ABSTRACT
In June 2015, an infectious disease with high prevalence causing severe hydropericardium syndrome (HPS) first appeared in duck farms of northeast China. The disease showed high morbidity of 35% and mortality of 15% in a commercial duck farm with 200,000 45-day-old ducks. One strain of hypervirulent fowl adenovirus serotype 4 was identified and designated as HLJDAd15. The whole genome of the duck isolate was sequenced and found to contain the same large deletions as a genotype that has become prevalent in chickens in China recently, indicating that this disease might be transmitted from chickens to ducks. The pathogenicity of HLJDAd15 was evaluated in SPF chickens and ducks. The results showed that chickens were more susceptible to this new genotype of fowl adenovirus, and it was more difficult to infect ducks than chickens with the duck origin virus. Thus, it appears that this severe HPS in ducks is far more likely to have been transmitted from chickens to ducks than from ducks to ducks. Therefore, transmission from chickens to ducks constitutes a threat to the duck farming industry, and this transmission route is a very important consideration for the prevention and control of the new genotype of fowl adenovirus. This is the first whole genome sequence of a FAdV-4 isolated from ducks, and this information is important for understanding the molecular characteristics and evolution of aviadenoviruses. The potential risks of infection with this new hypervirulent FAdV-4 genotype in chickens and ducks urgently require an effective vaccine.
Introduction
The Adenoviridae family is currently divided into five genera: Mastadenovirus, Aviadenovirus, Atadenovirus, Siadenovirus, and Ichtadenovirus (Marek et al., Citation2013). Fowl adenoviruses (FAdV), belonging to the genus Aviadenovirus are further grouped into five species (A to E) with 12 serotypes (1 to 8a and 8b to 11) based on serum cross-neutralization tests (Mazaheri et al., Citation1998; Hess, Citation2000). Recently, at least 12 genotypes within the five species were revealed by sequence analysis of the hexon loop 1 (L1) gene region (Ganesh et al., Citation2001). Diseases with inclusion body hepatitis (IBH), hydropericardium syndrome (HPS), and gizzard erosions (GE) are always associated with FAdV infection, but most HPS is caused by FAdV serotype 4 (FAdV-4), which can cause mortality as high as 30–70% (Mase et al., Citation2012; Asthana et al., Citation2013).
FAdVs are common infectious agents in poultry, although most of them have been isolated from chickens (Eregae et al., Citation2014; Mittal et al., Citation2014; Rahimi & Minoosh, Citation2015); therefore, most complete genome sequences of FAdVs are of strains isolated from chickens with or without significant clinical signs (Sheppard et al., Citation1995; Vera-Hernández et al., Citation2015; Li et al., Citation2016). However, very recently, hydropericardium hepatitis syndrome associated with FAdV-4 infection emerged in ducks in China (Chen et al., Citation2016). Several complete genome sequences of hypervirulent FAdVs isolated from chickens have been submitted to GenBank and show some characteristics of new genotypes (Ye et al., Citation2016), but no complete genome sequence of fowl adenovirus isolated from ducks was available until now. This is the first complete genome sequence of a hypervirulent FAdV-4 isolated from ducks, which will be important for understanding the prevalence, transmission, and evolution of fowl adenovirus in different hosts.
In this study, we report on an outbreak of severe HPS with high morbidity of 35% and mortality of 15% in a farm with 200,000 ducks in northeastern China. The 45-day-old ducks showed severe hydropericardium, enlarged discoloured livers, and IBH. A viral agent, designated as HLJDAd15, was isolated from the livers of dead ducks and characterized as FAdV-4. The whole genome was sequenced, and the pathogenicity of the virus was evaluated in SPF chickens and ducks.
Materials and methods
Ethics statement
The present study was approved by the Animal Care and Use Committee of Harbin Veterinary Research Institute (Harbin, China) and performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China). All of the SPF chickens and ducks were cared for in accordance with humane procedures.
Sample collection
After HPS was identified based on the emergence of massive pericardial effusion among 45-day-old ducks from the farm in Heilongjiang province, China, 20 dead ducks were collected for laboratory diagnosis to find the causative factor. Viscera were obtained aseptically from dead ducks with severe hydropericardium suspected of carrying FAdVs infection upon post-mortem examination and fixed in 10% neutral buffered formalin. Sections were stained with haematoxylin and eosin for microscopic examination.
