprygin et al., Citation2012). Chickens affected by HSS typically present enlarged livers, enlarged spleens and serosanguineous fluid in their coelomic cavities accompanied by a drop in egg production and high mortality rates (Meng & Shivaprasad, Citation2013). Characteristic histopathological changes may include massive coagulative necrosis, vasculatis, haemorrhage, amyloid deposition and non-specific hepatitis with a wide distribution of the aHEV through the parenchyma of liver tissue (Agunos et al., Citation2006; Morrow et al., Citation2008).
Based on serological evidence, aHEV is widespread in chicken flocks with seropositive rates of approximately 71% in the USA, 90% in Spain and 57% in Korea (Huang et al., Citation2002; Peralta et al., Citation2009; Kwon et al., Citation2012). However, the role of aHEV in HSS is unclear, as the virus has also been detected in flocks with no history of HSS (Sun et al., Citation2004; Peralta et al., Citation2009; Kwon et al., Citation2012). Differences in virus strain, virus dose, diet and age have been implicated as potential co-factors for the manifestation of the full spectrum of clinical HSS (Agunos et al., Citation2006; Meng & Shivaprasad, Citation2013). Nevertheless, aHEV strains recovered from healthy chickens in normal flocks and previously considered to be avirulent were only slightly attenuated in an experimental infection model (Billam et al., Citation2009). There was no clear clustering of aHEV sequences based on whether viruses were sampled from chickens displaying clinical signs or not (Marek et al., Citation2010).
The present report describes field outbreaks of HSS in two laying hen flocks during spring 2013 in the USA and documents the clinical, pathological and microbiological findings in addition to aHEV genetic characteristics.
Materials and Methods
Farm and clinical observations
In April 2013, disease outbreaks with elevated mortality were observed on two laying hen farms located in the Midwestern USA. Affected flocks on both farms consisted of white laying hens vaccinated against Marek's disease, infectious bronchitis, Newcastle disease, avian influenza, fowlpox, Mycoplasma gallisepticum and Salmonella Enteritidis. Vaccination against Salmonella Enteritidis on both farms consisted of administration of a live attenuated Salmonella Typhimurium vaccine via the water followed by intramuscular injection of killed Salmonella Enteritidis in an oil emulsion at 12 weeks of age. This vaccination protocol is typical of programmes utilized by Midwest egg producers in the USA.
Farm A was a 1,000,000 commercial laying hen facility, with an average house capacity of 111,000 white birds and a total of nine houses. Each house on Farm A was managed on the basis of all-in, all-out principles and received replacement pullet flocks from a single source. Unusually high mortality associated with a decline in food and water consumption was observed in one of nine houses after placement of a new lot of 16-week-old pullets. Weekly mortality peaked (0.78%) at 19 weeks of age and thereafter declined to normal levels (0.08%) during the ensuing 3 weeks (). Weekly mortality in the remaining eight unaffected houses continued to remain below 0.15% during this period. Egg production, which normally begins at 18 weeks, was delayed for 2 weeks in the affected pullet flock and did not begin until the flock reached 20 weeks of age. Egg production was reduced by 45% during the 8-week period from 19 to 27 weeks of age, after which time the flock's performance returned to near the breed standard (). No other clinical signs of disease were noted.
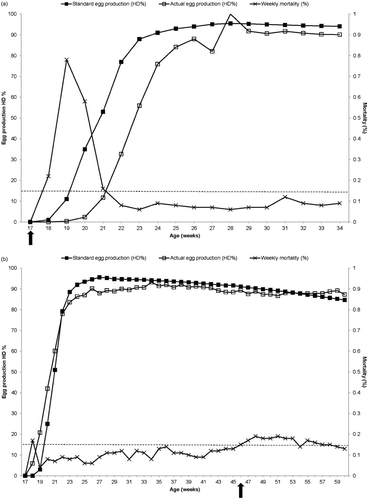
Farm B had 1,200,000 commercial white laying hens of a different breed to those in Farm A and was located in a different state than Farm A. Farm B had an average house capacity of 150,000 birds and a total of eight houses. Houses on this farm were also operated on all-in, all-out principles. On Farm B, increased mortality was observed in a single house containing 46-week-old hens. Maximum weekly mortality increased from an average of 0.09 to 0.17% in the affected flock, peaked (0.19%) at 48 weeks of age and thereafter declined to 0.13% during the following 13 weeks (). Egg production in Farm B remained within normal parameters and no other clinical signs were observed. Weekly mortality in the remaining seven unaffected houses continued to remain below 0.15% during this period and egg production was not affected ().
