Abstract
Ornithobacterium rhinotracheale (ORT) is a Gram-negative bacillus that causes respiratory disease in birds, and directly affects the poultry industry. The mechanisms behind these infections are not completely known. Currently, its capacity to form biofilms on inert surfaces has been reported; however, the conditions for biofilm development have not been described yet. The present work was aimed at identifying the conditions that enhance in vitro biofilm formation and development by ORT. For this, serovars A-E were analysed to assess their ability to induce biofilm development on 96-well flat-bottom polystyrene microtitre plates under diverse conditions: temperature, incubation time, and CO2 concentration. The results obtained showed not only that all serovars have the ability to produce in vitro biofilms, but also that the optimal conditions for biofilm density were 40°C after 72 h at an elevated CO2 concentration. In conclusion, ORT biofilm formation depends on the environmental conditions and may contribute to the persistence of this microorganism.
Introduction
Ornithobacterium rhinotracheale (ORT) is a Gram-negative bacterium associated with respiratory disease and septicaemia in poultry and other birds (Soriano et al., Citation2003; Espinosa et al., Citation2011). It has also been associated with retarded growth, reduction in egg production, and an increase in mortality (Gornatti et al., Citation2012). All of these conditions can lead to important economic losses to the poultry industry worldwide. To date, 18 serovars have been identified and designated from A to R (Chin et al., Citation2008); 95% of chicken infections correspond to serovar A, whereas serovars A, B, C, D, and E are the ones most often isolated in turkeys.
The ORT virulence factors are not yet fully described. This bacterium is able to adhere to tracheal cells in chickens and possesses a haemagglutinin and a neuraminidase (Soriano et al., Citation2003; Vega et al., Citation2008; De Haro-Cruz et al., Citation2013; Kastelic et al., Citation2013); additionally, it has been reported that some ORT isolates are able to produce a biofilm (Vichi et al., Citation2012). A biofilm is considered to be a complex microbial community where microorganisms synthesize an exopolysaccharide matrix that protects the microorganisms against the environment, antimicrobial agents and the immune response of the host, it can adhere to either an inert surface or a live tissue (Lasa et al., Citation2005; Vila et al., Citation2008; Castrillón et al., Citation2010). Despite the aforementioned, the conditions under which ORT is capable of forming a biofilm are still unknown; therefore, the objective of this study was to investigate these conditions: the bacterial concentration, temperature, incubation time, and oxygen potential needed by this bacterium to develop a biofilm.
Materials and methods
Bacterial strains and growth conditions
Five ORT reference strains were used, corresponding to serovars A, B, C, D, and E (). All the strains were cultured on 5% ovine blood agar (DIBICO, Mexico City, Mexico) and further incubated at 37°C in 5% CO2 for 48 h (Soriano et al., Citation2003, Vega et al., Citation2008). S. mutans ATCC 25125 was used as a positive control of biofilm-producing bacteria, and was cultured in the same medium and under the same culture conditions as ORT (Yoshida & Kuramitsu, Citation2002).
Table 1. Ornithobacterium rhinotracheale serovar reference strains used in this study.
Conditions of biofilm formation
Quantitative biofilm formation experiments were performed in 96-well flat-bottom polystyrene microtitre plates. The strains were grown in the described conditions; several colonies were gently re-suspended in 10 ml of brain-heart infusion broth (BHI) (Becton, Dickinson & Co., Mexico City, Mexico). After this, the cultures were adjusted to a final concentration of 9 × 108 CFU/ml and 200 μl of each cell suspension was transferred to microplates (Greiner, Bahlingen, Germany). To determine the effect of temperature, cultures were grown at 37°C, and 40°C for 24, 48, and 72 h in microplates without shaking. To establish the effect of elevated CO2 conditions, microplates were incubated at 37°C and 40°C for 24, 48, and 72 h in 5% CO2. After the establishment of the best conditions for biofilm formation, cells attached were carefully washed three times adding 200 μl sterile saline solution (0.85% NaCl) dried in an inverted position, and fixed with 2.5% (v/v) glutaraldehyde for 3 min at room temperature and washed again and stained with 100 μl of 0.4% crystal violet (CV) (Sigma-Aldrich Química, S.L. Toluca, Mexico) for 10 min at room temperature. The wells were then rinsed with water three times, air-dried, and biofilm-bound crystal violet was solubilized with 200 μl of 33% acetic acid. The optical density at 620 nm (OD620) was measured using an ELISA reader (BioTek-ELx808; Winooski, VT, USA). All experiments were performed in triplicate, and the mean-one standard deviation for each isolate was calculated from three independent experiments. The BHI medium and S. mutans ATCC 25125 were used as negative and positive controls for biofilm formation (Carrillo et al., Citation2010; Hu et al., Citation2010; Chen et al., Citation2012).
