Abstract
Fowl adenoviruses (FAdVs) cause diseases in domestic chickens, including inclusion body hepatitis (IBH), with immunosuppression believed to play a role in their pathogenesis. To gain a better understanding of the pathogenesis and chronology of disease caused by FAdVs, the gross pathology, histopathology and dissemination of virus were examined at several different time points, after inoculation of one-day-old specific pathogen-free chickens with FAdV-1, FAdV-8b or FAdV-11 via the ocular route. FAdV-8b had a slightly greater virulence than FAdV-11, but both were primary pathogens. The presence and severity of hepatic lesions were used to define the three stages of the disease: incubation (1–3 days post-inoculation, PI), degeneration (4–7 days PI) and convalescence (14 days PI). Both viruses were detected in the liver, kidney, bursa, thymus and gizzard of most birds during the degenerative stage, and persisted in the gizzard into convalescence. The FAdV-1 isolate was found to be apathogenic, but virus was detected in the bursa and/or gizzard of several birds between 2 and 7 days PI. This is the first study examining the chronology of gross and microscopic lesions of pathogenic and apathogenic FAdVs in association with viral presence in multiple tissues. It was concluded that both FAdV-8b and FAdV-11 are primary pathogens, and that these strains may play a role in immunosuppression.
Introduction
Fowl adenoviruses (FAdVs) have been associated with a number of diseases in poultry, with particular serotypes linked to each disease. Outbreaks of inclusion body hepatitis (IBH) are predominantly associated with FAdV-2, FAdV-7, FAdV-8a, FAdV-8b and FAdV-11 (Gomis et al., Citation2006; Hess, Citation2013), while hydropericardium syndrome (HPS) is primarily caused by FAdV-4 (Naeem et al., Citation1995). FAdV-1 can cause gizzard erosions in chickens (Okuda et al., Citation2001), with FAdV-8 implicated in some cases (Okuda et al., Citation2004), but FAdV-1 has also been associated with hepatitis in both chickens and quail (Kawamura & Horiuchi, Citation1964; Winterfield et al., Citation1973; Jack & Reed, Citation1990).
Traditionally, IBH is considered a secondary disease requiring concurrent infection with immunosuppressive agents, such as chicken anaemia virus or infectious bursal disease virus (Hess, Citation2013). However, several reports have shown or suggested FAdVs to be capable of causing naturally occurring outbreaks of IBH in the absence of immunosuppressive agents (Reece et al., Citation1986; Saifuddin & Wilks, Citation1990; Gomis et al., Citation2006; Steer et al., Citation2011). Attempts to experimentally reproduce the disease have had mixed findings (McDougall & Peters, Citation1974; Fadly et al., Citation1976; McCracken et al., Citation1976; Grimes et al., Citation1978; Erny et al., Citation1991; Mendelson et al., Citation1995; Toro et al., Citation2000; Grgic et al., Citation2011). The basis of the discrepancy between reports that investigated the role of FAdVs as primary or secondary pathogens may stem from variations in the route of administration, age of chickens and/or strains used for inoculation. For some strains, the signs of naturally occurring disease could not be replicated in an experimental setting, either in birds of an appropriate age (Nakamura et al., Citation2000) or by natural routes of infection (Hess, Citation2013), without some form of immunosuppression (Toro et al., Citation2000; Nakamura et al., Citation2003).
This study aimed to examine in detail the pathogenicity of three field isolates of FAdV of different serotypes, in the absence of immunosuppressive agents. We have previously reported that signs and lesions of IBH could be seen in birds as young as 9 days of age, with the speculation that these cases are the result of horizontal transmission occurring in the first few days of life (Steer et al., Citation2011). Therefore, birds of a susceptible age (1-day-old), free from exposure to a specific prescriptive list of avian pathogens (specific pathogen-free, SPF), were inoculated via the natural route of infection (oral/ocular) with an infective (but not lethal) dose of purified virus. Multiple sampling points were used in an effort to define the chronology of the disease process, including the incubation period, as well as pathological changes from degeneration through to convalescence.
Materials and Methods
Virus isolation and purification
Three field isolates of FAdV, belonging to FAdV-1, FAdV-8b and FAdV-11 serotypes, were used in this study. These isolates were selected as they were contemporary FAdV isolates available and their pathogenicity and role in IBH outbreaks in Australia were unknown. These viruses were identified as FAdV-1, FAdV-8b and FAdV-11 by virus neutralization and polymerase chain reaction–high resolution melt curve (PCR–HRM) genotyping (Steer et al., Citation2011), and their genotypes confirmed by sequencing the loop 1 region of the hexon gene, which showed they were identical in this region to GenBank sequences GU120272, GU120267 and GU120269, respectively. The viruses were propagated, as described previously (Steer et al., Citation2009, Citation2011), from the infected hepatic or bursal tissue of broiler chickens from flocks experiencing outbreaks of IBH.
Each virus was plaque purified three times, as described previously (Pallister et al., Citation1996), with some modifications. Briefly, virus dilutions of 10–0.5 to 10–4 were inoculated onto chicken embryo kidney cell (CEKC) monolayers in 28 cm2 cell culture dishes, and incubated for 1 h at 37°C in 5% CO2. The medium was then replaced with a 1:1 mixture of 2% agarose (Scientifix®) and BioWhittaker® Medium 199 (Cambrex Bioscience Walkersville, Inc., USA), prepared as described by Steer et al. (Citation2011). After 3–5 days of incubation at 37°C in 5% CO2, isolated plaques were picked and virus propagated in 6-well plates until cytopathic effect was evident (up to 6 days). The purity of the viruses was confirmed by PCR–HRM genotyping.
In order to obtain high titres of each virus, the purified viruses were propagated in chicken embryo liver cell cultures in 75 cm2 flasks, and the titre in median tissue culture infective doses (TCID50) was determined in CEKC cultures in 96-well titration plates, as described previously (Grimes et al., Citation1976). The chicken embryo liver cell and CEKC cultures were prepared, respectively, from 14- and 18-day-old SPF chicken embryos (Australian SPF Services, Pty Ltd., Woodend, Australia), as described previously (Schat & Sellers, Citation2008).