Sample collection, histopathology, and bacteriology
Six 20-week-old dead chickens from the affected house on Farm A and five 46-week-old dead chickens from Farm B were submitted to the Iowa State University Veterinary Diagnostic Laboratory for diagnostic work-up. In addition, 6 weeks after the initial outbreak on Farm A, livers were collected from 12 dead 23-week-old chickens from the affected house. Part of each liver was fixed in 10% neutral buffered formalin, dehydrated in a graded series of ethanol, embedded in paraffin, sectioned at 4 µm thickness, stained with haematoxylin and eosin and Congo red stain, and examined via light microscopy. Samples of the liver and spleen were cultured on agar plates using routine procedures under aerobic and anaerobic conditions at the Iowa State University Veterinary Diagnostic Laboratory. In addition, samples were cultured in tetrathionate broth medium in an attempt to isolate Salmonella spp. The remaining portions of fresh livers were stored at −80°C until processing for virological analysis.
Virology
Liver samples of approximately 1 g were minced and diluted 1:10 in phosphate-buffered saline, homogenized using a Stomacher® 80 (Seward Laboratory Systems Inc, Bohemia, NY, USA), and centrifuged at 1500 × g for 10 min to obtain supernatant. All samples were stored at −80°C until use. RNA extractions on liver homogenates were performed using the QIAamp® Viral RNA Mini Kit (Qiagen, Valencia, CA, USA). Extracts were subsequently used for detection of the helicase gene of aHEV using primers described previously (Sun et al., Citation2004) in a nested reverse transcriptase polymerase chain reaction (PCR) reaction. Briefly, external primer set 5′-TGTTATYACACCCACCAARACGYTG-3′ and 5′-CCTCRTGGACCGTWATCGACCC-3′ and internal primer set 5′-GCCACGGCTRTTACACCYCAYGT-3′ and 5′-GACCCRGGRTTCGACTGCTT-3′ were used. The reverse transcriptase reaction and first-round PCR were performed with the OneStep RT-PCR Kit (Qiagen) according to manufacturer's instructions under the following conditions: 50°C for 30 min, 95°C for 15 min, 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 1 min, followed by a final elongation step of 72°C for 10 min. The second-round PCR was performed with the ReadyMix® Taq PCR Reaction Mix (Sigma-Aldrich, St Louis, MO, USA) under the following conditions: 95°C for 5 min, 35 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec, followed by a final elongation step at 72°C for 7 min. PCR products with the expected size (386 base pairs) were examined on a 1% agarose gel, excised and purified using the QIAquick Gel Extraction Kit (Qiagen). Two pools of three liver samples each from 20-week-old chickens from Farm A and five liver samples from Farm B were submitted to the University of Georgia Veterinary Diagnostic Laboratory for adenovirus detection.
Sequencing and phylogenetic analysis
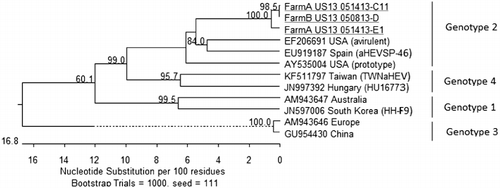
Sequencing of at least two aHEV RNA-positive samples from each farm was performed directly on both strands at the Iowa State University DNA Facility, Ames, IA, USA. Sequences were aligned with published data using BLAST at the national Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Sequences were compiled using Lasergene software and the Clustal V alignment algorithm (DNAStar, Madison, WI, USA). Identical nucleotide sequences were represented as one sequence and used in phylogenetic analysis. For sequence analysis, the 361-base-pair sequences of the helicase gene were compared with each other and with sequences of aHEV isolates from Australia (GenBank accession number AM943647) and South Korea HH-F9 (GenBank accession number JN597006) representing genotype 1, the US prototype (GenBank accession number AY535004), US avirulent (GenBank accession number EF206691) and Spain aHEVSP-46 (GenBank accession number EU919187) representing genotype 2, aHEV isolates from Europe (GenBank accession number AM943646) and China (GenBank accession number GU954430) representing genotype 3, and isolates from Hungary (GenBank accession number JN997392) and Taiwain (GenBank accession number KF511797) representing genotype 4. The nucleotide distance of the sequences was evaluated by neighbour-joining using Lasergene MegAlign (DNAStar). Confidence in the neighbour-joining tree was estimated by bootstrap replicates. Sequences reported in this paper have been deposited in the GenBank database under accession numbers KF573195 to KF573200.