Statistical analyses
All experimental data were analysed by descriptive analysis and three-way analysis of variance with ANOVA and Tukey tests using the SigmaPlot software. Statistical significance was set at P < .05.
Scanning electron microscopy (SEM)
To compare biofilm formation, two different conditions were used: 37°C in aerobic conditions, and 40°C in elevated CO2 conditions. Biofilms were obtained on glass coverslips (13 mm in diameter) at 24 to 48 h, without shaking. The biofilm was washed three times with saline solution. The sample was stained in 2.5% glutaraldehyde and 1% osmium tetroxide in PBS, ethanol dehydrated, critical point dried in a CO2 atmosphere in a Samdry-780A apparatus (Tousimis Research Corporation, Rockville, MD, USA), and gold coated in a Denton Vacuum Desk II (INXS Inc., FL., USA). Coverslips containing the biofilms were attached to aluminum holders and analysed using a scanning electron microscope model JSM5800LV (JEOL, Tokyo, Japan). Digital images were obtained and processed with Adobe Photoshop software (Adobe Systems Inc., San Jose, CA, USA).
Results
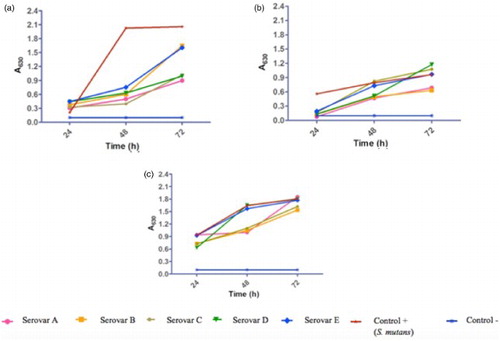
The semi-quantitative analysis of the biofilm density was performed by means of crystal violet detection. (a) and (b), show the relative densities of biofilm formed with the five ORT serovars, at different incubation times at 37°C and under aerobic conditions. All five serovars of ORT formed biofilm comparable to that formed by the S. mutans strain, which showed absorbance values of 0.126 ± 0.022, 2.016 ± 0.039, and 1.950 ± 0.021 at 24, 48, and 72 h, respectively. All ORT serovars showed increasing absorbance values that remained statistically significant until 72 h.
Figure 1. Influence of incubation time, temperature and CO2 concentration conditions in biofilm development of Ornithobacterium rhinotracheale. Serovars cells were grown in BHI medium without shaking at different experimental conditions. The amount of biofilm was determined by the crystal violet method, as described in materials and methods. Todas las cepas fueron ajustadas a un inoculo inicial de 9 × 108 (a) 37°C aerobic; (b) 40°C aerobic; (c) 40°C elevated CO2 concentration. Analysis was done with ANOVA and Tukey tests using the SigmaPlot software. Statistical significance was set at P < .05.
Influence of temperature on biofilm formation
Under aerobic conditions, we analysed the effect of temperature on biofilm formation; for S. mutans, absorbance values increased at 40°C with respect to those obtained at 37°C. Absorbance values obtained were: 0.25 ± 0.25, 0.99 ± 0.19, and 1.4 ± 0.72 at 24, 48, and 72 h, respectively. The increase in temperature yielded a higher production of biofilm at 72 h, mainly in serovars A and B, with values of 1.86 ± 0.82 and 1.91 ± 0.71, respectively, whereas serovars C, D, and E reached maximal absorbance values of 1.39 ± 0.27, 1.33 ± 0.31, and 1.54 ± 0.63, at this same time (P < .05) ((b)).