The purified FAdVs were tested by PCR or by reverse transcription-PCR (RT-PCR) to confirm the absence of known avian pathogens, including avian reovirus (Bruhn et al., Citation2005), chicken anaemia virus (Kaffashi et al., Citation2006), infectious bursal disease virus (Sapats & Ignjatovic, Citation2002), avian encephalomyelitis virus (Marvil et al., Citation1999; Xie et al., Citation2005), avian leukosis virus (Hauptli et al., Citation1997; Bagust et al., Citation2004; Fenton et al., Citation2005), Marek’s disease virus (Zhu et al., Citation1992), reticuloendotheliosis virus (Aly et al., Citation1993), egg drop syndrome virus (Raj et al., Citation2001), Mycoplasma gallisepticum (Ghorashi et al., Citation2010), Mycoplasma synoviae (Jeffery et al., Citation2007) and Chlamydia/Chlamydophila species (Robertson et al., Citation2009).
Chickens
One-day-old SPF Lohmann Select Leghorn chickens (Australian SPF Services Pty Ltd.) were used in this study. Throughout the experimental period the chickens were housed in high-efficiency particulate air-filtered negative pressure isolators, and provided irradiated food and acidified water ad libitum.
Experimental design
One hundred and eight 1-day-old chickens were separated into four groups of 27 birds. Three groups of birds were inoculated with FAdV-1, FAdV-8b or FAdV-11, and one group inoculated with sterile PBS and used as negative controls. Inoculation was performed via the ocular route with 200 µl of inoculum containing 103.7 tissue culture infective doses (TCID50) of virus (equivalent to a single dose of the live Intervet Nobilis® FAV vaccine). The dose administered was deemed to be infective, but not lethal, to allow birds to develop lesions for comparison of different viruses. Each day between days 1 and 7 post-inoculation (PI), three birds from each group were bled, euthanized and necropsied. All remaining birds were bled, euthanized and subjected to post-mortem examination on day 14 PI. All birds were monitored daily for clinical signs throughout the experiment.
All chickens were euthanized by intravenous injection of sodium pentobarbitone (Virbac Australia Pty Ltd.). Ethics approval for the animal experiment was granted by The University of Melbourne Animal Ethics Committee (Approval number 1011797).
Examination of gross and histological lesions
At each sampling point, between days 1 and 7 PI and on day 14 PI, each of the three birds per group was examined for gross lesions previously described as associated with FAdV infection. These included swelling, diffuse or focal paleness, and petechiae in the liver, paleness and swelling of the kidneys, flaccid small cloacal bursae, atrophied lobes of the thymus and erosions in the gizzard koilin/mucosa. Gross lesions were scored as 1 (present) or 0 (absent) in each tissue. Any gross lesions observed in other tissues were also noted.
The liver, kidney, thymus, cloacal bursa and gizzard were sampled and processed for histopathological examination. Samples from spleen were also taken when gross lesions were seen in these tissues. All tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned and stained with haematoxylin and eosin.
Histopathological examination of tissue sections was performed in random order and with the pathologist blinded to the group of origin. Tissues were examined for histological lesions associated with FAdV infection, including intranuclear inclusion bodies (INIBs) in the liver and kidney, lymphoid depletion in the thymus and cloacal bursa, and epithelial erosion and INIBs in the gizzard. Histological lesions were scored as 1 (present) or 0 (absent).
Examination of FAdV tissue tropism
The liver, kidney, thymus, cloacal bursa and gizzard were sampled aseptically for viral detection and identification by PCR–HRM genotyping, as described previously (Steer et al., Citation2011). A 10–25 mg sample of each tissue was used for DNA extraction, performed using the QIAxtractor and DXT extraction reagents according to the manufacturer’s instructions (Qiagen, Pty Ltd., Doncaster, Australia). Each tissue was tested in duplicate. A sample was regarded positive when both replicates amplified with similar Ct values (±3) and the products had matching HRM curve profiles.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL). The gross pathology scores, histopathology scores and virus detection results were analysed based on the disease stages characterized during the study, and the proportion of positives compared between groups and disease stages using Fisher’s exact test, with a Bonferroni correction for multiple comparisons. Differences were considered significant where P < 0.05. The strength of association between specific gross lesions, histological lesions and virus detection was determined by calculating the odds ratio (OR).
Results
Clinical signs and mortality
FAdV-8b inoculated birds exhibited mild signs of lethargy and slight greenish discoloration of urate component of the droppings (not shown) on days 5 and 6 PI. These signs were not detected in birds in any other group at any time point. Two of the FAdV-8b inoculated birds died on days 5 and 6 PI. No mortalities occurred in any other group.
Gross lesions observed in infected chickens
shows the proportion of birds in each group with gross and histological liver lesions over the course of the experiment, and the proposed stages of disease derived from these data. No gross lesions were seen in the liver of any birds from any groups from days 1 to 3 PI (, incubation). Multiple gross liver lesions were seen in all 14 FAdV-8b inoculated birds and in 9 out of 12 FAdV-11 inoculated birds examined over days 4–7 PI (, degeneration). Then only minimal swelling was observed in three out of four and five out of six birds from the FAdV-8b and FAdV-11 inoculated groups, respectively, on day 14 PI (, convalescence).
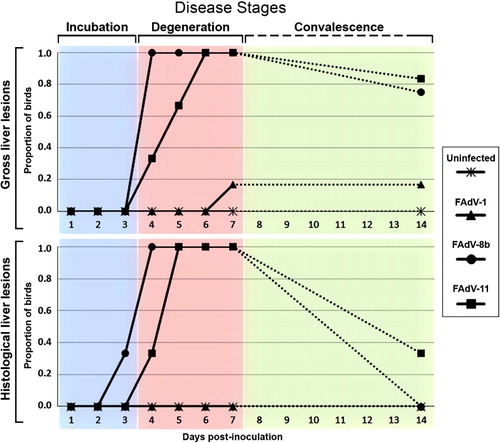
No gross lesions were seen in any tissues in the uninfected negative control birds throughout the experiment (, and ). Very few gross lesions were observed in the FAdV-1 inoculated birds, with only two birds showing liver swelling (days 7 and 14 PI) and four birds showing kidney swelling (days 6 and 14 PI; and ). No gizzard erosions were seen in any bird throughout the experiment.