Results
Post-mortem examination
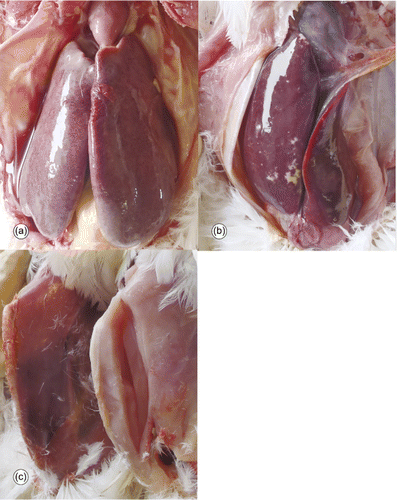
Macroscopic examination of 20-week-old dead chickens from Farm A and 46-week-old dead chickens from Farm B revealed unusually dark livers with hepatomegaly and splenomegaly in all submitted chickens. Feather cover on these chickens was normal and no other macroscopic abnormalities were observed. Examination of 23-week-old dead chickens from the affected house on Farm A, collected after mortality levels had declined to normal levels, revealed that 5/12 had severely enlarged livers (), 2/12 had livers that were moderately larger than normal, and livers in the remaining 5/12 chickens were normal in appearance. Enlarged livers were friable and stippled with red, yellow or tan foci with frequent subcapsular haemorrhages (). In 4/5 chickens with severe liver lesions, approximately 15 ml clear straw-coloured or blood-tinged fluid was present in the coelomic cavity. In these chickens, skeletal muscles were pale (). To investigate possible causes for the presence of the coelomic cavity fluid, the keel bones and rib bones were examined and fractures were not observed.
Histopathogical examination
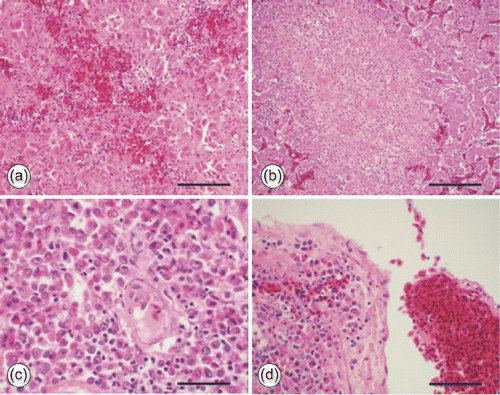
Grossly affected livers were microscopically characterized by multifocal moderate-to-severe necrotizing hepatitis with large, multifocal-to-coalescing areas of hepatocellular necrosis and haemorrhage (). Amorphous eosinophilic material was present in the space of Disse between the Kupffer cells and the hepatocytes. Liver sections stained with Congo red and viewed with polarized light did not show apple-green birefringence associated with amyloid. In acutely affected livers, low numbers of inflammatory cells (heterophils) were present. In subacutely infected livers, large numbers of dead and dying heterophils and pools of condensed fibrin were present in affected areas. Hepatocytes did not have increased cytoplasmic deposition of fat.
Bacteriology
Low numbers of Escherichia coli were isolated from livers. No other agent was isolated using standard aerobic and anaerobic bacteria isolation protocols.