Nucleic acid extraction and PCR assay
DNA was extracted from liver samples using a DNeasy Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, while RNA was acquired using Trizol reagent (Ambion, Carlsbad, CA, USA) and reverse transcribed into cDNA, which was used as a template for PCR amplification.
The primers used in this study to detect the genotype of FAdV were designed based on the L1 region of the Hexon gene (FAd-F: 5′-AACTTCGACCCCATGTCGCGTCAGG-3′ and FAd-R: 5′-TGGCGAAAGGCGTACGGAAGTAAGC-3′). The PCR products were subjected to electrophoresis in a 1% (w/v) agarose gel. The PCR procedure consisted of an initial incubation for 5 min at 95°C; 35 cycles of 30 sec at 95°C, annealing for 60 sec at 55°C, and extension for 60 sec at 72°C; and a final extension for 5 min at 72°C. Samples were examined by blood agglutination assay, PCR, or RT-PCR for the presence of other DNA or RNA viruses, including avian reovirus (ARV) (Zhong et al., Citation2016), Marek’s disease virus (MDV) (Lv et al., Citation2016), infectious bursal disease virus (IBDV) (Lu et al., Citation2015), reticuloendotheliosis virus (REV) (Jiang et al., Citation2013), avian influenza virus (AIV) (Li et al., Citation2006), Newcastle disease virus (NDV) (Zhu et al., Citation2016), and avian leukosis virus (ALV) (Wang et al., Citation2014), according to previously described methods.
Complete genome sequencing
Based on the available FAdV nucleotide sequences of strains KR5 (GenBank Accession No. HE608152) and JSJ13 (KM096544), 36 pairs of specific primers () were designed to amplify the complete genome sequence of the duck isolate HLJDAd15. PCR products with expected lengths were sequenced directly or cloned into the pEASY-T1 cloning vector (TransGen, Beijing, China) according to the manufacturer’s instructions and sequenced at Jilin Comate Bioscience Co., Ltd., Changchun, China. The complete sequence was manually assembled using the Seqman programme in the DNAstar software package (version 5.01, Madison, WI, USA). A phylogenetic tree based on the complete Hexon gene was constructed using MEGA 6.0 software by the neighbour-joining method (1000 bootstrap replicates).
Table 1. The primers for complete genome sequencing of HLJDAd15.
Virus stock
Liver samples collected from five affected dead ducks were homogenized in sterile phosphate-buffered saline (PBS) to generate a 15% suspension (w/v). After three cycles of freezing and thawing, the homogenates were centrifuged at 3000×g for 30 min, and each supernatant was then passed through a 0.22-μm syringe filter and stored at −70°C until use (Kaján et al., Citation2013).
Pathogenicity in embryos
In this experiment, all SPF chicken embryos and Jinding duck (Anas platyrhynchos domesticus) embryos were obtained from the Experimental Animal Center of Harbin Veterinary Research Institute (HVRI) of the Chinese Academy of Agricultural Sciences (CAAS), China. 135 9-day-old SPF chicken embryos were randomly divided into nine groups with 15 embryos per group. Different 10-fold dilutions from 10−1 to 10−8 of HLJDAd15 (0.2 ml) were injected onto the chorioallantoic membranes of each embryo. The eggs were incubated in a humidified atmosphere (55%) at 37°C. The livers of the embryos died between 3 and 7 days were harvested and fixed in 10% neutral buffered formalin for pathological analysis. The median embryo lethal dose (ELD50) of HLJDAd15 in SPF chicken embryos was determined using the Reed–Muench method (Reed & Muench, Citation1938).
In another experiment, 105 9-day-old SPF Jinding duck embryos were randomly assigned to seven groups with 15 eggs per group. Ten-fold dilutions from 10−1 to 10−6 of liver homogenate supernatant of HLJDAd15 (0.2 ml) were injected onto the chorioallantoic membranes of the embryos. The eggs were incubated at 37°C and observed daily.