In the FAdV-8b and FAdV-11 inoculated groups, enlarged spleens were observed in 10 birds from each group between 5 and 14 days PI, and 6 and 14 days PI, respectively. In all except one of these birds gross liver lesions were also present. Bilateral lung congestion was seen on days 6 and 7 PI in four out of seven birds in the FAdV-8b inoculated group and in five out of six birds in the FAdV-11 inoculated group.
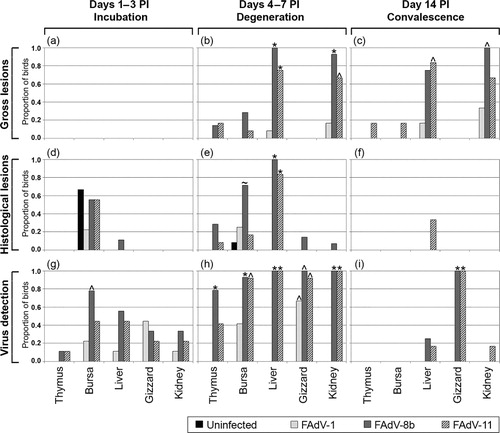
Statistical analysis based on the proposed three disease stages, as shown in , revealed no significant differences in the gross lesions of any tissues between the groups in the incubation stage (days 1–3 PI). However, during the degenerative stage (days 4–7 PI), significant differences were evident in the proportion of birds with gross lesions in the liver and kidneys between the uninfected group and the FAdV-8b and FAdV-11 inoculated groups, as well as between the FAdV-1 inoculated group and the FAdV-8b inoculated group (). In the convalescence stage (day 14 PI), there were significant differences between the proportion of uninfected birds and the proportion of FAdV-8b inoculated birds with kidney lesions, and between the proportion of uninfected birds and the proportion of FAdV-11 inoculated birds with liver lesions (). No significant differences were seen in the gross lesions of the thymuses, bursae and gizzards of birds between the different groups throughout the three disease stages.
Histological lesions in liver, kidney and gizzard
There were no IBH-related histopathological changes in the liver (Supplementary Figure 1a and b) or kidneys, nor any epithelial erosions in the gizzard, of the uninfected or FAdV-1 inoculated birds at any stage of the study. On day 6 PI, the gizzard of two FAdV-1 inoculated birds had small focal areas of koilin disruption, admixed with cell debris, with small numbers of heterophils in the underlying lamina propria. Two uninfected birds had similar lesions in the gizzard on day 14 PI.
In the FAdV-8b inoculated group, hepatic necrosis, often in combination with hepatitis (Supplementary Figure 1c), was observed in all birds between days 3 and 14 PI, with the exception of one bird on day 3 PI. There were INIBs in the hepatocytes (Supplementary Figure 2a) of 15 of these 21 birds. Histopathological changes were seen in the kidneys of two birds, with a focal area of necrosis and lymphocytic infiltration seen in one bird on day 4 PI, and rare INIBs seen in another on day 6 PI (Supplementary Figure 3a). Rare foci of koilin disruption were seen in the gizzard of one bird each on days 4, 7 and 14 PI, similar to the changes seen in birds in the uninfected and FAdV-1 inoculated groups. INIBs were infrequently seen in superficial mucosal epithelial cells in the gizzard of one bird each on days 6 and 7 PI (Supplementary Figure 3b).
Histological lesions similar to those seen in the FAdV-8b inoculated group were seen in the FAdV-11 inoculated group, with hepatic necrosis and hepatitis (Supplementary Figure 1d) in one bird on day 4 PI, and in all birds thereafter, with INIBs seen in the hepatocytes (Supplementary Figure 2b) of 12 of these 16 birds. Rare foci of koilin disruption were seen in the gizzard of two birds on day 7 PI and one bird on day 14 PI. No changes were seen in the kidneys of any FAdV-11 inoculated birds during the study.
Statistical analysis revealed no significant differences in the proportions of birds with histological lesions between any groups for any tissues examined in the incubation stage (days 1–3 PI; ), or convalescence stage (day 14 PI; ). Significant differences were evident during the degenerative stage (4–7 days PI) in the proportion of birds with histological liver lesions between the FAdV-8b inoculated group and both the uninfected and FAdV-1 inoculated groups, as well as between the FAdV-11 inoculated group and both the uninfected and FAdV-1 inoculated groups (). There was no significant difference in the histological observations of the gizzards and kidneys of birds in the different groups during the degenerative stage.
Histological lesions in primary and secondary lymphoid tissues
No histological lesions were found in the thymuses of uninfected and FAdV-1 inoculated birds throughout the study. Mild atrophy of the bursal follicles was observed in seven uninfected birds between 1 and 4 days PI, and in five FAdV-1 inoculated birds between 1 and 7 days PI.
In the FAdV-8b inoculated birds, the thymus was found to have atrophy of the cortex and medulla in one and three birds on days 6 and 7 PI, respectively (Supplementary Figure 4b). Cloacal bursal lymphoid atrophy, occasionally with clusters of heterophils within the follicles, was also seen in 15/21 birds between days 1 and 7 PI (Supplementary Figure 5b).
In the FAdV-11 inoculated group, thymic atrophy was seen in one bird on day 6 PI, and bursal atrophy in 7 birds between days 1 and 6 PI.
The spleen was also collected for histopathological examination from those birds with enlarged spleens, as well as from birds with normal spleens that were necropsied on the same day. All spleens examined from uninfected and FAdV-1 inoculated birds were histologically unremarkable. Histopathological changes were seen in the spleens of 7/11 birds in the FAdV-8b inoculated group between days 5 and 7 PI, and in one bird on day 14 PI, as well as in 5/6 birds in the FAdV-11 inoculated group on days 6 and 7 PI. The lesions included diffuse lymphoid depletion in the white pulp (Supplementary Figure 6b), and change in morphology (from spindloid to polygonal with abundant eosinophilic cytoplasm) of the cells surrounding the sheathed capillaries (Supplementary Figure 7b and c). In some birds the red pulp was also depleted (Supplementary Figure 7c). With the exception of the FAdV-8b inoculated bird on day 14 PI, these changes in the spleen were seen only in birds in which INIBs were detected in the liver.