Virology
On Farm A, all six livers from 20-week-old hens submitted immediately after the period of peak of mortality were positive for aHEV helicase gene RNA. In samples collected 3 weeks later, only 50% (3/6) of samples from chickens that died and had hepatomegaly were positive for aHEV RNA. On Farm B, all five livers submitted were positive for aHEV helicase gene RNA. Adenovirus DNA was detected in one out of two sample pools from Farm A, while all five samples from Farm B were negative.
Sequencing and phylogenetic analysis
For Farm A, two HEV RNA-positive samples collected at 20 weeks of age and one sample collected at 23 weeks of age were selected for sequencing. For Farm B, two HEV RNA-positive samples collected at 46 weeks of age were sequenced. Sequencing results showed that strains from Farms A and B presented 98.6 to 100% nucleotide identity for the 361-base-pair fragment of the helicase gene. Between sequences described here and prototype and avirulent USA strains, there was 89% identity. Between 89.9 and 90.5% identity was found with genotype 2 strains recovered from healthy chicken flocks in Spain (aHEVSP-46) (). Similarity with genotype 1, 3 and 4 isolates varied between 75.4 and 83.4%.
Discussion
The present report describes the detection and characterization of aHEV RNA in two layer flocks experiencing clinical disease. Increases in mortality, a drop in egg production in one flock, hepatomegaly, splenomegaly and haemorrhage into the coelomic cavity were the main features observed, and these findings were consistent with observations described previously for HSS in other field cases (Massi et al., Citation2005; Agunos et al., Citation2006; Morrow et al., Citation2008; Sprygin et al., Citation2012).
A presumptive diagnosis of HSS can be made on the basis of clinical signs and gross and microscopic lesions (Meng & Shivaprasad, Citation2013); however, confirmation of aHEV infection requires detection of virus RNA by reverse transcriptase PCR (Troxler et al., Citation2011). HSS must be differentiated from haemorrhagic fatty liver syndrome that occurs in caged layers fed high-energy diets and is characterized by accumulation of fat in liver hepatocytes and liver haemorrhages (Agunos et al., Citation2006). These two syndromes can be differentiated by histological examination, because hepatocytes in HSS livers do not contain excessive lipid and haemorrhagic fatty liver syndrome livers do not have massive necrosis (Agunos et al., Citation2006). Liver fractures caused by external trauma to the body wall should also be considered as a differential diagnosis. Liver fractures result in massive haemorrhage into the coelomic cavity and coagulated blood can often be observed adhering to the liver surface at the fracture site (Meng & Shivaprasad, Citation2013). In the present study, no bacterial agents other than E. coli were recovered from affected birds and no keel or rib fractures were seen, suggesting that mortality and lesions observed in these flocks were associated with aHEV infection. Although adenovirus DNA was recovered from one sample from Farm A, adenoviruses are commonly found in laying hens and no histopathological lesions suggestive of inclusion body hepatitis were present in any of the studied chickens.
Interestingly, clinical signs of HSS occurred in chickens younger than 20 weeks on Farm A, which is unusual because HSS is typically associated with above-normal mortality in broiler breeder hens and laying hens 30 to 72 weeks of age, with the highest incidence occurring between 40 and 50 weeks of age (Agunos et al., Citation2006; Morrow et al., Citation2008; Sprygin et al., Citation2012; Meng & Shivaprasad, Citation2013). Clinical HSS in pullets on Farm A was associated with a 2-week delay in onset of egg production even though lighting and nutritional programmes were the same as those used for other flocks on this farm. Reduced egg production after onset of lay persisted for several weeks before production approached the breed standard. It has been suggested that natural infection of chicken flocks with aHEV occurs around 12 weeks of age (Sun et al., Citation2004) and subclinical infections appear to be frequent (Sun et al., Citation2004; Peralta et al., Citation2009; Kwon et al., Citation2012). To the author's knowledge, HSS in young chickens has not been reported to date. HSS observed in pullets in the present case report could have been associated with variation in viral virulence and tropism, timing and dose of infection, interaction with other management factors, an impaired immune response or additional co-infections. Under field conditions, HSS may also pass unnoticed or be masked by other diseases in pullets.
