ABSTRACT
In 2010, 81 confirmed cases of Salmonella Typhimurium DT8 were reported across England and Northern Ireland – an increase of 26% from 2009 and 41% since 2008. Five cases were hospitalized and one death reported, with a strong association found between cases and the consumption of duck eggs. Once present on farms, Salmonella may become persistent and can survive for long periods of time in residual organic matter, increasing risk of infection for follow-on flocks if cleaning and disinfection is not carried out effectively. The aim of this study was to investigate the efficacy of a range of disinfectants used by the duck industry against Salmonella using laboratory models. Sixteen products were selected from seven chemical groups and the Minimum Inhibitory Concentration and Minimum Bactericidal Concentrations determined. Each product was also tested at the recommended general orders (GO) concentration using a faecal suspension model to mimic boot dips and a surface contamination model to simulate contaminated building fabric and equipment. In the faecal suspension model, all products were effective at 2 × GO concentration, and activity was more inconsistent at GO concentration. At 0.5 × GO concentration, iodine-based and quaternary-ammonium-compound-based products were significantly less effective than products within other chemical groups (P < 0.001). Glutaraldehyde-based products were significantly more effective than the other products in the surface contamination tests (P < 0.001). Chlorocresol-based products were found to be most effective for use in boot dips and aldehyde-based products for surface disinfection, although there was variability between products within a chemical group.
Introduction
In 2012 Salmonella Enteritidis and S. Typhimurium represented 41.3% and 22.1%, respectively, of all 82,409 confirmed human cases in people within the European Union (EFSA/ECDC, Citation2014). Salmonella has been linked to poultry eggs and meat for many years, with occurrences of serovars important to public health monitored and reported to the European Union under statutory regulations (EC) No 517/2011 and (EC) No 1190/2012 when isolated from chickens or turkeys (ANON, Citation2011, Citation2012). Ducks are not included in these regulations; however, there is a Duck Assurance Scheme in place in Great Britain (GB) which encourages voluntary monitoring by the industry. Denmark, Poland and Germany, also collect and submit data on Salmonella from ducks, with overall EU prevalence in flocks, reported as combined data for ducks and geese, being 4.9% for S. Typhimurium and/or S. Enteritidis in 2013; this had increased from the 3.8% reported in 2012 (EFSA/ECDC, Citation2014). During 2013, S. Indiana was the most commonly isolated serovar (39.0%) from ducks in GB, whereas in 2010 and 2011 it had been S. Typhimurium (60.4% and 77.8% of all duck Salmonella isolations, respectively); however, levels of S. Typhimurium decreased to 4.5% in 2013 (ANON, Citation2013, Citation2014).
Between January and October 2010, a total of 81 confirmed cases of ST DT8 in people had been reported across England and Northern Ireland, an increase of 26% compared with 2009 and 41% since 2008. Five cases were hospitalized and one death was reported (Noble et al., Citation2012); there were similar outbreaks in Northern Ireland and Eire (Garvey et al., Citation2013), with many cases linked with consumption of duck eggs. Once present on a farm, Salmonella can persist, even after cleaning and disinfection, can survive for long periods of time in residual organic matter and can multiply if warmth and moisture/nutrient source are provided, for example, in poorly cleaned feeders or drinkers within a re-stocked poultry house. This presents a risk for follow-on flocks introduced in previously contaminated houses, as naïve birds, especially within the first week of life or during the onset of lay, are susceptible to low levels of residual contamination (Poppe, Citation2000). The effectiveness of disinfection is largely dependent on the disinfectant chosen, its concentration and the cleanliness of the surfaces to which it is applied, and the use of a suitable disinfectant in the cleaning process has been identified as a factor likely to reduce the risk of Salmonella infection in turkey flocks (Featherstone et al., Citation2010). The environment where ducks are housed can be particularly wet, when compared to housing for chickens and the wet organic matter in the duck environment may encourage high levels of contamination. Other studies have reported successful Salmonella reduction or elimination in a farm setting (Davies & Breslin, Citation2003; Payne et al., Citation2005; Carrique-Mas et al., Citation2009; Mueller-Doblies et al., Citation2010); however, on-farm investigations are labour intensive and require access to suitable contaminated farm buildings.