Statistical analysis of the proportion of birds with histological lesions revealed that the FAdV-8b inoculated group had a significantly higher proportion of birds with histological bursal lesions than both the uninfected and FAdV-11 inoculated groups during the degenerative stage (days 4–7 PI; ). There was no significant difference in the histological observations of the bursae of birds in the different groups during the incubation and degenerative stages, or that of the thymuses during any stage of the study (, and ).
Virus detection in tissues of infected chickens
No FAdV was detected by PCR-HRM in the tissues of the uninfected birds at any time point. In the FAdV-1 inoculated group virus was first detected on day 2 PI in the gizzard of one bird and in the liver of another bird. Virus was also detected in the bursa and/or the gizzard of one or more birds each day on days 3–7 PI and in the kidney of one bird on day 4 PI. No virus was detected in the thymus of any FAdV-1 inoculated birds at any stage.
In the FAdV-8b inoculated group, virus was detected in the bursae of all birds between 1 and 7 days PI, with the exception of two birds on day 1 PI, and one bird on day 4 PI. Virus was detected in the liver of two birds on day 2 PI, and in the thymus of one bird on day 3 PI, then in the liver, gizzard and kidney of all birds between days 3 and 7 PI, as well as in the thymus of all birds between days 5 and 7 PI. On day 14 PI, virus was detected in the gizzard of all FAdV-8b inoculated birds and in the liver of one bird.
In the FAdV-11 inoculated group, virus was first detected on day 2 PI in all five tissues of one bird, and in the bursa of another bird. On day 3 PI, FAdV-11 was detected in the bursa, liver and kidney of one bird, in the bursa and liver of one bird, and in the liver and gizzard of one bird. FAdV-11 was then detected in the bursa, liver, gizzard and kidney of most birds between days 4 and 7 PI, as well as in the thymus on days 6 and 7 PI. On day 14 PI, virus was detected in the gizzard of all birds, as well as in the liver and kidney of one bird.
Over the incubation stage (1–3 days PI) there was a significant difference between the FAdV-8b inoculated group and the uninfected group in the proportion of birds with detectable virus in the bursa (). Significant differences were evident during the degenerative stage (4–7 days PI) between the FAdV-8b inoculated group and both the uninfected and FAdV-1 inoculated groups in the frequency of virus detection in the thymus, bursa, liver and kidney. Also a significant difference was detected between the FAdV-11 inoculated group and both the uninfected and FAdV-1 inoculated groups in the frequency of virus detection in the liver and kidney (). The frequency of virus detection in the bursa in the FAdV-11 inoculated group differed significantly from that in the uninfected group, and the frequency of detection in the gizzard of all three FAdV inoculated groups differed significantly from that in the uninfected group. On day 14 PI, both the FAdV-8b and FAdV-11 inoculated groups had significantly higher rates of virus detection in the gizzard than either the FAdV-1 inoculated group or uninfected group ().
Relationships between studied parameters
There was found to be a relationship between certain lesions in various tissues and the presence of virus. In the degenerative stage between days 4 and 7 PI, there was a positive relationship between gross and histological lesions in the liver, with an OR of 575 (P < 0.001), as well as between gross lesions and virus detection in the liver (OR = 176, P < 0.001), and between histological lesions and virus detection in the bursa (OR = 4.9, P = 0.029). There was also a positive relationship between gross lesions in the kidney and the liver in both the degenerative (OR = 84, P < 0.001) and convalescence stages (OR = 11.7, P = 0.02), and between virus detection in the liver and virus detection in the bursa, gizzard or kidney in the degenerative stage (P < 0.001).
Where spleen was collected at necropsy and examined histologically, there was a positive relationship between the gross lesion of enlarged spleen and histological lesions in the spleen (OR = 64, P < 0.001), as well as histological lesions in the liver (OR = 100.4, P < 0.001).
Discussion
FAdV-8b and FAdV-11 have been identified in several countries in association with IBH (Ojkic et al., Citation2008; Lim et al., Citation2011; Zadravec et al., Citation2011; Hess, Citation2013), as well as with HPS (Nakamura et al., Citation2000) and gizzard erosion (Okuda et al., Citation2004). The primary purpose of this investigation was to determine if field strains of FAdV-1, FAdV-8b and FAdV-11, all isolated in association with IBH field outbreaks without evidence of immunosuppression, are primary pathogens. It is known that IBH may start in birds as young as 7 days old (Hess, Citation2013). Our previous study has also shown that signs and lesions of IBH could be seen in birds as young as 9 days of age, with the speculation that these cases are the result of vertical transmission occurring in the first few days of life (Steer et al., Citation2011). Therefore, birds of a susceptible age (1-day-old) were used in this study. The selection of time points was designed to capture the full disease period and monitor the progress, development and severity of disease, as well as indicators associated with recovery. Sequential pathology of recombinant FAdV-1 CELO strains (Le Goff et al., Citation2005) and a FAdV-8 strain (Reece et al., Citation1987) has been studied in normal and cyclophosphamide treated chickens, respectively. The pathology of FAdV-11 infection was examined over four time points (Mendelson et al., Citation1995), and several FAdV-E serotypes were studied with dose rate and mortality as a measure of virulence (Erny et al., Citation1991). Also kinetics of FAdV-8 has been studied in detail in SPF chickens (Saifuddin & Wilks, Citation1991). However, there appears to be no report comparing in detail the gross and microscopic lesions as well as viral presence of three different serotypes of FAdV belonging to different species groups. It is notable that Cook (Citation1983) investigated the serological response of six different FAdVs and their viral load in a large number of tissues but their study did not include histological examinations. The present study provides a more detailed insight into the pathogenesis of three FAdV serotypes following infection through a natural route. The inclusion of FAdV-11 in this study, as an emerging serotype in many countries, was particularly important since there are limited reports examining its pathogenicity and tissue tropism in detail, especially in side-by-side comparison with those of FAdV-8b.
In this study, both FAdV-8b and 11 produced gross and histological lesions in the absence of any immunosuppression using ocular route of inoculation. Very few experimental studies have been able to induce IBH disease using natural routes of infection in young SPF chickens (Hess, Citation2013), and where this has been achieved the dose of virus inoculated was greater than that used in this study (Cook, Citation1983; Saifuddin & Wilks, Citation1991; Lim et al., Citation2011). Ocular inoculation of the birds with serotypes 8b and 11 led to an incubation period of 3 days, followed by a degenerative period between days 4 and 7 PI, when the severity and prevalence of gross and histological lesions were greatest (). Although still present in some birds, overall fewer and less severe gross and histological lesions were seen on day 14, indicating commencement of convalescence. The absence of histological liver/kidney lesions in infected birds, despite the presence of minor gross lesions (), was likely due to the criteria used for evaluation of histological lesions. For example, liver/kidney paleness or enlargement could be caused by mild oedema or congestion, which were not used as criteria for assessment of the histological lesions here.
