Abstract
Two 1-year old Roulroul partridges (Rollulus rouloul), one male and one female, were presented because of eye problems and anorexia. Twenty of the 30 Roulroul partridges in the owner's collection had already died. The affected birds stopped eating, became thinner, and eventually died. Antibiotic treatment, which started because of the suspicion of a septicaemic process, was unsuccessful. At clinical examination of the two partridges it was found that in both birds, one eye ball was filled with a whitish yellow amorphous material and the other eye ball of the female showed a distinct corneal opacity. Both presented birds were euthanized. Necropsy revealed no significant abnormalities in addition to the eye lesions. Histology and immunohistochemistry of the female's eye revealed an infiltrate of T-lymphocytes corresponding to ocular lymphoma. Herpesvirus genus-specific PCR, followed by Sanger sequencing confirmed the presumptive diagnosis of Marek's disease in both birds. To our knowledge, this is the first confirmed case of infection with Gallid Herpesvirus 2 (Marek's disease virus) in partridges and the first case in this specific species.
Introduction
Marek's disease is a well-known disease in poultry (Marek, Citation1907; Biggs, Citation1967) caused by a cell-associated lymphotropic alpha-herpesvirus (Parcells et al., Citation2003; Biggs & Nair, Citation2012) which can induce tumours in different organs (e.g. liver, lungs and ovary) including the eyes (Smith et al., Citation1974; Pandiri et al., Citation2008). Chronic Marek's disease is known to affect nerves, particularly of the lumbo-sacral plexus (Biggs & Nair, Citation2012). The ocular form of the disease consists of different types of lesions depending on the anatomical structures involved. These lesions may result in blindness leading to death caused by starvation (Pandiri et al., Citation2008). The disease was first described in chickens (laying hens as well as broilers), and thoroughly studied in this species (Marek, Citation1907). Cases in turkeys (Davidson et al., Citation2002; Pennycott & Venugopal, Citation2002; Blake-Dyke & Baigent, Citation2013), quail (Coturnix coturnix japonica) (Pradhan et al., Citation1985; Imai et al., Citation1990; Pennycott et al., Citation2003) and pheasants (experimental) (Phasianus colchicus) (Lesnik et al., Citation1981), and one case in a flock of geese (Anser albifrons) (Murata et al., Citation2007) have been reported. According to Murata et al. (Citation2012), Marek's disease virus is widespread among waterfowl without causing clinical signs. These species could be considered a reservoir for other avian species. Pettit et al. (Citation1976) described macroscopic and histopathological lesions similar to those caused by Marek's disease in a black francolin (Francolinus francolinus) without the confirmation of the aetiologic agent. Jennings (Citation1954) reported a case of neural lymphomatosis in a partridge (Perdix perdix) in the UK. This bird showed enlargement of the lumbo-sacral plexus in combination with corresponding histological lesions, similar to those described in chickens (Biggs, Citation1967), but, again, an aetiologic agent could not be assigned.
Roulroul partridges (R. rouloul) are medium-sized partridges originating from Thailand and Malaysia, which are frequently kept in private and zoo collections. Marek's disease virus has not been reported previously in this species.
Materials and methods
History
In a breeding group of 30 adult Roulroul partridges (R. rouloul), over a period of 2.5 months, 20 birds died after developing a whitish yellow, amorphous material in their eyes or an opaque cornea. These Roulroul partridges were bought at the age of 2–3 months from a breeding facility in which previously chickens had been kept for several years. Other species such as black francolins (F. francolinus), blue-scaled quails (Callipepla squamata), European partridges (P. perdix) and Chinese bamboo partridges (Bambusicola thoracicus) were kept in separate cages in the same room, and showed no clinical signs or mortality. These species were bought from another breeding facility. The Roulrouls became anorectic and died approximately 10 days after the first signs. They were unsuccessfully treated orally with enrofloxacine (Baytril®, Bayer Animal Health Care) via drinking water and locally with chloramphenicol ointment (unknown origin), because of the suspicion of septicaemia after a bacterial infection. Two chicks from the affected birds (eggs laid at the onset of the eye signs), which were artificially incubated and reared (no vaccination was performed), were completely normal and in good health at 10 weeks of age (the time of presenting the adults).