Pathogenicity in SPF chickens and ducks
To evaluate the pathogenicity of HLJDAd15, 27 35-day-old SPF chickens and 27 35-day-old SPF Jinding ducks were purchased from the Experimental Animal Center of HVRI, CAAS, China, and orally inoculated with 103.0 ELD50 doses of the HLJDAd15 strain in 0.2 ml of PBS, while another 10 SPF chickens and ducks served as negative controls. The challenged and control groups were separately housed in different negative-pressure isolators and monitored daily for 14 days. All birds were sacrificed 14 days post infection by intraperitoneal injection of sodium pentobarbital. The birds were inspected for gross lesions, and the main organs were collected for pathogen recovery and histological analysis.
Results
Clinical signs and pathological analysis
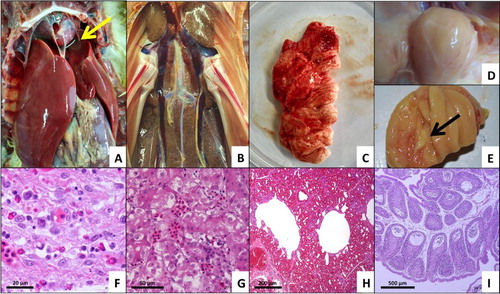
The dead ducks showed severe hydropericardium, with fluid volumes ranging from 5 to 10 ml, and enlarged, discoloured livers ((A)). Multifocal areas of necrosis and petechial haemorrhage were observed in livers and kidneys ((B)). Fibrinous and foam-like exudates were observed around the lung tissue ((C)), and the bursa was filled with caseous inflammatory exudates ((D,E)). Histological analysis showed typical IBH in the enlarged, discoloured liver ((F)), degeneration of partial renal tubular epithelial cells ((G)), and congestion in the kidney and lung ((H)). Lymphocyte reduction, follicular atrophy, and vacuole formation were also found in some of the follicles of the bursa ((I)).
Figure 1. Gross lesions and histopathology in tissues from dead ducks. (A) Severe hydropericardium with a fluid volume ranging from 5 to 10 ml, enlarged discoloured liver, and point-like necrosis in the liver (arrow). (B) Multifocal areas of necrosis and petechial haemorrhage in kidneys. (C) Fibrinous and foam-like exudates around the lung tissue. (D&E) Caseous inflammatory exudates filling the bursa (arrow). (F) Typical virus inclusion bodies in liver cells (H&E stain). (G) Degeneration of partial renal tubular epithelial cells and congestion in kidneys (H&E stain). (H) Congestion in lungs (H&E stain). (I) Lymphocyte reduction, follicular atrophy, and vacuole formation in some of the follicles of the bursa (H&E stain).
Characterization of HLJDAd15
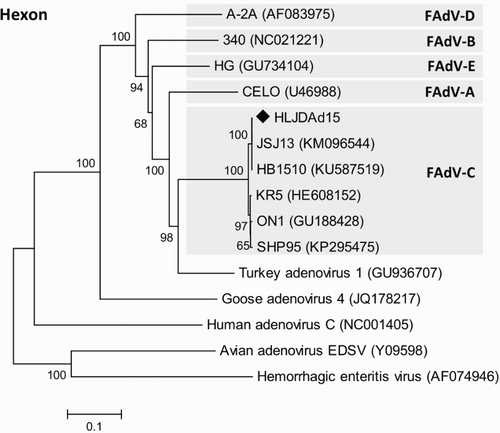
ARV, MDV, IBDV, REV, AIV, NDV, and ALV were excluded by blood agglutination assay, PCR, or RT-PCR. A 564 bp PCR product of fowl adenovirus was amplified and sequenced based on the serotype-specific primers for the L1 region of the Hexon gene. The pathogenic factor causing HPS in ducks was identified as FAdV-4 coupled with several hypervirulent strains isolated from chickens, and the isolate was designated as HLJDAd15 based on the phylogenetic analysis (). The similarity between HLJDAd15 and other FAdV-4 strains or other species of FAdVs varied from 98.6% to 100% and 50.1% to 76.2%, respectively.