The peak in the disease in the FAdV-8b and FAdV-11 inoculated groups, as determined by the quantity and severity of hepatic lesions, was at days 5 and 6 PI, respectively. This suggested that FAdV-8b may have a shorter incubation period than FAdV-11. As the lesions observed were overall slightly more severe in the FAdV-8b inoculated group and this was the only group in which mortalities occurred, this suggests that this virus may also be more virulent than the FAdV-11 strain. Elucidation of the detailed molecular pathogenesis of these FAdVs, including sequence and expression analysis of the hexon and fibre genes (Pallister et al., Citation1996), may shed light on the differences in expression of disease observed between groups inoculated with these different FAdV serotypes.
The association between gross lesions, histological lesions and virus detection in the liver during the degenerative stage is logical, since this represents the most virulent and destructive stage of the infection. Although the tissue tropism differed, similar findings were reported for experimentally induced gizzard erosions by inoculation with FAdV-1 (Grafl et al., Citation2013). Noteworthy is the relationship between histopathology and virus detection in the bursa during the degenerative stage, indicating the involvement of this primary lymphoid organ in the pathology of FAdV infection.
FAdV-1 was the first FAdV to be characterized and was found to be lethal for chicken embryos when vertically transmitted (Hess, Citation2013). During the last 15 years some FAdV-1 strains have been shown to cause gizzard erosions in broiler chickens in Japan (Abe et al., Citation2001; Okuda et al., Citation2001; Nakamura et al., Citation2002; Ono et al., Citation2003), several European countries (Manarolla et al., Citation2009; Marek et al., Citation2010; Domanska-Blicharz et al., Citation2011; Grafl et al., Citation2012; Kaján et al., Citation2013; Schade et al., Citation2013) and in Korea (Lim et al., Citation2012). The FAdV-1 field isolate used in this study was isolated from the bursa of a healthy broiler chicken, as in other studies in which serotype 1 isolates were obtained from healthy birds (Saifuddin et al., Citation1992; Nakamura et al., Citation2002). It is believed that the strain of FAdV-1 used in our study may not have been a true representative of this serotype. Inter-strain variation in pathogenicity of a FAdV serotype has been reported (Pallister et al., Citation1993; Okuda et al., Citation2006; Marek et al., Citation2010; Grafl et al., Citation2014). The FAdV-1 isolate used in our study was included since it was the only contemporary FAdV-1 isolate available in Australia and its pathogenicity and role in IBH outbreaks in Australia were unknown. The contrast in pathogenicity of the FAdV-1 isolate used here and that of the ones examined by other workers (Okuda et al., Citation2001; Domanska-Blicharz et al., Citation2011; Lim et al., Citation2012; Grafl et al., Citation2013) was highlighted in our study. Interestingly, some strains of FAdV-8 have been implicated in cases with both gizzard erosions and IBH (Okuda et al., Citation2004).
In the present study no gross lesions were observed in the gizzard of any birds, regardless of group, with only a small number of minor histopathological changes noted in a few birds and not attributable to FAdV infection. However, the high prevalence of virus in the gizzard during the degenerative and convalescent stages, in the absence of gross and histological lesions, is interesting and may indicate that serotypes 8b and 11, and perhaps other IBH associated FAdVs, have a predilection for gizzard, contributing to their infectivity and in-vivo propagation. These findings, together with the fact that FAdV-1 was detectable in the gizzard, but not in the liver (with the exception of one bird during the incubation stage), suggests that the epithelial lining of the gizzard, and perhaps also of the remaining sections of the alimentary tract, may serve as a reservoir of FAdV and play a role in transmission by shedding it long after typical liver lesions have resolved. Interestingly, this is consistent with pathogenic FAdV-1 strains that can induce gizzard erosions in chickens (Grafl et al., Citation2013), as well as some human adenoviruses that specifically attach to and infect epithelial cells of the alimentary system (Favier et al., Citation2004). In a previous study, following ocular inoculation with FAdV-8 at a similar age but with a higher dose of virus than that used in this study, viral antigens were detected in the epithelial cells of the intestine up to 13 days PI, but only low levels of antigen were detected in the proventriculus in a small proportion of birds between 2 and 5 days PI (Saifuddin & Wilks, Citation1991). The application of techniques such as DNA in situ hybridization (Latimer et al., Citation1997) or immunocytochemistry (Saifuddin & Wilks, Citation1991) on the histological samples acquired in this study would further elucidate the role that subclinical infection of the epithelial lining of the gizzard might play in persistent dissemination of the virus.
In this study gross and microscopic evidence of lymphoid depletion was observed in primary lymphoid tissues, thymus and cloacal bursa, of the birds inoculated with FAdV-8b and 11. It is possible that this is at least partially due to recruitment of lymphocytes in other (target) organs, especially liver. However, lymphocytic inflammatory cell infiltration is not a main feature of IBH, particularly during the degenerative phase of the disease. This, together with the association of lesions with the presence of virus in the bursa, suggests that these FAdV-8b and 11 strains may have a role to play as immunosuppressive agents. It is also notable that FAdV-1 was detected in cloacal bursa too. Interestingly, this isolate was originally isolated from cloacal bursa of an asymptomatic healthy chicken (Steer et al., Citation2011). Previous reports have suggested the possible immunosuppressive function of certain strains of FAdV-1 (Singh et al., Citation2006) and FAdV-8 (Saifuddin & Wilks, Citation1992). Evaluation of bursal lesions was made visually in this study but given that all gross lesions were examined by a single operator, and that a scoring system was applied, the results are considered non-subjective. Further studies investigating the role of FAdV in bursal atrophy should use bursa/body weight ratio. Caecal tonsils were not included in this examination as there was no previous report on involvement of this tissue in field cases of IBH, nor were lesions detected in caecal tonsils of the birds examined in this study.