Disinfectant efficacy has been evaluated in-vitro previously using a faecal suspension model and a surface contamination model to mimic conditions on chicken farms (McLaren et al., Citation2011). Chlorocresol-based products were reported to be the most efficient for eliminating Salmonella in a boot dip model, whereas quaternary-ammonium-compound (QAC)-based products, iodine-based products and peroxygen-based products were only moderately effective. Aldehyde-based disinfectants proved to be superior for disinfection of contaminated surfaces.
The objective of this study was to investigate the efficacy of a range of disinfectants against duck-related Salmonella in a laboratory setting using tests which aim to recreate on-farm situations.
Materials and methods
Bacterial isolates and inocula
Two Salmonella isolates recovered from duck-related cases, S. Typhimurium DT8 (SO5712-08) and S. Indiana (SO2626-13), were selected for inclusion in this study. The isolates were incubated overnight at 37°C on nutrient agar, and then a subculture from a single colony was placed in 10 ml nutrient broth No. 2 and incubated aerobically for 18 h at 37°C. The broth cultures were allowed to stand at room temperature for 24 h to produce a stationary phase culture before use in the testing models.
Disinfectants
The disinfectants used, their active ingredients and concentrations approved by Department for Environment, Food and Rural Affairs (Defra) general orders (GO) are detailed in . All concentrations were made using World Health Organisation (WHO) Standard Hard Water.
Table 1. Disinfectants used in the present study with active ingredients and Defra GO dilution.
Minimum inhibitory concentrations and minimum bactericidal concentrations (MIC/MBC)
MIC/MBC data were obtained for the panel of products by adding Salmonella Typhimurium DT8 (SO5712-08) and Salmonella Indiana (SO2626-13) cultures at 1 × 106 CFU/ml to a decreasing concentration of product in a 96 micro-well plate, observing at what point the disinfectant inhibited growth or became bactericidal (Andrews, Citation2001). Each product was tested in duplicate on three separate occasions. MBC was determined by taking a 10 µl aliquot from the MIC micro-well plate and incubating with 90 µl of fresh nutrient broth No. 2. Turbidity within a micro-well indicated bacterial growth and the MBC value was taken as the last well in which no growth occurred.
Model systems
Two testing models were used to simulate the use of boot dips (faecal suspension) and surface disinfection (surface contamination). These models follow the wet and dry model systems of McLaren et al. (Citation2011). Amendments to the McLaren methodology included the use of Salmonella-free duck faeces, and the inclusion of 0.3% lecithin as a neutralizer for disinfectants containing QACs.
In brief, the faecal suspension model used 1 g of duck faeces that were artificially inoculated with 5 × 108 S. Typhimurium DT8, added to 9 ml of disinfectant. Each disinfectant was prepared in triplicate at each concentration: 0.5, 1× and 2× Defra GO rate as specified on the Defra website at the time of the study. Where a product was not Defra-approved for GO, the manufacturer’s recommended concentration was used. After contact times of 30 min, 2 h and 4 h, 0.1 ml aliquots were removed, neutralized in 10 ml nutrient broth plus 5% horse serum, or 10 ml 0.3% lecithin for QACs, for at least 5 min and then 1 ml was transferred to 10 ml nutrient broth No. 2 for overnight incubation.
For the surface contamination model, wooden dowels (940 mm long × 10 mm diameter) were thoroughly coated in a slurry of duck faeces with 5 × 108 S. Typhimurium DT8 added. Dowels were allowed to air-dry for three days at room temperature. Three dowels were then placed into fresh dilutions of each disinfectant at 0.5, 1 and 2 × GO for 10 min. Dowels were removed and stored for 18 h at 15°C before being vortex-mixed for 10 s in neutralizer solution which was serially diluted in nutrient broth and incubated overnight at 37°C for semi-quantitative enumeration.