Figure 2. Phylogenetic analysis of HLJDAd15 based on the hexon gene. Strain HLJDAd15 was sequenced in this study, and the sequences of the other strains were downloaded from GenBank. The phylogenetic tree was generated by the neighbour-joining method in the MEGA6.0 software. Accession numbers of published adenovirus sequences are given in the brackets.
Genome properties of the isolated HLJDAd15
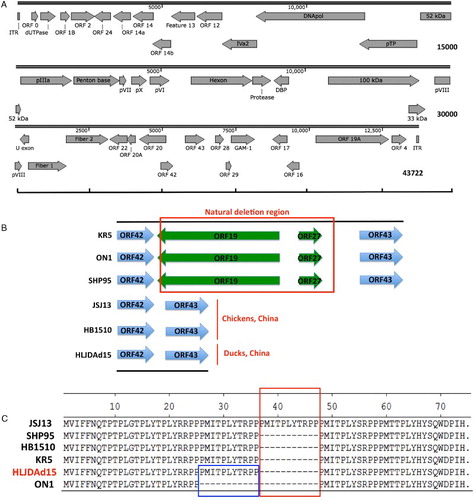
The whole genome nucleotide sequence of the HLJDAd15 isolate is available in the GenBank database under accession number KX538980. The full genome is 43722 bp in length, and the inverted terminal repeat sequence is 56 bp. A diagrammatic overview of the open reading frames (ORFs) is shown in (A). Compared with strains isolated from chickens outside of China, HLJDAd15, as well as the other two strains (JSJ13 and HB1510) isolated from chickens in China, showed a natural large genomic deletion mutation (1966 bp), and the deleted region includes two ORFs, ORF19 and ORF27 ((B)). Sequence analysis of ORF29 showed an 11-amino-acid deletion relative to the JSJ13 strain and an 11-amino-acid insertion relative to the ON1 strain and the other three strains ((C)).
Figure 3. Genome properties of the isolate HLJDAd15. (A) Schematic representation of genome size and organization of the HLJDAd15 isolate. (B) The natural deletion occurs between ORF42 and ORF43, removing ORF19 and ORF27. (C) Amino acid alignment of ORF29 of HLJDAd15 JSJ13 and ON1 showing the 11-amino-acid deletion (large box) and 11-amino-acid insertion (small box), respectively.
Pathogenicity in embryos
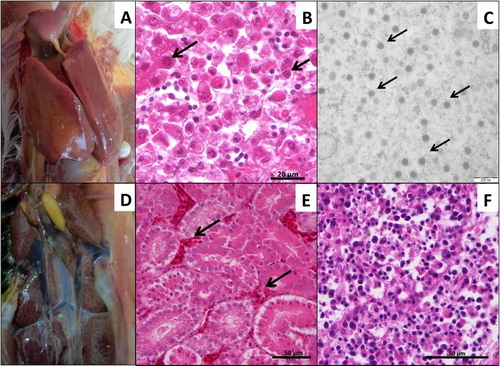
The SPF chicken embryos died between 3 and 7 days post inoculation with HLJDAd15. The highest mortality (60%) was observed in the 10−1 dilution group, whereas no embryos died in 10−7, 10−8 dilution groups or the PBS control group until 14 days post inoculation (). The infectivity titre of the virus in SPF chicken embryos was determined to be 103.28 ELD50/200 μl using the formula of Reed and Muench. Friable and discoloured liver ((A)) appeared in the dead embryos. Severe congestion in the liver was observed in the pathological analysis ((B)). Virions of HLJDAd15 in livers of the dead embryos were observed with a transmission electron microscope ((C)). However, there were no mortalities in any of the SPF duck embryo groups until 14 days post inoculation with the virus ().
Figure 4. Pathogenicity in SPF chicken embryos. (A) Friable and discoloured livers of dead embryos. (B) Severe congestion in the liver (H&E stain). (C) Virions of HLJDAd15 in liver viewed by transmission electron microscopy (arrows).
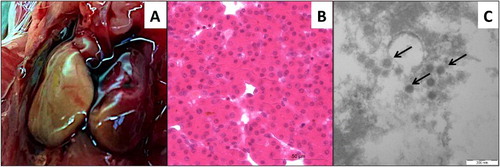
Table 2. The determination of the ELD50 of HLJFAd15 in SPF chicken embryos.