Enlarged spleen (as a secondary lymphoid organ) was also observed in birds inoculated with FAdV-8b or FAdV-11 (evident at day 5 PI and thereafter), prompting the collection of this tissue for histological examination. An enlarged spleen is rarely reported in association with IBH in the literature (Saifuddin & Wilks, Citation1992; Zadravec et al., Citation2013), and when reported has been more frequently associated with HPS (Cowen, Citation1992; Asrani et al., Citation1997). The observation of an enlarged spleen at necropsy in FAdV-8b and FAdV-11 inoculated birds in this study coincided with the histological observation of morphological changes of some cells and depletion of the white pulp, as well as the observation of INIBs in the liver. Together with the results seen in the primary lymphoid tissues, this supports the concept that infection with pathogenic FAdVs can suppress the humoral and cellular immune response of chickens (Singh et al., Citation2006; Schonewille et al., Citation2008).
In conclusion, this study emphasizes the potential of FAdVs to act as primary pathogens, and adds support to their role in immunosuppression. Given the global nature of the poultry industry and the detection of FAdV-8b and FAdV-11 in many countries (Gomis et al., Citation2006; Lim et al., Citation2011; Hess, Citation2013), this information has implications for the poultry industry worldwide, as control of immunosuppressive agents such as chicken anaemia virus and infectious bursal disease virus alone may not fully control IBH.
Supplemental Data
Supplemental data for this article can be accessed here.
Supplementary figure 7.jpg
Download JPEG Image (1.3 MB)Supplementary figure 6.jpg
Download JPEG Image (1.2 MB)Supplementary figure 5.jpg
Download JPEG Image (912.1 KB)Supplementary figure 4.jpg
Download JPEG Image (902.8 KB)Supplementary figure 3.jpg
Download JPEG Image (533.1 KB)Supplementary figure 2.jpg
Download JPEG Image (528.1 KB)Supplementary figure 1.jpg
Download JPEG Image (1.7 MB)Acknowledgements
This research was partly conducted within the Poultry Cooperative Research Centre, established and supported under the Australian Government’s Cooperative Research Centres Program. The primary author was supported by scholarships from The University of Melbourne and the Poultry Cooperative Research Centre. The authors would like to thank Garry Anderson for assistance with statistical analyses, Kylie Hewson, Anthony Chamings and Mauricio Coppo for technical assistance, and Cheryl Colson, June Daly and Jenece Wheeler for assistance with animal experiment procedures.
References
- Abe, T., Nakamura, K., Tojo, T. & Yuasa, N. (2001). Gizzard erosion in broiler chicks by group I avian adenovirus. Avian Diseases, 45, 234–239.
- Aly, M.M., Smith, E.J. & Fadly, A.M. (1993). Detection of reticuloendotheliosis virus infection using the polymerase chain reaction. Avian Pathology, 22, 543–554.
- Asrani, R.K., Gupta, V.K., Sharma, S.K., Singh, S.P. & Katoch, R.C. (1997). Hydropericardium-hepatopathy syndrome in Asian poultry. Veterinary Record, 141, 271–273.
- Bagust, T.J., Fenton, S.P. & Reddy, M.R. (2004). Detection of subgroup J avian leukosis virus infection in Australian meat-type chickens. Australian Veterinary Journal, 82, 701–706.
- Bruhn, S., Bruckner, L. & Ottiger, H.-P. (2005). Application of RT-PCR for the detection of avian reovirus contamination in avian viral vaccines. Journal of Virological Methods, 123, 179–186.
- Cook, J.K. (1983). Fowl adenoviruses: studies on aspects of the pathogenicity of six strains for 1-day-old chicks. Avian Pathology, 12, 35–43.
- Cowen, B.S. (1992). Inclusion body hepatitis-anaemia and hydropericardium syndromes: aetiology and control. World’s Poultry Science Journal, 48, 247–254.
- Domanska-Blicharz, K., Tomczyk, G., Smietanka, K., Kozaczynski, W. & Minta, Z. (2011). Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poultry Science, 90, 983–989.
- Erny, K.M., Barr, D.A. & Fahey, K.J. (1991). Molecular characterization of highly virulent fowl adenoviruses associated with outbreaks of inclusion body hepatitis. Avian Patholology, 20, 597–606.
- Fadly, A.M., Winterfield, R.W. & Olander, H.J. (1976). Role of the bursa of Fabricius in the pathogenicity of inclusion body hepatitis and infectious bursal disease viruses. Avian Diseases, 20, 467–477.
- Favier, A.L., Burmeister, W.P. & Chroboczek, J. (2004). Unique physicochemical properties of human enteric Ad41 responsible for its survival and replication in the gastrointestinal tract. Virology, 322, 93–104.
- Fenton, S.P., Reddy, M.R. & Bagust, T.J. (2005). Single and concurrent avian leukosis virus infections with avian leukosis virus-J and avian leukosis virus-A in Australian meat-type chickens. Avian Pathology, 34, 48–54.
- Ghorashi, S.A., Noormohammadi, A.H. & Markham, P.F. (2010). Differentiation of Mycoplasma gallisepticum strains using PCR and high-resolution melting curve analysis. Microbiology, 156, 1019–1029.
- Gomis, S., Goodhope, A.R., Ojkic, A.D. & Willson, P. (2006). Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Diseases, 50, 550–555.
- Grafl, B., Aigner, F., Liebhart, D., Marek, A., Prokofieva, I., Bachmeier, J. & Hess, M. (2012). Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathology, 41, 599–604.
- Grafl, B., Liebhart, D., Gunes, A., Wernsdorf, P., Aigner, F., Bachmeier, J. & Hess, M. (2013). Quantity of virulent fowl adenovirus serotype 1 correlates with clinical signs, macroscopical and pathohistological lesions in gizzards following experimental induction of gizzard erosion in broilers. Veterinary Research, 44, 1–8.
- Grafl, B., Prokofleva, I., Wernsdorf, P., Steinborn, R. & Hess, M. (2014). Infection with an apathogenic fowl adenovirus serotype-1 strain (CELO) prevents adenoviral gizzard erosion in broilers. Veterinary Microbiology, 172, 177–185.
- Grgic, H., Yang, D.H. & Nagy, E. (2011). Pathogenicity and complete genome sequence of a fowl adenovirus serotype 8 isolate. Virus Research, 156, 91–97.