A filter paper disc method was also included in order to develop a standardized testing method that was not reliant on Salmonella-free faeces, similar to that used in model 2 by Rabie et al. (Citation2015). Whatman 6 mm filter discs (GE Healthcare, Little Chalfont, UK) were saturated by immersion with S. Typhimurium DT8 at approximately 1.8 × 107 CFU/disc. Discs were exposed to an aliquot of the disinfectant at GO concentration for either 30 or 60 min at 4°C then placed into 10 ml nutrient broth and incubated for 18 h at 37°C (Rabie et al., Citation2015).
Bacterial resuscitation and culture
Eighteen-hour cultures were inoculated onto Modified Semi-Solid Rappaport-Vassiliadis including 0.01% novobiocin (MSRV; MAST, Bootle, UK), incubated at 41.5°C for 24 h and a 1 µl loop from the edge of the opaque growth zone streaked onto Rambach agar and incubated for 18 h at 37°C. The absence of detectable Salmonella at this stage was taken as indicating an effective combination of product and concentration.
Statistical analysis
The results were analysed as binary data with either no growth observed or growth observed for each sample. Counts of the number of samples positive or negative were analysed for differences between product, and concentration effect where applicable. Data from the faecal suspension model and surface contamination model were analysed using Fishers Exact test in Stata12. MIC/MBC data for each serotype were compared using a paired t-test.
Faecal suspension data was collected on three separate occasions. Poisson distribution analysis of the counts at each occasion indicated it is reasonable to treat the three trial times as equivalent; hence the results have been pooled as independent observations. Thus the observations were treated as nine replicates for each treatment at 0.5 × GO. The small numbers in the surface contamination model required exact analysis, which was simplified by using the approximating assumption that observations at different concentrations were equivalent. Although there was evidence that the probability of growth was influenced by concentration, the analysis was balanced for the effect of concentration and can be considered robust to this inaccuracy; hence samples are pooled and treated as independent observations. The power of the analysis is therefore slightly reduced relative to a hypothetical analysis that would take account of the effect of concentration on the contrasts between disinfectants.
Results
MIC/MBC
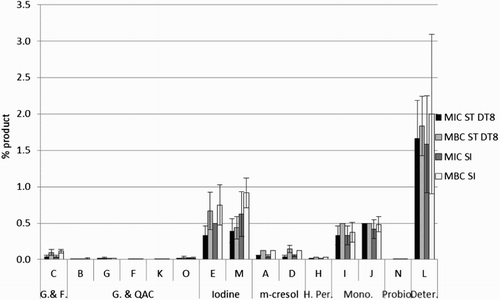
There were no significant differences between S. Typhimurium DT8 and S. Indiana for the MIC or MBC, P = 0.098 and 0.304, respectively. The QAC-based products and glutaraldehyde/QAC combination products performed well at high dilutions, whereas the iodine- and peroxymonosulphate-based products only displayed inhibitory and bactericidal properties at higher concentrations (). Products within the same chemical group had widely different MIC and MBC values. Products demonstrated bactericidal action at concentrations within one or two doubling dilutions of their MIC concentration.
Figure 1. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration for each of the disinfectant products tested against Salmonella Typhimurium (ST DT8) and Salmonella Indiana (SI). Note: G. – Glutaraldehyde, F. – Formaldehyde, QAC – Quaternary Ammonium Compound, H. Per. – Hydrogen Peroxide, Mono. – Peroxymonosulphate, Probio. – Probiotic detergent, Deter. – alkaline detergent.
Faecal suspension model
As there was no difference between the ST DT8 and S. Indiana for the MIC/MBC the DT8 strain was selected for inclusion in the faecal suspension model. The glutaraldehyde/formaldehyde/QAC combinations and chlorocresol-based products consistently eliminated Salmonella in the boot dip model, even when they were used at half the Defra GO concentration (). The majority of the other products were effective at GO and twice GO concentrations and one of the iodine-based products was effective at all concentrations tested when the contact time was greater than 30 min.
