ABSTRACT
The pathogen Salmonella Pullorum is the causative agent of persistent systemic infection of poultry, leading to economic losses in developing countries due to morbidity, mortality and reduction in egg production. These infections may result in vertical transmission to eggs or progeny. Limited information is available regarding the mechanisms involved in the survival of Salmonella Pullorum in egg albumen and developing chicken embryos. Hence, we investigated the role of O-polysaccharide in the contamination of eggs and the colonization of chicken embryos. Compared with the wild-type strain, the isogenic waaL mutant exhibited an O-antigen-deficient rough phenotype, and increased sensitivity to egg albumen and chicken serum, as well as reduced adherence to DF-1 cells. Infection with Salmonella Pullorum lacking O-polysaccharide resulted in significantly reduced embryo lethality and bacterial colonization. These results suggest that O-polysaccharide is essential for Salmonella Pullorum colonization in eggs, both post-lay and developing embryos. The chicken embryo infection model could be used to characterize the interaction between Salmonella Pullorum and developing embryos, and it will also contribute to the development of more rational vaccines to protect laying hens and embryos.
Introduction
Salmonella enterica serovar Gallinarum biovar Pullorum (Salmonella Pullorum) is the causative agent of pullorum disease, which is characterized by an acute systemic disease that results in high mortality in very young chicks (Wigley et al., Citation2002; Guo et al., Citation2016). In addition, Salmonella Pullorum is known to produce a persistent infection in hens, in which localization in the reproductive tract is associated with vertical transmission. Eggs contaminated with Salmonella Pullorum are the most commonly identified route of transmission from one generation to the next (Berchieri et al., Citation2001). Survival in splenic macrophages is one of the major mechanisms underlying the persistence of Salmonella Pullorum infection in laying hens and transmission to eggs (Wigley et al., Citation2001); infection of the reproductive tract and eggs with Salmonella Pullorum is associated with suppression of cellular immunity at sexual maturity (Wigley et al., Citation2005). However, little is known regarding the mechanism by which Salmonella Pullorum survives in eggs after laying, and regarding the factors required for the non-fatal colonization of chicken embryos during incubation.
Previous studies have shown that Salmonella lipopolysaccharide (LPS) is important for the colonization of the chicken reproductive tract, egg contamination and survival in egg albumen (Gantois et al., Citation2009; Shah et al., Citation2012; Coward et al., Citation2013; Raspoet et al., Citation2014). LPS is a main component of the outer membrane of Gram-negative bacteria, and can be structurally divided into three distinct domains: lipid A, core oligosaccharide and O-antigen polysaccharide. These three components of LPS play different roles in bacterial pathogenesis. O-polysaccharide is highly variable and immunogenic in Salmonella, and has been reported to be essential for serotyping, adhesion and environmental persistence (Liu et al., Citation2014). Salmonella Enteritidis lacking O-polysaccharide chains was more susceptible to egg albumen containing antimicrobial compounds such as lysozyme and ovotransferrin (Guard-Bouldin et al., Citation2004). However, it remains unclear whether the O-polysaccharide of Salmonella Pullorum is required for contaminating eggs and colonizing chicken embryos.
Therefore, we constructed a rough mutant lacking O-polysaccharide chains by deleting the waaL gene encoding the unique O-antigen ligase from the well-characterized Salmonella Pullorum strain S06004. Interestingly, we found that the O-polysaccharide-negative Salmonella Pullorum mutant showed significant sensitivity to egg albumen, as well as reduced lethality and colonization in a chicken embryo infection model. The findings further our understanding of vertical transmission and virulence associated with this pathogen, and contribute to the development of efficient control strategy against salmonellosis.
Materials and methods
Ethics statement
All experimental work involving birds was performed according to the guidelines of the Animal Welfare and Ethics Committee of Yangzhou University. All laboratory health and safety procedures were complied with during the course of the experimental work.
Bacteria, cells and chicken embryos
Salmonella Pullorum virulent strain S06004, originally isolated from a newly hatched chick with pullorum disease, is naturally resistant to nalidixic acid (Nal) (Guo et al., Citation2016). The S06004ΔwaaL::cat mutant strain was constructed using the one-step inactivation method, as previously described (Datsenko & Wanner, Citation2000). All strains were cultured aerobically at 37°C in Luria-Bertani (LB) broth. When required, media was supplemented with antibiotics (Nal, 50 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml). DF-1 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% foetal bovine serum in a 5% CO2 incubator. Fertile HY-line white chicken embryos, obtained from Jiangsu Institute of Poultry Sciences (China), were hatched in the laboratory.