Table 3. The determination of the ELD50 of HLJFAd15 in SPF duck embryos.
Pathogenicity in SPF chickens and ducks (Figure 5)
All SPF chickens in the control group remained alive and did not show any clinical signs until 14 days post inoculation with PBS. Three of the 27 SPF chickens orally inoculated with 103.0 ELD50 of HLJDAd15 died within 4–7 days post inoculation (11.1% mortality rate). Dead chickens in the infected group showed severe hydropericardium ((A)) and basophilic intranuclear inclusion bodies ((B)). The livers of dead chickens were FAdV-4 positive by PCR identification. Furthermore, electron microscopy of the livers of dead SPF chickens confirmed the presence of adenovirus virions ((C)). The infected and dead chickens showed mild or severe enlargement of the kidneys ((D)). Protein casts in the renal tubules and extensive congestion in the renal interstitium were observed ((E)). Large numbers of necrotic lymphocytes were present in the lymph follicles in the bursa of the infected chickens ((F)). Conversely, no mortality occurred in the infected or control SPF duck groups until 14 days post infection. Although the virus was identified in the livers of the infected ducks by PCR at 7 days post infection, none of the typical clinical signs were observed throughout the progress.
Figure 5. Pathogenicity in SPF chickens. (A) Severe hydropericardium and swollen and friable liver with multifocal areas of necrosis. (B) Basophilic intranuclear inclusion bodies in liver cells (arrows, H&E stain). (C) Adenovirus virions in liver tissues (arrows). (D) Mild or severe enlargement of kidney of dead chickens. (E) Protein casts in renal tubules and extensive congestion in renal interstitium (arrows, H&E stain). (F) Large numbers of necrotic lymphocytes in lymph follicles in the bursa (H&E stain).
Discussion
Since June 2015, severe hydropericardium hepatitis syndrome caused by hypervirulent FAdV-4 in broilers and layers has been observed in China. Compared with more mild disease caused by FAdV-4, the recent outbreak of FAdV-4 has resulted in huge economic losses in the poultry industry. According to the genome sequence analysis, this epidemic FAdV-4 is a novel genotype (Ye et al., Citation2016). Furthermore, hydropericardium hepatitis syndrome has been reported to be emerging in Cherry Valley ducks in China, and the disease is also induced by FAdV-4 (Chen et al., Citation2016). However, no complete genome sequence of FAdV-4 isolated from ducks has been available, which is very important for understanding the evolution and cross-transmission between different hosts.
Therefore, a hypervirulent FAdV-4 was isolated from dead ducks with severe IBH and HPS, and the complete genome was sequenced and submitted to GenBank. Sequence analysis showed that HLJDAd15 isolated from ducks carries the same large natural deletion of ORF19 and ORF27 as the strains currently prevalent in chickens as well as another deletion of 11 amino acids in ORF29 (Zhao et al., Citation2015). The complete genome suggests that the HPS currently prevalent in chickens and ducks might be caused by the same hypervirulent FAdV-4 strain with a novel genotype. Despite the small variation between strains isolated from chickens and ducks, the cross-transmission potential between chickens, ducks, and other birds increases the difficultly of controlling the spread of FAdV-4 infection. Furthermore, the large deletion occurs in a non-essential site for viral replication (Johnson et al., Citation2003; Francois et al., Citation2004). Thus, potentially, the exogenous genes could be inserted into this region of the other mildly virulent adenovirus strains for vaccine production. This could be very important information for vaccine development but needs further investigation.