Table 2. Faecal suspension model results as counts of positive samples at 0.5, 1 and 2 times Defra GO concentrations for 30, 120 and 240 min contact times.
As there were no positive results after any treatment used at 2 × GO concentration, all products were equally effective. Data were pooled across time and the seven disinfectant groups were compared using a Fisher’s exact test at each concentration, which found strong evidence that the efficacy at 0.5 × GO differed between groups (P < 0.001). The least effective disinfectant groups contained the iodine-based products and the QAC-based products; there were insufficient data to rank the other disinfectants. There was very little evidence for differences between treatments using the same chemical group except for the two iodine treatments (P = 0.009). Salmonella survival occurred after longer contact times for four products at 0.5 × GO concentration, in one to two samples. It is likely that this concentration was on the border line in its ability to eliminate Salmonella.
Filter disc model
The only product to fail testing using the filter disc model, at GO concentration, was the glutaraldehyde/formaldehyde-based product, where Salmonella was not eliminated from eight of nine samples after 30 min contact time at 4°C. All products eliminated Salmonella after 120 min contact time.
Surface contamination
Statistical analysis found that the glutaraldehyde/formaldehyde and glutaraldehyde/QAC-based products were more effective than the other product groups, which did not significantly differ from each other. Fisher’s exact test result for all product groups P < 0.001; for product groups excluding glutaraldehyde/formaldehyde and glutaraldehyde/QAC P > 0.1. There was also marginal statistically significant evidence of differences among glutaraldehyde/QAC-based products, Fisher’s exact test P = 0.04. At GO concentration and above the glutaraldehyde/formaldehyde and four of the glutaraldehyde/QAC-based products were able to eliminate Salmonella from the wooden dowels, although effectiveness was reduced at half GO ().
Table 3. Surface contamination model results at 0.5, 1 and 2 times Defra GO concentrations (n = 3).
Discussion
This study has identified differences between disinfectant products when tested for use as a boot dip (faecal suspension model) and for surface disinfection (surface contamination model). The iodine, QAC and hydrogen peroxide-based products all performed poorly in the faecal suspension model compared to the other products tested. The same products also performed poorly in the surface contamination model, as did chlorocresol-based products, and peroxymonosulphates and the performance of glutaraldehyde and QAC-based products varied. Overall the glutaraldehyde/formaldehyde-based product and some glutaraldehyde and QAC-based products consistently inhibited Salmonella, even in the presence of faecal material and for short contact times. However in terms of suitability for use in a boot dip environment, chlorocresol-based products have been demonstrated to have a superior ability to remain stable and effective over a 14-day period than glutaraldehyde-based products (unpublished work by the author).
The surface contamination model results are comparable to those reported by McLaren et al. (Citation2011), with similar failure rates occurring between the S. Typhimurium DT8 in the present study and S. Enteritidis; however, differences were noted between products tested using the faecal suspension model. GO concentrations for disinfectants are revised over time; therefore, direct comparisons with previous studies are not always possible. The hydrogen-peroxide-based product performed well in the present study, whereas it had previously performed poorly against S. Enteritidis and chicken faeces, at a lower concentration. The glutaraldehyde/formaldehyde-based product also performed better in the present study whereas previously it had not inhibited S. Enteritidis growth after 30 min contact time; the concentration for this product was the same for both studies. McLaren et al. (Citation2011) hypothesized a difference in disinfectant effect between faecal challenges from different species and Salmonella challenges, and the variation in results with the present study may be due to the use of duck faeces containing different flora.