Influence of CO2 concentration on biofilm formation
The effect of CO2 concentration on biofilm production was assessed when ORT incubation conditions were kept at 40°C and using a concentration of 9 × 108 CFU/ml. Results showed biofilm development starting at 24 h of incubation, being higher at 72 h. Absorbance values obtained from the five serovars (Abs620 > 1) were close to the absorbance value of S. mutans(1.805 ± 0.26, Abs620,) at 72 h of incubation. The significant difference (P < 0.05) among the incubation times indirectly indicates the relevance of the incubation time, and reveals that all serovars have the same ability to form biofilm in vitro ((c)).
Scanning electron microscopy (SEM)
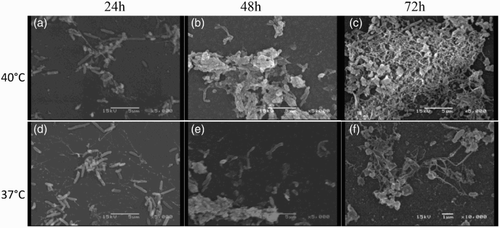
Finally, we studied, through SEM, the ultrastructural composition of the ORT biofilm obtained on a polystyrene surface at optimal growth conditions (40°C and 5% CO2) at different incubation times. At 24 h, we observed few rod-shaped bacteria on the surface of the polystyrene dish at a 5000 × magnification ((a)) with approximately 1.2 μm in length. At 48 h of cultivation, an early stage in the biofilm formation was observed ((b)). After 72 h of incubation, a flat biofilm structure was found corresponding to the typical morphology of mature biofilms ((c)). In contrast to 37°C at 24 and 48 h, we observed few rod-shaped bacteria on the surface of the polystyrene dish beginning to adhere and cluster on the surface ((d–e)), and at 72 h a biofilm that shows a less dense distribution could be observed ((f))
Figure 2. SEM micrographs of Ornithobacterium rhinotracheale serovar A. Cells were grown on glass coverslips at 37°C (aerobic) and 40°C (microaerobic) in BHI and gently resuspended in PBS liquid medium without shaking after the following incubation times: (a) 24 h (few bacterial cells attached to the surface), (b) 48 h biofilm started to form (more attached cells), and (c) 72 h (micro colonies, mature biofilm) at 40°C., (d and e) 24 and 48 h (adherence and clustering, respectively), (f) 72 h (biofilm less dense) at 37°C. Scale bar = 5 μm.
Discussion
Biofilm formation by diverse bacteria is considered an ecological niche and a protection against antimicrobial agents as of the immune response of host (Stewart & Costerton, Citation2001; Hu et al., Citation2010; Chen et al., Citation2012). In this research, we were able to confirm the capacity of ORT to form biofilm, as occurred with S. mutans, which was the strain used as the positive control for biofilm formation. It has been described that S. mutans is able to produce biofilm under diverse pH values, as well as under aerobiosis or elevated CO2 conditions and at different temperatures (Shumi et al., Citation2010; Nishimura et al., Citation2012; Welch et al., Citation2012).
The results obtained in this investigation reveal that the five ORT serovars were biofilm producers under in vitro conditions. This phenomenon was favoured by changing the temperature, and the highest biofilm production was observed at 40°C in elevated CO2 conditions. These conditions suggest a bacterial persistence that could correlate with the handling of the birds, such as overpopulation in poultry-rearing farms that could be provoking an increase in temperature and CO2 as well as a diminished oxygen concentration. Similar conditions have been reported in other bacteria that favour biofilm production, for example, Salmonella typhimurium in metallic surfaces at 42 °C produce a higher biofilm density than at 37°C. (Bottje et al., Citation1998; Barrasa et al., Citation2012; Nguyen et al., Citation2014). Another factor that could foster bacterial colonization is the increase in moisture and ammonia produced by the degradation of the poultry's excrement, leading to a more microaerobic environment (Al-Homidan et al., Citation2003; Estrada-Pareja & Márquez-Girón, Citation2005; Estrada-Pareja et al., Citation2007), as occurs with other pathogens, such as Campylobacter jejuni, in which the incubation temperature and oxygen pressure can influence biofilm formation, as a low oxygen pressure and an increased temperature favour biofilm formation (Reeser et al., Citation2007).
It is well established that biofilm formation by bacteria that cause human infections can contaminate plumbing, medical implants, heating and ventilation systems, air conditioning, and other systems. This may also occur in sheds, cages, and other surfaces in poultry farms. However, to define better the role of ORT biofilm in the persistence of the bacterium, more studies are required. Regarding the ultrastructural analysis of ORT biofilm, it is very similar to the biofilm produced by Riemerella anatipestifer, becoming more compact as time elapses and conferring greater protection against antimicrobial agents and sanitizing substances, differing only in the time of maturation, which is 24 h for R. anatipestifer whereas it is 72 h for ORT.