Differences in timing and dose of infection could partially explain differences in the clinical presentation of HSS in both farms, as no decline in egg production was noticed in Farm B and the rise of mortality in this farm was more subtle than that observed in Farm A. Different clinical presentations of HSS outbreaks have been reported previously, with increases in mortality up to 1.0% a week that may return to previous levels after 4 weeks or may last until the end of the laying cycle (Agunos et al., Citation2006; Morrow et al., Citation2008; Meng & Shivaprasad, Citation2013). On Farm A, atypical food and water consumption and increased mortality were observed immediately after placement at 16 weeks of age, suggesting that initial aHEV infection may have occurred on the pullet farm prior to moving chickens to the laying house. Infectious aHEV particles have been detected in egg albumen, but vertical transmission has not been confirmed (Guo et al., Citation2007). Although aHEV transmission within and between flocks appears to occur readily (Meng & Shivaprasad, Citation2013), clinical signs associated with HSS were limited to one affected house on both farms. Unidentified co-factors in each of these houses may have potentiated and synergized aHEV-induced disease. Lesions and clinical signs associated with HEV have been reported to be dose dependent in non-human primates. Animals infected with a high dose typically progress to significant lesions, whereas animals receiving low doses have subclinical infection without development of hepatic lesions (Tsarev et al., Citation1994). Dose dependency could partially explain why chickens in only one house on each farm presented clinical signs. Although aHEV could have spread to other flocks in nearby houses on the farm, the number of virus particles circulating in other houses may have been insufficient to induce significant lesions or clinical signs. However, one limitation of this case study is that chickens from the unaffected flocks on these farms were not investigated for aHEV infection.
Sequences of aHEV obtained in this study shared 99% nucleotide identity in the helicase gene to each other and between 75.4 and 90.5% identity with previously published sequences. Prior studies reported 76 to 100% identity among aHEV isolates recovered in different regions of the USA based on partial fragments of the helicase gene (Huang et al., Citation2002; Sun et al., Citation2004). Isolates described in this paper clustered with previously reported USA and Spanish genotype 2 isolates.
In this report, characterization of aHEV detected in young and adult layers with HSS on two US egg farms is described. Genetic analysis of the obtained helicase gene sequences showed that the aHEVs on both farms clustered together and were closely related to genotype 2.
References
- Agunos, A.C., Yoo, D., Youssef, S.A., Ran, D., Binnington, B. & Hunter, D.B. (2006). Avian hepatitis E virus in an outbreak of hepatitis–splenomegaly syndrome and fatty liver haemorrhage syndrome in two flaxseed-fed layer flocks in Ontario. Avian Pathology, 35, 404–412. 10.1080/03079450600920976
- Bányai, K., Tóth, Á.G., Ivanics, É., Glávits, R., Szentpáli-Gavallér, K. & Dán, Á. (2012). Putative novel genotype of avian hepatitis E virus, Hungary, 2010. Emerging Infectious Diseases, 18, 1365–1368.
- Bilic, I., Jaskulska, B., Basic, A., Morrow, C.J. & Hess, M. (2009). Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. Journal of General Virology, 90, 863–873. 10.1099/vir.0.007179-0
- Billam, P., LeRoith, T., Pudupakam, R.S., Pierson, F.W., Duncan, R.B. & Meng, X.J. (2009). Comparative pathogenesis in specific-pathogen-free chickens of two strains of avian hepatitis E virus recovered from a chicken with Hepatitis–Splenomegaly syndrome and from a clinically healthy chicken. Veterinary Microbiology, 139, 253–261. 10.1016/j.vetmic.2009.06.008
- Guo, H., Zhou, E.M., Sun, Z.F. & Meng, X.-J. (2007). Egg whites from eggs of chickens infected experimentally with avian hepatitis E virus contain infectious virus, but evidence of complete vertical transmission is lacking. Journal of General Virology, 88, 1532–1537. 10.1099/vir.0.82689-0
- Haqshenas, G., Shivaprasad, H.L., Woolcock, P.R., Read, D.H. & Meng, X.J. (2001). Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. Journal of General Virology, 82, 2449–2462.