Clinical examination
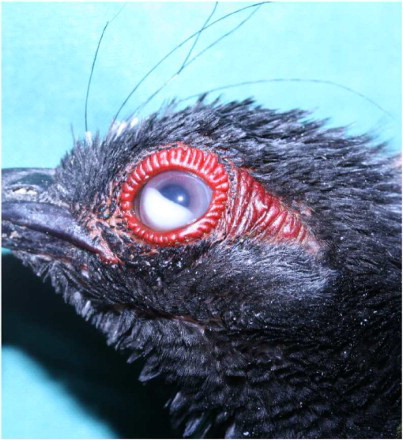
Two of the birds, one male and one female, both 1-year old, were presented. The birds displayed a poor body condition (210 g, normal bodyweight 230–250 g), were depressed and showed eye lesions, resulting in reduced eyesight. The left eye of the female had exophthalmia and a whitish yellow, amorphous granular material in the anterior eye chamber that seemed to be attached to the cornea () and the right eye showed an opacity of the cornea (). The male had exophthalmia at the right side and similar material as described in the female. The left eye appeared normal. Because of the high mortality, poor prognosis and the importance of a correct diagnosis, the birds were euthanized by an intravenous injection in the vena ulnaris of sodiumpentobarbital 0.5 ml/kg body weight (Natrium Pentobarbital®, Kela Laboratoria, Belgium) for necropsy and further examination.
Necropsy and further diagnostic procedures
The two birds were submitted for necropsy and, for each, a macroscopic evaluation of the organs and cytology (Hemacolor®, VWR International, Leuven, Belgium) was done on smears from lung, spleen, kidney, liver, crop and eye. Small and large intestines, as well as caecal content were evaluated for endoparasites.
A swab of the eyes (cornea and anterior eye chamber) and faecal material from the female were collected and routinely processed for bacteriological and mycological examination. Faecal material of the same bird was examined for the presence of Salmonella spp. Eyes including optical nerve, spleen, liver, lung, kidney, heart, proventriculus, ventriculus, intestines and adrenal glands were sampled and fixed in 10% buffered formalin. After fixation, the samples were processed for histological examination. Paraffin sections were stained with haematoxylin-eosin. Paraffin sections of the eye of the female were also stained for CD3 (T-lymphocytes) (Polyclonal Rabbit Anti-Human CD3, Dako, Glostrup, Denmark) and CD20 (B-lymphocytes) (Polyclonal Rabbit anti-Human CD20, Thermo Scientific, Fremont, CA, USA) immunohistochemistry. The former polyclonal antibody has been tested in our laboratory, and shows cross-reactivity with chicken B-lymphocytes. The latter was tested by Jones et al. (Citation1993) and found appropriate to use on chicken tissue.
A swab from the eye of the female and samples from the liver of both birds were preserved at −20°C for further molecular diagnostic procedures. DNA from these samples was extracted using the DNeasy Blood and Tissue kit (Qiagen Ltd., Crawley, UK). A nested Herpesvirus genus-specific polymerase chain reaction (PCR) was done as described by VanDevanter et al. (Citation1996) with adjustment of the annealing temperature to 43°C and 48°C for the first and second assay, respectively. This assay targeted a region of the herpes viral DNA-directed DNA polymerase gene. DNA from an avian herpesvirus (Columbid Herpesvirus 1) served as a positive control in these assays. All PCR assays were done using a Mastercycler thermal cycler (Eppendorf, Hamburg, Germany). Secondary PCR products were run on a 1.5% agarose gel stained with gelred for 75 min at 170 V, and under UV-light to evaluate the PCR results. Positive PCR products were submitted for Sanger sequencing (GATC-Biotech, Constance, Germany) using the primers from the second PCR assay. Reticuloendotheliosis virus (REV) PCR, which targeted the gp90 gene, was done as described previously by Li et al. (Citation2012). REV antigen concentrate (Charles Rivers Laboratories, Wilmington, MA, USA) served as a positive control and water as a negative. Equipment and gel electrophoresis were similar as mentioned above.