Pathogenicity analysis showed that it was much easier to infect chickens with the HLJDAd15 virus isolated from ducks than to infect ducks. The clinical signs in infected chickens induced by duck isolate HLJDAd15 were almost identical to those of the naturally infected ducks. However, HLJDAd15 was not able to induce mortality in experimentally infected ducks, although the virus was present in their livers. The different kinds of breeds and husbandry managements of birds might be responsible for the different mortalities between ducks in clinical cases and experimental infection. Nevertheless, the HPS prevalent in ducks was far more likely transmitted from chickens to ducks than from ducks to ducks. Although the cross-transmission between different species needs further investigation, this finding has revealed a potential novel translation pattern. Furthermore, the cross-transmission makes it more difficult to block the transmission route of the disease. Unfortunately, there is currently no commercial vaccine against FAdV-4 for chickens or ducks in China. The potential risk of increased spread of the FAdV-4 hypervirulent strain urgently requires an effective vaccine.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Asthana, M., Chandra, R. & Kumar, R. (2013). Hydropericardium syndrome: current state and future developments. Archives of Virology, 158, 921–931. doi: 10.1007/s00705-012-1570-x
- Chen, H., Dou, Y., Zheng, X., Tang, Y., Zhang, M., Zhang, Y., Wang, Z. & Diao, Y. (2016). Hydropericardium hepatitis syndrome emerged in cherry valley ducks in China. Transboundary and Emerging Diseases. [Epub ahead of print]. doi:10.1111/tbed.12500.
- Eregae, M.E., Dewey, C.E., McEwen, S.A., Ouckama, R., Ojkić, D. & Guerin, M.T. (2014). Flock prevalence of exposure to avian adeno-associated virus, chicken anemia virus, fowl adenovirus, and infectious bursal disease virus among Ontario broiler chicken flocks. Avian Diseases, 58, 71–77. doi: 10.1637/10612-071113-Reg.1
- Francois, A., Chevalier, C., Delmas, B., Eterradossi, N., Toquin, D., Rivallan, G. & Langlois, P. (2004). Avian adenovirus CELO recombinants expressing VP2 of infectious bursal disease virus induce protection against bursal disease in chickens. Vaccine, 22, 2351–2360. doi: 10.1016/j.vaccine.2003.10.039
- Ganesh, K., Suryanarayana, V., Raghavan, R. & Gowda, S. (2001). Nucleotide sequence of L1 and part of P1 of hexon gene of fowl adenovirus associated with hydropericardium hepatitis syndrome differs with the corresponding region of other fowl adenoviruses. Veterinary Microbiology, 78, 1–11. doi: 10.1016/S0378-1135(00)00288-1
- Hess M. (2000). Detection and differentiation of avian adenoviruses: a review. Avian Pathology, 29, 195–206. doi: 10.1080/03079450050045440
- Jiang, L., Qi, X., Gao, Y., Hua, Y., Li, K., Deng, X., Wang, Q., Zhang, L., Chai, H., Chen, Y., Yin, C., Gao, H., Qin, L., Wang, Y., Qu, Y., Chen, Q., Fan, Z. & Wang, X. (2013). Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in northeast China. Veterinary Microbiology, 166, 68–75. doi: 10.1016/j.vetmic.2013.05.008
- Johnson, M.A., Pooley, C., Ignjatovic, J. & Tyack, S.G. (2003). A recombinant fowl adenovirus expressing the S1 gene of infectious bronchitis virus protects against challenge with infectious bronchitis virus. Vaccine, 21, 2730–2736. doi: 10.1016/S0264-410X(03)00227-5
- Kaján, G.L., Kecskeméti, S., Harrach, B. & Benkő, M. (2013). Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Veterinary Microbiology, 167, 357–363. doi: 10.1016/j.vetmic.2013.09.025
- Li, L., Luo, L., Luo, Q., Zhang, T., Zhao, K., Wang, H., Zhang, R., Lu, Q., Pan, Z., Shao, H., Zhang, W. & Wen, G. (2016). Genome sequence of a fowl adenovirus serotype 4 strain lethal to chickens, isolated from China. Genome Announcements, 4, e00140–16.