- Hsu, I.W.-Y. & Tsai, H.-J. (2014). Avian hepatitis E virus in chickens, Taiwan, 2013. Emerging Infectious Diseases, 20, 149–151. 10.3201/eid2001.131224
- Huang, F.F., Haqshenas, G., Shivaprasad, H.L., Guenette, D.K., Woolcock, P.R., Larsen, C.T., Pierson, F.W., Elvinger, F., Toth, T.E. & Meng, X.J. (2002). Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States. Journal of Clinical Microbiology, 40, 4197–4202. 10.1128/JCM.40.11.4197-4202.2002
- Kwon, H.M., Sung, H.W. & Meng, X.-J. (2012). Serological prevalence, genetic identification, and characterization of the first strains of avian hepatitis E virus from chickens in Korea. Virus Genes, 45, 237–245. 10.1007/s11262-012-0761-6
- Marek, A., Bilic, I., Prokofieva, I. & Hess, M. (2010). Phylogenetic analysis of avian hepatitis E virus samples from European and Australian chicken flocks supports the existence of a different genus within the Hepeviridae comprising at least three different genotypes. Veterinary Microbiology, 145, 54–61. 10.1016/j.vetmic.2010.03.014
- Massi, P., Tosi, G., Gelmetti, D., Lavazza, A., Lombardi, G. & Torcoli, G. (2005). Big liver and spleen disease in broiler breeders in Italy. Italian Journal of Animal Science, 4, 303–305.
- Meng, X.J., Anderson, D., Arankalle, V.A., Emerson, S.U., Harrison, T.J., Jameel, S. & Okamoto, H. (2012). Hepeviridae. In A.M.Q. King, M.J. Adams, E.B. Carstens, & E.J. Lefkowitz (Eds.). Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses (pp. 1021–1028). London: Academic Press.
- Meng, X.J. & Shivaprasad, H.L. (2013). Avian Hepatitis E virus infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V.L. Nair (Eds.). Diseases of Poultry 13th edn (pp. 494–512). Ames, IA: John Wiley & Sons.
- Morrow, C.J., Samu, G., Matrai, E., Klausz, A., Wood, A.M., Richter, S., Jaskulska, B. & Hess, M. (2008). Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathology, 37, 527–535. 10.1080/03079450802356946
- Payne, C.J., Ellis, T.M., Plant, S.L., Gregory, A.R. & Wilcox, G.E. (1999). Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Veterinary Microbiology, 68, 119–125. 10.1016/S0378-1135(99)00067-X
- Peralta, B., Biarnés, M., Ordóñez, G., Porta, R., Martín, M., Mateu, E., Pina, S. & Meng, X.J. (2009). Evidence of widespread infection of avian hepatitis E virus (avian HEV) in chickens from Spain. Veterinary Microbiology, 137, 31–36. 10.1016/j.vetmic.2008.12.010
- Sprygin, A.V., Nikonova, Z.B. & Zinyakov, N.G. (2012). Avian hepatitis E virus identified in Russian chicken flocks exhibits high genetic divergence based on the ORF2 capsid gene. Avian Pathology, 41, 459–463. 10.1080/03079457.2012.711464
- Sun, Z.F., Larsen, C.T., Dunlop, A., Huang, F.F., Pierson, F.W., Toth, T.E. & Meng, X.J. (2004). Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. Journal of General Virology, 85, 693–700. 10.1099/vir.0.19582-0
- Troxler, S., Marek, A., Prokofieva, I., Bilic, I. & Hess, M. (2011). TaqMan real-time reverse transcription-PCR assay for universal detection and quantification of avian hepatitis E virus from clinical samples in the presence of a heterologous internal control RNA. Journal Clinical Microbiology, 49, 1339–1346. 10.1128/JCM.01626-10
- Tsarev, S.A., Tsareva, T.S., Emerson, S.U., Yarbough, P.O., Legters, L.J., Moskal, T. & Purcell, R.H. (1994). Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. Journal of Medical Virology, 43, 135–142. 10.1002/jmv.1890430207
- Zhao, Q., Zhou, E.M., Dong, S.W., Qiu, H.K., Zhang, L., Hu, S.B., Zhao, F.F., Jiang, S.J. & Sun, Y.N. (2010). Analysis of avian hepatitis E virus from chickens, China. Emerging Infectious Diseases, 16, 1469–1472. 10.3201/eid1609.100626