- Grimes, T.M., Fletcher, O.J. & Munnell, J.F. (1978). Comparative study of experimental inclusion body hepatitis of chickens caused by two serotypes of avian adenovirus. Veterinary Pathology, 15, 249–263.
- Grimes, T.M., King, D.J. & Kleven, S.H. (1976). Application of a microtiter cell-culture method to characterization of avian adenoviruses. Avian Diseases, 20, 299–301.
- Hauptli, D., Bruckner, L. & Ottiger, H.P. (1997). Use of reverse transcriptase polymerase chain reaction for detection of vaccine contamination by avian leukosis virus. Journal of Virological Methods, 66, 71–81.
- Hess, M. (2013). Avidenovirus infections. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V. L. Nair (Eds.). Diseases of Poultry 13th edn (pp. 290–300). Ames, IA: Wiley-Blackwell.
- Jack, S.W. & Reed, W.M. (1990). Further characterization of an avian adenovirus associated with inclusion body hepatitis in bobwhite quails. Avian Diseases, 34, 526–530.
- Jeffery, N., Gasser, R.B., Steer, P.A. & Noormohammadi, A.H. (2007). Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single-copy region. Microbiology, 153, 2679–2688.
- Kaffashi, A., Noormohammadi, A.H., Allott, M.L. & Browning, G.F. (2006). Viral load in 1-day-old and 6-week-old chickens infected with chicken anaemia virus by the intraocular route. Avian Pathology, 35, 471–474.
- Kaján, G.L., Kecskeméti, S., Harrach, B. & Benko, M. (2013). Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Veterinary Microbiology, 167, 357–363.
- Kawamura, H. & Horiuchi, T. (1964). Pathological changes in chickens inoculated with CELO virus. National Institute of Animal Health Quarterly, 4, 31–39.
- Latimer, K.S., Niagro, F.D., Williams, O.C., Ramis, A., Goodwin, M.A., Ritchie, B.W. & Campagnoli, R.P. (1997). Diagnosis of avian adenovirus infections using DNA in situ hybridization. Avian Diseases, 41, 773–782.
- Le Goff, F., Mederle-Mangeot, I., Jestin, A. & Langlois, P. (2005). Deletion of open reading frames 9, 10 and 11 from the avian adenovirus CELO genome: effect on biodistribution and humoral responses. Journal of General Virology, 86, 2019–2027.
- Lim, T.H., Kim, B.Y., Kim, M.S., Jang, J.H., Lee, D.H., Kwon, Y.K., Lee, J.B., Park, S.Y., Choi, I.S. & Song, C.S. (2012). Outbreak of gizzard erosion associated with fowl adenovirus infection in Korea. Poultry Science, 91, 1113–1117.
- Lim, T.H., Lee, H.J., Lee, D.H., Lee, Y.N., Park, J.K., Youn, H.N., Kim, M.S., Youn, H.S., Lee, J.B., Park, S.Y., Choi, I.S. & Song, C.S. (2011). Identification and virulence characterization of fowl adenoviruses in Korea. Avian Diseases, 55, 554–560.
- Manarolla, G., Pisoni, G., Moroni, P., Gallazzi, D., Sironi, G. & Rampin, T. (2009). Adenoviral gizzard erosions in Italian chicken flocks. Veterinary Record, 164, 754–756.
- Marek, A., Schulz, E., Hess, C. & Hess, M. (2010). Comparison of the fibers of fowl adenovirus A serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. Journal of Veterinary Diagnostic Investigation, 22, 937–941.
- Marvil, P., Knowles, N.J., Mockett, A.P., Britton, P., Brown, T.D. & Cavanagh, D. (1999). Avian encephalomyelitis virus is a picornavirus and is most closely related to hepatitis A virus. Journal of General Virology, 80, 653–662.
- McCracken, R.M., McFerran, J.B., Evans, R.T. & Connor, T.J. (1976). Experimental studies on the aetiology of inclusion body hepatitis. Avian Pathology, 5, 325–339.
- McDougall, J.S. & Peters, R.W. (1974). Avian adenoviruses: a study of 8 field isolates. Research in Veterinary Science, 16, 12–18.
- Mendelson, C., Nothelfer, H.B. & Monreal, G. (1995). Identification and characterization of an avian adenovirus isolated from a ‘spiking mortality syndrome’ field outbreak in broilers on the Delmarva Peninsula, USA. Avian Pathology, 24, 693–706.
- Naeem, K., Niazi, T., Malik, S.A. & Cheema, A.H. (1995). Immunosuppressive potential and pathogenicity of an avian adenovirus isolate involved in hydropericardium syndrome in broilers. Avian Diseases, 39, 723–728.
- Nakamura, K., Mase, M., Yamaguchi, S. & Yuasa, N. (2000). Induction of hydropericardium in one-day-old specific-pathogen-free chicks by adenoviruses from inclusion body hepatitis. Avian Diseases, 44, 192–196.
- Nakamura, K., Ohyama, T., Yamada, M., Abe, T., Tanaka, H. & Mase, M. (2002). Experimental gizzard erosions in specific-pathogen-free chicks by serotype 1 group I avian adenoviruses from broilers. Avian Diseases, 46, 893–900.
- Nakamura, K., Shoyama, T., Mase, M., Imada, T. & Yamada, M. (2003). Reproduction of hydropericardium syndrome in three-week-old cyclophosphamide-treated specific-pathogen-free chickens by adenoviruses from inclusion body hepatitis. Avian Diseases, 47, 169–174.
- Ojkic, D., Martin, E., Swinton, J., Vaillancourt, J.P., Boulianne, M. & Gomis, S. (2008). Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathology, 37, 95–100.
- Okuda, Y., Ono, M., Shibata, I. & Sato, S. (2004). Pathogenicity of serotype 8 fowl adenovirus isolated from gizzard erosions of slaughtered broiler chickens. Journal of Veterinary Medical Science, 66, 1561–1566.
- Okuda, Y., Ono, M., Shibata, I., Sato, S. & Akashi, H. (2006). Comparison of the polymerase chain reaction-restriction fragment length polymorphism pattern of the fiber gene and pathogenicity of serotype-1 fowl adenovirus isolates from gizzard erosions and from feces of clinically healthy chickens in Japan. Journal of Veterinary Diagnostic Investigation, 18, 162–167.