Results
Gross pathologic examination of both Roulrouls revealed no abnormalities except for the eye lesions. Cytology of the internal organs and the eyes of the female showed no abnormalities. Cytology of the right eye of the male showed heterophils, lymphocytes and coccoid bacteria, however, bacteriological and mycological examination of the eyes of both birds was negative. The faecal material tested negative for Salmonella spp.
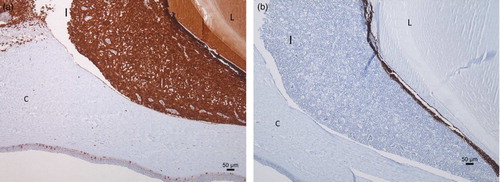
Histopathological examination of the eyes revealed a diffuse infiltration of the iris with round cells with a large central nucleus and a narrow rim of cytoplasm (). There was moderate anisokaryosis and anisocytosis. There was an average of two mitoses per high power field (HPF). These cells were also infiltrating in the corneal stroma and the corpus ciliare. Additionally, paraffin sections of the eye ball were stained with a CD3- and CD20-specific staining. The CD3-specific staining was positive ((a)) and the CD20 staining negative ((b)), meaning that the eye was infiltrated by a monomorphic population of T-lymphocytes in the absence of B-lymphocytes. Histology of the other organs revealed an infiltration of lymphoblasts in the optic nerve, ventriculus, heart, kidney, lung and adrenal glands.
Figure 3. Histopathological section (HE) of the female's iris (I) showing a diffuse infiltration with round cells with a large central nucleus and a large amount of apoptotic cell bodies (C: Cornea).
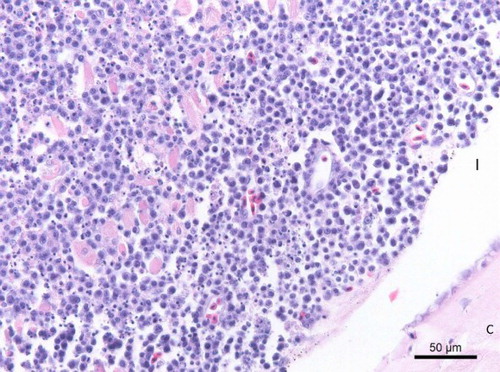
Figure 4. Immunohistochemistry of the female's eye shows a T-lymphocyte infiltration in the iris (I) (CD-3 immunohistochemistry) (a) and absence of B-lymphocyte infiltration (CD-20 immunohistochemistry) (b) (C: Cornea; L: lens).
REV PCR was negative and herpesvirus genus-specific PCR positive for the female eye swab, female and male liver. These three PCR products revealed a single band on agarose gel. To confirm the diagnosis of Marek's disease, the PCR products were sequenced. The eye revealed a sequence of 240 basepairs (bp) and the liver one of 245 bp. These sequences were compared with known sequences using the online Basic Local Alignment Search Tool (BLAST). Both sequences matched 99% with the Gallid Herpesvirus 2 (Marek's Disease virus serotype 1).
Discussion
Ocular neoplasia in birds is a rare disease, with ocular lymphomatosis in chickens being the most prevalent (Cho, Citation1974; Dukes & Pettit, Citation1983). Previous cases describing clinical signs and histologic characteristics suggestive for Marek's disease in partridges or closely related birds such as quail and francolins are rare and the aetiology has never been confirmed (Jennings, Citation1954; Biggs, Citation1967; Pettit et al., Citation1976). With recent techniques, and especially PCR, confirming the diagnosis of Marek's disease should be easier. To our knowledge, this is the first confirmed diagnosis of Marek's disease in partridges. It is remarkable that this virus has a tropism for ocular tissue in this species, and that there were no macroscopic abnormalities noticed at the internal organs, although an infiltration of lymphoblasts was present in many organs and the birds’ livers tested positive in the PCR. Ocular lesions, as the only gross anomaly in Marek's disease, have been reported previously in chickens (Ficken et al., Citation1991). This condition appears to be caused by specific isolates. But in quail, a bird species closely related to partridges, nerve lesions and ocular lesions due to Marek's disease are rare (Kenzy & Cho, Citation1969; Imai et al., Citation1991).