- Li, Y., Li, C., Liu, L., Wang, H., Wang, C., Tian, G., Webster, R.G., Yu, K. & Chen, H. (2006). Characterization of an avian influenza virus of subtype H7N2 isolated from chickens in northern China. Virus Genes, 33, 117–122. doi: 10.1007/s11262-005-0042-8
- Lu, Z., Zhang, L., Wang, N., Chen, Y., Gao, L., Wang, Y., Gao, H., Gao, Y., Li, K., Qi, X. & Wang, X. (2015). Naturally occurring reassortant infectious bursal disease virus in northern China. Virus Research, 203, 92–95. doi: 10.1016/j.virusres.2015.04.003
- Lv, H., Zhang, Y., Sun, G., Bao, K., Gao, Y., Qi, X., Cui, H., Wang, Y., Li, K., Gao, L., Pan, Q., Wang, X. & Liu, C. (2016). Genetic evolution of Gallid herpesvirus 2 isolated in China. Infection, Genetic and Evolution, 51, 263–274. doi: 10.1016/j.meegid.2016.04.027
- Marek, A., Kosiol, C., Harrach, B., Kaján, G.L., Schlötterer, C. & Hess, M. (2013). The first whole genome sequence of a Fowl adenovirus B strain enables interspecies comparisons within the genus Aviadenovirus. Veterinary Microbiology, 166(1–2), 250–256. doi: 10.1016/j.vetmic.2013.05.017
- Mase, M., Nakamura, K. & Minami, F. (2012). Fowl adenoviruses isolated from chickens with inclusion body hepatitis in Japan, 2009–2010. Journal of Veterinary Medicine Science, 74, 1087–1089. doi: 10.1292/jvms.11-0443
- Mazaheri, A., Prusas, C., Voss, M. & Hess, M. (1998). Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathology, 27, 269–276. doi: 10.1080/03079459808419335
- Mittal, D., Jindal, N., Tiwari, A.K. & Khokhar, R.S. (2014). Characterization of fowl adenoviruses associated with hydropericardium syndrome and inclusion body hepatitis in broiler chickens. VirusDisease, 25, 114–119. doi: 10.1007/s13337-013-0183-7
- Rahimi, M. & Minoosh, S.H.Z. (2015). Adenovirus-like inclusion body hepatitis in a flock of broiler chickens in Kermanshah province, Iran. Veterinary Research Forum, 6, 95–98.
- Reed, L.J. & Muench, H. (1938). A simple method of estimation of 50% end points. American Journal of Hygiene, 27, 493–497.
- Sheppard, M., McCoy, R.J. & Werner, W. (1995). Genomic mapping and sequence analysis of the fowl adenovirus serotype 10 hexon gene. Journal of General Virology, 76, 2595–2600. doi: 10.1099/0022-1317-76-10-2595
- Vera-Hernández, P.F., Morales-Garzón, A., Cortés-Espinosa, D.V., Galiote-Flores, A., García-Barrera, L.J., Rodríguez-Galindo, E.T., Toscano-Contreras, A., Lucio-Decanini, E. & Absalon, A.E. (2015). Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl aviadenovirus serotype 4. Avian Pathology, 27, 1–32.
- Wang, Q., Li, X., Ji, X., Wang, J., Shen, N., Gao, Y., Qi, X., Wang, Y., Gao, H., Zhang, S. & Wang, X. (2014). A recombinant avian leukosis virus subgroup J for directly monitoring viral infection and the selection of neutralizing antibodies. PLoS One, 9, e115422. doi: 10.1371/journal.pone.0115422
- Ye, J., Liang, G., Zhang, J., Wang, W., Song, N., Wang, P., Zheng, W., Xie, Q., Shao, H., Wan, Z., Wang, C., Chen, H., Gao, W. & Qin, A. (2016). Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerging Microbes and Infections, 5, e50. doi: 10.1038/emi.2016.50
- Zhao, J., Zhong, Q., Zhao, Y., Hu, Y.X. & Zhang, G.Z. (2015). Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One, 10, e0133073. doi: 10.1371/journal.pone.0133073
- Zhong, L., Gao, L., Liu, Y., Li, K., Wang, M., Qi, X., Gao, Y. & Wang, X. (2016). Genetic and pathogenic characterisation of 11 avian reovirus isolates from northern China suggests continued evolution of virulence. Scientific Reports, 6, 35271. doi: 10.1038/srep35271
- Zhu, J., Hu, S., Xu, H., Liu, J., Zhao, Z., Wang, X. & Liu, X. (2016). Characterization of virulent Newcastle disease viruses from vaccinated chicken flocks in Eastern China. BMC Veterinary Research, 12, 113. doi: 10.1186/s12917-016-0732-6