Finally, it is important to emphasize that the conditions assessed in this study, that is, temperature of 40°C and elevated CO2, point towards a significantly higher formation in the farm when these conditions are fostered by mismanagement such as overcrowding, poor sanitary conditions, too many birds in a cage, etc. Whilst here we show the basic conditions ORT requires for biofilm formation, it is important to further elucidate the multifactorial process that leads to biofilm formation, as well as its active involvement in bacterial pathogenesis and persistence.
Acknowledgements
We thank Edgardo Soriano-Vargas for providing strain isolates.
Funding
This study was funded by Instituto Politécnico Nacional (SIP code 20130601 and 20140864). The SIP-IPN was not involved in the development of the study design, collection, analysis, and interpretation of the data, the writing of the report nor in the decision to submit the paper for publication. Edgar Oliver López-Villegas, Graciela Castro-Escarpulliare fellows of Estímulos al Desempeño en Investigación and Comisión y Fomento de Actividades Académicas (Instituto Politécnico Nacional) and Sistema Nacional de Investigadores (SNI, CONACyT). Fernando M Guerra-Infante received SNI support and Miguel A De la Rosas-Ramos held a scholarship from CONACyT.
References
- Al-Homidan, A., Robertson, J.F. & Petchey, A.M. (2003). Review of effect of ammonia and dust concentrations on broiler performance. World's Poultry Science Journal, 59, 340–349.
- Barrasa, M., Lamosa, S., Fernandez, M.D. & Fernandez, E. (2012). Occupational exposure to carbón dioxide, ammonia and hydrogen sulphide on livestock farms in North-west Spain. Annals of Agricultural Enviromental Medicine, 19, 17–24.
- Bottje, W.G., Wang, S., Kelly, F.J., Dunster, C., Williams, A. & Mudway, I. (1998). Antioxidant defenses in lung lining fluid of broilers: impact of poor ventilation conditions. Poultry Science, 77, 516–522.
- Carrillo, Z.G., Redondo, S.M. & Arias, E.M.L. (2010). Capacidad de formación de biopelículas de cepas de Listeria monocytogenes aisladas a partir de queso de origen costarricense (Biofilm formation ability of Listeria monocytogenes strains isolated from cheese from Costa Rica). Archivos Latinoamericano de Nutrición, 60, 175–178.
- Castrillón, R.L.E., Palma, R.A. & Desgarennes, P.M.C. (2010). Importancia de las biopelículas en la práctica médica (Importance of biofilms in medical practice). Revista Mexicana de Dermatología, 54, 14–24.
- Chen, H., Yu, S., Hu, M., Han, X., Chen, D., Qiu, X. & Ding, C. (2012). Identification of biofilm formation by Mycoplasma gallisepticum. Veterinary Microbiology, 161, 96–103.
- Chin, R.P., van Empel, P.C.M. & Hafez, H.M. (2008). Ornithobacterium rhinotracheale infection. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, N.K. Nolan, & D.E. Swayne (Eds.). Diseases of Poultry 12th edn (pp. 765–774). Blackwell: Oxford, United Kingdom.
- De Haro-Cruz, M.J., Ixta-Avila, L. & Guerra-Infante, F.M. (2013). Adherence of five serovars of Ornithobacterium rhinotracheale to chicken tracheal epitelial cells. British Poultry Science, 54, 425–429.
- Espinosa, I., Colas, M., Vichi, J., Báez, M. & Martínez, S. (2011). Isolation and identification of Ornithobacterium rhinotracheale from laying hens in farms of la Habana province. Revista Salud Animal, 33, 38–43.
- Estrada-Pareja, M.M. & Márquez-Girón, S.M. (2005). Interacción de los factores ambientales con la respuesta del comportamiento productivo en pollos de engorde (Interaction of environmental factors with the response of the productive performance in broilers). Revista Colombiana de Ciencias Pecuarias, 18, 246–257.