Pandiri et al. (Citation2008) reported that the distribution of the lymphoid infiltrates in the eye differs according to the time after infection. Lymphocytic infiltration of the iris is classified as an early lesion while late lesions consist of aggregates of lymphocytes and macrophages in the anterior chamber resulting in granular material often attached to the cornea. In this case, however, both lesions were present at the same time in one bird. Additionally, corneal oedema was present. Most likely early eye lesions were present, but obviously it was only the granular material which drew the owner's attention. These ocular changes probably result in impaired vision, followed by the inability to find food, resulting in wasting and eventually death. Blindness due to Marek's disease-associated miosis and grey iris discoloration has been described in chickens (Ficken et al., Citation1991), but was not present in this case.
Differential diagnosis in these cases includes Salmonella spp., Pasteurella multocida and Mycoplasma gallisepticum septicaemia (Bayón et al., Citation2007) and intraocular aspergillosis (Beckman et al., Citation1994). P. multocida associated ophthalmia has been reported in Turkeys (Olson, Citation1981) resulting in a similar granular material in the anterior chamber. Beckman et al. (Citation1994) reported intraocular aspergillosis in chicks which resulted in similar lesions as in the present case. Nunoya et al. (Citation1995) reported a corneal opacity in layer chickens infected with M. gallisepticum. Salmonella typhimurium has been reported as the causative agent of eye changes in young broilers (Hinz & Kaleta, Citation1970). The authors described a similar material in the anterior chamber as reported here. Bacteriological and mycological examination of the eye swab and faecal material obtained from the female was negative. Furthermore, the high morbidity and mortality, combined with the fast onset of signs and progression of the infection, are more likely to be associated with a viral pathogen. Reticuloendotheliosis virus (REV), an oncogenic retrovirus, has been described in a number of species including chickens (Robinson & Twiehaus, Citation1974), quail (C. coturnix japonica) (Carlson et al., Citation1974) and partridges (P. perdix) (Trampel et al., Citation2002) and can cause similar gross lesions to Marek's disease virus, but often limited to the intestinal tract, liver and spleen (Carlson et al., Citation1974; Trampel et al., Citation2002; Cheng et al., Citation2007). Eye lesions caused by REV are not mentioned. In the present case, there were no gross lesions seen in the internal organs as described in REV cases. Besides, both liver samples and the eye sample tested negative in the REV PCR assay.
Coccoid bacteria were observed in cytology smears of the male's eye, but cultures were not obtained. These bacteria could be part of the normal conjunctival flora (Zenoble et al., Citation1983) or could be secondary to the viral primary pathogen.
In the present case, it was not possible to identify the source of infection with certainty. The other species and specifically the other partridges showed no clinical signs. Most likely, the Roulroul partridges were infected at a young age in the breeding facility from which the birds were bought. In this breeding facility chickens were kept during the previous years. Pradhan et al. (Citation1985) already described the occurrence of Marek's disease in quail located at the same farm where there was a problem of recurrent Marek's disease among chickens.
In the present outbreak, chicks from these infected parents showed no problems (at the time of diagnosis 10 weeks old). Artificial incubation and rearing is a good preventive measure as vertical transmission of this virus has not been reported (Solomon et al., Citation1970). The other partridges showed no signs, probably because they came into contact with the virus from the Roulrouls when they already gained age-resistance. Furthermore, these partridges were bought from a different breeding facility to the Roulrouls.
In conclusion, we can state that partridges are indeed susceptible to Marek's disease virus. In the present case, noteworthy is the presence of different ocular lesions in different birds in the absence of any other signs or macroscopic lesions.
Acknowledgements
We would like to thank Dr C. Adriaensen and Dr P. Van Rooij for their skilful technical assistance.