Payne et al. (Citation2005) reported potassium-peroxymonosulphate-based products to be the most effective products at reducing Salmonella counts in Salmonella-challenged soil samples, with the use of a QAC returning results similar to those of the control. However Mueller-Doblies et al. (Citation2010) reported that products containing a mixture of formaldehyde, glutaraldehyde and QAC performed significantly better in reducing Salmonella in turkey houses than products containing hydrogen peroxide and peracetic acid. They also reported that cleaning and disinfection was least effective in nest boxes. This may be due to the material the nest boxes are made of (often wood) being difficult to disinfect if organic matter has adhered to the surface. This was demonstrated in the surface contamination model in the present study where only the glutaraldehyde/formaldehyde and one of the glutaraldehyde/QAC-based products were effective at GO concentration. Stringfellow et al. (Citation2009) also reported a decrease in disinfectant ability to eliminate Salmonella growth when levels of organic matter were increased. Phenol-based products have been reported to be effective at reducing bacterial challenge (Stringfellow et al., Citation2009), but they are not permitted for use in many chicken layer farms due to the potential for tainting the eggs; however, the Duck Assurance Scheme does not specify any restrictions as long as the product is approved by Defra. Formaldehyde-based products were reported to be more effective at cleaning a range of surfaces found in poultry houses than glutaraldehyde/benzalkonium-chloride-based products, which performed better than a peroxygen compound (Gradel et al., Citation2004). However Wales et al. (Citation2006) identified that it was more important that the cleaning and disinfection process was carried out to a high standard rather than relying on applying the “best” disinfectant at the end of a poor process.
In conclusion, this study has demonstrated that there is a difference between disinfectants in their effectiveness to inhibit Salmonella in laboratory tests designed to mimic on-farm situations. It has also highlighted differences in efficacy depending on the situation the product is being used in, that is, boot dips or surface disinfection. The iodine, QAC and hydrogen peroxide-based products all performed poorly in the faecal suspension model compared with the other products tested, suggesting that they would not be suitable products to be used in a boot dip. They also performed poorly in the surface contamination model, designed to mimic poultry house cleaning requirements, as did the chlorocresol-based products, and peroxymonosulphates and one of the glutaraldehyde and QAC-based products. Overall the glutaraldehyde and formaldehyde-based product and one glutaraldehyde and QAC-based product consistently inhibited Salmonella growth, even in the presence of faecal material and at concentrations below GO. It is therefore vital to consider both the intended use of the disinfectant and its concentration when designing a disinfection programme. It is particularly important to use antibacterial concentrations of disinfectants when bacterial pathogens are present in poultry houses, as the concentrations that are recommended for control of exotic viral pathogens are not effective for most bacteria.
Additional information
Funding
References
- Andrews, J.M. (2001). Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 48, 5–16. doi: 10.1093/jac/48.suppl_1.5
- ANON. (2011). Commission Regulation (EU) No 517/2011 of 25 May 2011 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards a Union target for the reduction of the prevalence of certain Salmonella serotypes in laying hens of Gallus gallus and amending Regulation (EC) No 2160/2003 and Commission Regulation (EU) No 200/2010. Official Journal of the European Union, 138 (26.5.2011), 45–51.
- ANON. (2012). COMMISSION REGULATION (EU) No 1190/2012 of 12 December 2012 concerning a Union target for the reduction of Salmonella Enteritidis and Salmonella Typhimurium in flocks of turkeys, as provided for in Regulation (EC) No 2160/2003 of the European Parliament and of the Council. Official Journal of the European Union, 340 (13.12.2011), 29–34.
- ANON. (2013). Zoonoses Report 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/236983/pb13987-zoonoses-report-2012.pdf: PHE.
- ANON. (2014). Salmonella in Livestock, Quarterly update. http://ahvlaintranet/SiteCollectionDocuments/science-salmonella-quarterly-report.pdf: AHVLA.