- Okuda, Y., Ono, M., Yazawa, S., Imai, Y., Shibata, I. & Sato, S. (2001). Pathogenicity of serotype 1 fowl adenovirus in commercial broiler chickens. Avian Diseases, 45, 819–827.
- Ono, M., Okuda, Y., Yazawa, S., Shibata, I., Sato, S. & Okada, K. (2003). Outbreaks of adenoviral gizzard erosion in slaughtered broiler chickens in Japan. Veterinary Record, 153, 775–779.
- Pallister, J., Wright, P.J. & Sheppard, M. (1996). A single gene encoding the fiber is responsible for variations in virulence in the fowl adenoviruses. Journal of Virology, 70, 5115–5122.
- Pallister, J.A., Erny, K.M. & Fahey, K.J. (1993). Serological relationships within the group E fowl adenoviruses. Intervirology, 36, 84–90.
- Raj, G.D., Sivakumar, S., Manohar, B.M., Nachimuthu, K. & Nainar, A.M. (2001). An in vitro and in vivo evaluation of the virulence of egg drop syndrome virus for the chicken reproductive tract. Avian Pathology, 30, 13–21.
- Reece, R.L., Barr, D.A. & Grix, D.C. (1987). Pathogenicity studies with a strain of fowl adenovirus serotype 8 (VRI-33) in chickens. Australian Veterinary Journal, 64, 365–367.
- Reece, R.L., Grix, D.C. & Barr, D.A. (1986). An unusual case of inclusion body hepatitis in a cockerel. Avian Diseases, 30, 224–227.
- Robertson, T., Bibby, S., O’Rourke, D., Belfiore, T., Lambie, H. & Noormohammadi, A.H. (2009). Characterization of Chlamydiaceae species using PCR and high resolution melt curve analysis of the 16S rRNA gene. Journal of Applied Microbiology, 107, 2017–2028.
- Saifuddin, M. & Wilks, C.R. (1990). Reproduction of inclusion body hepatitis in conventionally raised chickens inoculated with a New Zealand isolate of avian adenovirus. New Zealand Veterinary Journal, 38, 62–65.
- Saifuddin, M. & Wilks, C.R. (1991). Pathogenesis of an acute viral hepatitis: inclusion body hepatitis in the chicken. Archives of Virology, 116, 33–43.
- Saifuddin, M. & Wilks, C.R. (1992). Effects of fowl adenovirus infection on the immune system of chickens. Journal of Comparative Pathology, 107, 285–294.
- Saifuddin, M., Wilks, C.R. & Murray, A. (1992). Characterisation of avian adenoviruses associated with inclusion body hepatitis. New Zealand Veterinary Journal, 40, 52–55.
- Sapats, S.I. & Ignjatovic, J. (2002). Restriction fragment length polymorphism analysis of the VP2 gene of Australian strains of infectious bursal disease virus. Avian Pathology, 31, 559–566.
- Schade, B., Schmitt, F., Böhm, B., Alex, M., Fux, R., Cattoli, G., Terregino, C., Monne, I., Currie, R.J.W. & Olias, P. (2013). Adenoviral gizzard erosion in broiler chickens in Germany. Avian Diseases, 57, 159–163.
- Schat, K.A. & Sellers, H.S. (2008). Cell culture methods. In L. Dufour-Zavalar, D.E. Swayne, J.R. Glisson, J.E. Pearson, W.M. Reed, M.W. Jackwood, & P.R. Woolcock (Eds.). A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens (pp. 195–203). Athens, GA: American Association of Avian Pathologists.
- Schonewille, E., Singh, A., Gobel, T.W., Gerner, W., Saalmuller, A. & Hess, M. (2008). Fowl adenovirus (FAdV) serotype 4 causes depletion of B and T cells in lymphoid organs in specific pathogen-free chickens following experimental infection. Veterinary Immunology and Immunopathology, 121, 130–139.
- Singh, A., Grewal, G.S., Maiti, N.K. & Oberoi, M.S. (2006). Effect of fowl adenovirus-1 (IBH isolate) on humoral and cellular immune competency of broiler chicks. Comparative Immunology, Microbiology and Infectious Diseases, 29, 315–321.
- Steer, P.A., Kirkpatrick, N.C., O’Rourke, D. & Noormohammadi, A.H. (2009). Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. Journal of Clinical Microbiology, 47, 311–321.
- Steer, P.A., O’Rourke, D., Ghorashi, S.A. & Noormohammadi, A.H. (2011). Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Australian Veterinary Journal, 89, 184–192.
- Toro, H., Gonzalez, C., Cerda, L., Hess, M., Reyes, E. & Geissea, C. (2000). Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/hydropericardium syndrome. Avian Diseases, 44, 51–58.
- Winterfield, R.W., Fadly, A.M. & Gallina, A.M. (1973). Adenovirus infection and disease. I. Some characteristics of an isolate from chickens in Indiana. Avian Diseases, 17, 334–342.
- Xie, Z., Khan, M.I., Girshick, T. & Xie, Z. (2005). Reverse transcriptase-polymerase chain reaction to detect avian encephalomyelitis virus. Avian Diseases, 49, 227–230.
- Zadravec, M., Slavec, B., Krape, U., Kaján, G.L., Ranik, J., Juntes, P., Cizerl, R.J., Benko, M. & Rojs, O.Z. (2011). Inclusion body hepatitis associated with fowl adenovirus type 8b in broiler flock in Slovenia – a case report. Slovenian Veterinary Research, 48, 107–113.
- Zadravec, M., Slavec, B., Krapež, U., Kaján, G.L., Račnik, J., Juntes, P., Juršič Cizerl, R., Benkõ, M. & Zorman Rojs, O. (2013). Inclusion body hepatitis (IBH) outbreak associated with fowl adenovirus type 8b in broilers. Acta Veterinaria, 63, 101–110.
- Zhu, G.S., Ojima, T., Hironaka, T., Ihara, T., Mizukoshi, N., Kato, A., Ueda, S. & Hirai, K. (1992). Differentiation of oncogenic and nononcogenic strains of Marek’s disease virus type 1 by using polymerase chain reaction DNA amplification. Avian Diseases, 36, 637–645.