Funding
This research was supported by the Research Fund of Ghent University, Belgium [BOF grant number 01D20312].
References
- Bayón, A., Almela, R.M. & Talavera, J. (2007). Avian ophthalmology. European Journal of Companion Animal Practice, 17, 253–266.
- Beckman, B.J., Howe, C.W., Trampel, D.W., DeBey, M.C., Richard, J.L. & Niyo, Y. (1994). Aspergillus fumigatus keratitis with intraocular invasion in 15-day-old chicks. Avian Diseases, 38, 660–665.
- Biggs, P.M. (1967). Marek's disease. Veterinary Record, 81, 583–592.
- Biggs, P.M. & Nair, V. (2012). The long view: 40 years of Marek's disease research and Avian Pathology. Avian Pathology, 41, 3–9.
- Blake-Dyke, C. & Baigent, S. (2013). Marek's disease in commercial turkey flocks. Veterinary Record, 173, 376.
- Carlson, H.C., Seawright, G.L. & Pettit, J.R. (1974). Reticuloendotheliosis in Japanese quail. Avian Pathology, 3, 169–175.
- Cheng, Z., Shi, Y., Zhang, L., Zhu, G., Diao, X. & Cui, Z. (2007). Occurrence of reticuolendotheliosis in Chinese partridge. Journal of Veterinary Medical Science, 69, 1295–1298.
- Cho, B.R. (1974). Case report: an isolation of Marek's disease herpesvirus from aqueous humor of a chicken with ocular form of Marek's disease. Avian Diseases, 18, 267–270.
- Davidson, I., Malkinson, M. & Weisman, Y. (2002). Marek's disease in turkeys. I. A seven-year survey of commercial flocks and experimental infection using two field isolates. Avian Diseases, 46, 314–321.
- Dukes, T.W. & Pettit, J.R. (1983). Avian ocular neoplasia – a description of spontaneously occurring cases. Canadian Journal of Comparative Medicine, 47, 33–36.
- Ficken, M.D., Nasisse, M.P., Boggan, G.D., Guy, J.S., Wages, D.B., Witter, R.L., Rosenberger, J.K. & Nordgren, R.M. (1991). Marek's disease virus isolates with unusual tropism and virulence for ocular tissues: clinical findings, challenge studies and pathological features. Avian Pathology, 20, 461–474.
- Hinz, K.-H. & Kaleta, E.F. (1970). Augenveränderungen bei Hühnerküken infolge Salmonella typhimurium-Infektion. [Eye changes in chickens due to Salmonella typhimurium infection]. Archiv für Geflügelkunde, 34, 37–39.
- Imai, K., Yuasa, N., Kobayashi, S., Nakamura, K., Tsukamoto, K. & Hihara, H. (1990). Isolation of Marek's disease virus from Japanese quail with lymphoproliferative disease. Avian Pathology, 19, 119–129.
- Imai, K., Yuasa, N., Furuta, K., Narita, M., Banba, H., Kobayashi, S. & Horiuchi, T. (1991). Comparative studies on pathological, virological and serological properties of Marek's disease virus isolated from Japanese quail and chicken. Avian Pathology, 20, 57–65.
- Jennings, A.R. (1954). Diseases in wild birds. Journal of Comparative Pathology and Therapeutics, 64, 356–359.
- Jones, M., Cordell, J.L., Beyers, A.D., Tse, A.G.D. & Mason, D.Y. (1993). Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. The Journal of Immunology, 150, 5429–5435.
- Kenzy, S.G. & Cho, B.R. (1969). Transmission of classical Marek's disease by affected and carrier birds. Avian Diseases, 13, 211–214.
- Lesnik, F., Pauer, T., Vrtiak, O.J., Danihel, M., Gdovinova, A. & Gergely, K. (1981). Transmission of Marek's disease to wild feathered game. Veterinarni Medicina, 26, 623–630.
- Li, K., Gao, H., Gao, L., Qi, X., Qin, L., Gao, Y., Xu, Y. & Wang, X. (2012). Development of taqman real-time PCR assay for detection and quantitation of reticuloendotheliosis virus. Journal of Virological Methods, 179, 402–408.