- Carrique-Mas, J.J., Marin, C., Breslin, M., McLaren, I. & Davies, R. (2009). A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathology, 38, 419–424. doi: 10.1080/03079450903193768
- Davies, R. & Breslin, M. (2003). Persistence of Salmonella Enteritidis Phage type 4 in the environment and arthropod vectors on an empty free-range chicken farm. Environmental Microbiology, 5, 79–84. doi: 10.1046/j.1462-2920.2003.00387.x
- EFSA/ECDC. (2014). The European Union summary report on trends and sources of Zoonoses, Zoonotic agents and food-borne outbreaks in 2012. EFSA Journal 2014, 12, 3547.
- Featherstone, C.A., Reichel, R., Snow, L.C., Davies, R.H., Christiansen, K.H., Carrique-Mas, J.J. & Evans, S.J. (2010). Investigation of risk factors for Salmonella on fattening-turkey farms. Epidemiology and Infection, 138, 1427–1438. doi: 10.1017/S0950268810000312
- Garvey, P., McKeown, P., Kelly, P., Cormican, M., Anderson, W., Flack, A., Barron, S., De Lappe, N., Buckley, J., Cosgrove, C., Molloy, D., O’Connor, J., O’Sullivan, P., Matthews, J., Ward, M., Breslin, A., O’Sullivan, M.B., Kelleher, K., McNamara, A., Foley-Nolan, C., Pelly, H. & Cloak, F. (2013). Investigation and management of an outbreak of Salmonella Typhimurium DT8 associated with duck eggs, Ireland 2009 to 2011. Eurosurveillance, 18, 17–23.
- Gradel, K.O., Sayers, A.R. & Davies, R. H. (2004). Surface disinfection tests with Salmonella and a putative indicator bacterium, mimicking worst-case scenarios in poultry houses. Poultry Science, 83, 1636–1643. doi: 10.1093/ps/83.10.1636
- McLaren, I., Wales, A., Breslin, M. & Davies, R. (2011). Evaluation of commonly-used farm disinfectants in wet and dry models of Salmonella farm contamination. Avian Pathology, 40, 33–42. doi: 10.1080/03079457.2010.537303
- Mueller-Doblies, D., Carrique-Mas, J.J., Sayers, A.R. & Davies, R.H. (2010). A comparison of the efficacy of different disinfection methods in eliminating Salmonella contamination from turkey houses. Journal of Applied Microbiology, 109, 471–479.
- Noble, D.J., Lane, C., Little, C.L., Davies, R., Pinna, E.D., Larkin, L. & Morgan, D. (2012). Revival of an old problem: an increase in Salmonella enterica serovar Typhimurium definitive phage type 8 infections in 2010 in England and Northern Ireland linked to duck eggs. Epidemiology and Infection, 140, 146–149. doi: 10.1017/S0950268811000586
- Payne, J.B., Kroger, E.C. & Watkins, S.E. (2005). Evaluation of disinfectant efficacy when applied to the floor of poultry grow-out facilities. Journal of Applied Poultry Research, 14, 322–329. doi: 10.1093/japr/14.2.322
- Poppe, C. (2000). Salmonella infections in the domestic fowl. In C. Wray & A. Wray (Eds.), Salmonella in Domestic Animals (pp. 107–132). Oxon: CABI
- Rabie, A.J., McLaren, I.M., Breslin, M.F., Sayers, R. & Davies, R.H. (2015). Assessment of anti-Salmonella activity of boot dip samples. Avian Pathology, 44, 129–134. doi: 10.1080/03079457.2015.1012046
- Stringfellow, K., Anderson, P., Caldwell, D., Lee, J., Byrd, J., McReynolds, J., Carey, J., Nisbet, D. & Farnell, M. (2009). Evaluation of disinfectants commonly used by the commercial poultry industry under simulated field conditions. Poultry Science, 88, 1151–1155. doi: 10.3382/ps.2008-00455
- Wales, A., Breslin, M. & Davies, R. (2006). Assessment of cleaning and disinfection in Salmonella-contaminated poultry layer houses using qualitative and semi-quantitative culture techniques. Veterinary Microbiology, 116, 283–293. doi: 10.1016/j.vetmic.2006.04.026