- Estrada-Pareja, M.M., Márquez-Girón, S.M. & Restrepo, B.L.F. (2007). Efecto de la temperaturay la humedad relativa en los parámetros productivos y la transferencia de calor en pollos de engorde (Effect of temperature and relative humidity in the production parameters and heat transfer in broilers). Revista Colombiana de Ciencias Pecuarias, 20, 288–303.
- Gornatti, C.C.D., Machuca, M.A., Vigo, G.B. & Petrucelli, M.A. (2012). Ornithobacterium rhinotracheale Infection in Poultry: an update review. International Journal of Molecular Zoology, 2, 23–38.
- Hu, Q., Han, X., Zhou, X., Ding, S., Ding, C. & Yu, S. (2010). Characterization of biofilm formation by Riemerella anatipestifer. Veterinary Microbiology, 144, 429–436.
- Kastelic, S., Lucijana, R., Cizelj, I., Bencina, M., Makrai, L., Zorma, R.O., Narat, M., Bisgaard, M., Christensen, H. & Bencina, D. (2013). Ornithobacterium rhinotracheale has neuraminidase activity causing desialylation of chicken and turkey serum and tracheal mucus glycoproteins. Veterinary Microbiology, 162, 707–712.
- Lasa, J.L., Del Pozo, L., Penadés, J.R. & Leiva, J. (2005). Biofilms Bacterianos e Infección (Bacterial biofilms and infection). Anales del Sistema Sanitario Navarra, 28, 163–175.
- Nguyen, H.D.N., Yang, Y.S., & Yulk, H.G. (2014). Biofilm formation of Salmonella typhimurium on stainless steel and acrylic surfaces as affected by temperature and pH level. LWT- Food Science and Technology, 55, 383–388.
- Nishimura, J., Saite, T., Yonemaya, H., Bai, L.L., Okumura, K. & Isogai, E. (2012). Biofilm formation by Streptococcus mutans and related bacteria. Advances in Microbiology, 2, 208–215.
- Reeser, R.J., Medler, R.T., Billington, S.J., Jost, B.H. & Joens, L.A. (2007). Characterization of Campylobacter jejuni biofilms under defined growth conditions. Applied and Environmental Microbiology, 73, 1908–1913.
- Shumi, W., Lim, J., Nam, S.W., Lee, K., Kim, S.H., Kim, M.H., Cho, K.S. & Park, S. (2010). Environmental factors that affect Streptococcus mutans biofilm formation in aminofluid device mimicking teeth. Bio Chip Journal, 4, 527–563.
- Soriano, V.E., Longinos, M.G. & Fernández, R.P. (2003). Aislamiento y caracterización de Ornithobacterium rhinotracheale obtenido de pavos con enfermedad respiratoria (Isolation and characterization of Ornithobacterium rhinotracheale obtained from turkeys with respiratory disease). Veterinaria México, 34, 283–288.
- Stewart, P.S. & Costerton, J.W. (2001). Antibiotic resistance of bacteria in biofilms. The Lancet, 358, 135–138.
- Vega, V., Zepeda, A., Ramírez, S., Morales, V., Fernández, P., Montez de Oca, R., Guerra-Infante Fernando, M., Haro, C.M.J., Blackall, J.P. & Soriano, V.E. (2008). Hemagglutinating activity of serovar reference strains of Ornithobacterium rhinotracheale. Journal of Veterinary Diagnostic Investigation, 20, 353–355.
- Vichi, J., Hernández, M., Espinosa, I., Báez, M. & Martínez, S. (2012). Ornithobacterium rhinotracheale biofilm formation in abiotic surface. Revista Salud Animal, 34, 65–66.
- Vila, J., Soriano, A. & Mensa, J. (2008). Bases moleculares de la adherencia microbiana sobre los materiales protésicos. Papel de las biocapas en las infecciones asociadas a los materiales protésicos (Molecular basis of microbial adherence to prosthetic materials. Role of biofilms in infections associated with prosthetic materials). Enfermedades Infecciosas y Microbiología Clínica, 26, 48–55.
- Welch, K., Cai, Y. & Stromme, M. (2012). A method for quantitative determination of biofilm variability. Journal of Functional Biomaterials, 3, 418–431.
- Yoshida, A. & Kuramitsu, H.K. (2002). Multiple Streptococcus mutans genes are involved in biofilm formation. Applied and Environmental Microbiology, 68, 6283–6291.