- Marek, J. (1907). Multiple nervenentzuedung (polyneuritis) bei huehnern. [Multiple polyneuritis in chickens]. Deutsche Tierarztliche Wochenschrift, 15, 417–421.
- Murata, S., Chang, K.-S., Yamamoto, Y., Okada, T., Lee, S.-I., Konnai, S., Onuma, M., Osa, Y., Asakawa, M. & Ohashi, K. (2007). Detection of the virulent Marek's disease virus genome from feather tips of wild geese in Japan and the far East region of Russia. Archives of Virology, 152, 1523–1526.
- Murata, S., Hayashi, Y., Kato, A., Isezaki, M., Takasaki, S., Onuma, M., Osa, Y., Asakawa, M., Konnai, S. & Ohashi, K. (2012). Surveillance of Marek's disease virus in migratory and sedentary birds in Hokkaido, Japan. The Veterinary Journal, 192, 538–540.
- Nunoya, T., Yagihashi, T., Tajima, M. & Nagasawa, Y. (1995). Occurrence of keratoconjunctivitis apparently caused by Mycoplasma gallisepticum in layer chickens. Veterinary Pathology, 32, 11–18.
- Olson, L.D. (1981). Ophthalmia in turkeys infected with Pasteurella multocida. Avian Diseases, 25, 423–430.
- Pandiri, A.K.R., Cortes, A.L., Lee, L.F. & Gimeno, I.M. (2008). Marek's disease virus infection in the eye: chronological study of the lesions, virus replication, and vaccine-induced protection. Avian Diseases, 52, 572–580.
- Parcells, M.S., Arumugaswami, J.T., Prigge, J.T., Pandaya, K. & Dienglewicz, R.L. (2003). Marek's disease virus reactivation from latency: changes in gene expression at the origin of replication. Poultry Science, 82, 893–898.
- Pennycott, T.W. & Venugopal, K. (2002). Outbreak of Marek's disease in a flock of turkeys in Scotland. Veterinary Record, 150, 277–279.
- Pennycott, T.W., Duncan, G. & Venugopal, K. (2003). Marek's disease, candidiasis and megabacteriosis in a flock of chickens (Gallus gallus domesticus) and Japanese quail (Coturnix japonica). Veterinary Record, 153, 293–297.
- Pettit, J.R., Taylor, P.A. & Gough, A.W. (1976). Microscopic lesions suggestive of Marek's Disease in a Black Francolin (Francolinus f. francolinus). Avian Diseases, 20, 410–415.
- Pradhan, H.K., Mohanty, G.C. & Mukit, A. (1985). Marek's disease in Japanese quails (Coturnix coturnix japonica): a study of natural cases. Avian Diseases, 29, 575–582.
- Robinson, F.R. & Twiehaus, M.J. (1974). Historical note: isolation of the avian reticuloendothelial virus (Strain T). Avian Diseases, 18, 278–288.
- Smith, T.W., Albert, D.M., Robinson, N., Calnek, B.W. & Schwabe, O. (1974). Ocular manifestations of Marek's disease. Investigative Ophthalmology & Visual Science, 13, 586–592.
- Solomon, J.J., Witter, R.L., Stone, H.A. & Champion, L.R. (1970). Evidence against embryo transmission of Marek's disease virus. Avian Diseases, 14, 752–762.
- Trampel, D.W., Pepper, T.M. & Witter, R.L. (2002). Reticuloendotheliosis in Hungarian partridge. Journal of Wildlife Diseases, 38, 438–442.
- VanDevanter, D.R., Warrener, P., Bennett, L., Schultz, E.R., Coulter, S., Garber, R.L. & Rose, T.M. (1996). Detection and analysis of diverse herpesviral species by consensus primer PCR. Journal of Clinical Microbiology, 34, 1666–1671.
- Zenoble, R.D., Griffith, R.W. & Clubb, S.L. (1983). Survey of bacteriologic flora of conjunctiva and cornea in healthy psittacine birds. American Journal of Veterinary Research, 44, 1966–1967.