LPS extraction and silver staining
Bacterial cultures grown overnight at 37°C in LB broth were diluted to a concentration of approximately 1 × 109 colony-forming units (CFU)/ml. LPS was extracted using the LPS extraction kit (iNtRON, Gyeonggi, Korea) according to the manufacturer’s instructions; 20 μl of each sample was separated by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE), and visualized using a silver stain kit (Thermo Scientific, Rockford, IL, USA).
Survival in egg albumen
The ability of both wild-type and mutant Salmonella Pullorum strains to survive in egg albumen was quantified, as previously described (Shah et al., Citation2012), with minor modifications. Organic, unfertilized, antibiotic-free eggs from a local farm were disinfected by immersion in 70% ethanol, and aseptically broken to collect egg albumen into a sterile container. Overnight bacterial cultures were mixed with 1 ml aliquots of egg albumen at a concentration of approximately 1 × 103 CFU/ml. Bacteria–egg albumen mixtures were incubated at 37°C for 24 h following thorough mixing, and then plated on LB agar to enumerate the viable bacterial counts. This assay was repeated three times.
Serum sensitivity
To analyse the sensitivity of the mutant strain to serum, overnight bacterial cultures were washed three times in phosphate buffered saline (PBS, pH 7.2), and normalized to a concentration of 1 × 106 CFU/ml, then 100 μl of bacteria was added to 100 μl SPF chicken serum yielding a 200 μl sample containing approximately 1 × 105 CFU in 50% serum. Aliquots were removed following incubation at 37°C for 1 h, serially diluted in PBS and then plated to determine viability.
Adherence assay
The bacterial adherence assay was performed using chicken embryo fibroblast DF-1 cells, as previously described (Han et al., Citation2014; Szmolka et al., Citation2015). Briefly, DF-1 cells were seeded (approximately 1 × 105 cells per well) in 24-well tissue culture plates and cultured in DMEM supplemented with 10% foetal bovine serum without antibiotics at 37°C under 5% CO2. Following a single wash with DMEM, cells were infected for 2 h at 37°C under 5% CO2 with overnight bacterial cultures at a multiplicity of infection of 100. Following incubation, cells were washed to remove non-adherent bacteria, then lysed directly with 0.5% Triton X-100, and plated on LB agar plates for quantification of bacterial cells. Giemsa staining was performed as previously described (El-Malah et al., Citation2014). DF-1 cells alone were included in all experiments as negative controls, and infection with each strain was performed in triplicate.
Chicken embryo infection model
The chicken embryo lethality assay was carried out, as previously described (Seo et al., Citation2013), with minor modifications. To determine the appropriate dose for Salmonella Pullorum inoculation, a preliminary experiment was performed for allantoic cavity injection of chicken embryos. Chicken embryos were incubated in an automatic incubator at 37.5°C and 50–60% relative humidity. Embryo viability was confirmed prior to inoculation, and non-viable embryos were discarded. Once the optimal dose was determined, an overnight bacterial culture grown at 37°C in LB broth was washed twice with PBS (pH 7.2), then resuspended and diluted to 103 CFU/ml. The number of CFU inoculated into the embryos was quantified using the viable count method. For inoculation, 0.1 ml of the diluted bacterial cell culture was administered into the allantoic cavity of 12-day-old embryonated eggs using an 18-gauge needle (20 embryos per group). PBS inoculated embryos were used as the control. The holes were resealed with paraffin, and the embryos were incubated at 37.5°C and 50–60% relative humidity. The treated embryos were examined by candling to monitor viability, which were marked as alive or dead based on the integrity of the venous system and embryo movement, and the number of deaths was recorded. At the end of the experiment, all embryos were dissected, and various internal organs and fluids were collected aseptically for bacteria counting.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). An analysis of bacterial survival in all assays was performed using unpaired t-tests. Statistical significance was established at P < 0.05.
Results
Phenotypic characterization of the ΔwaaL mutant
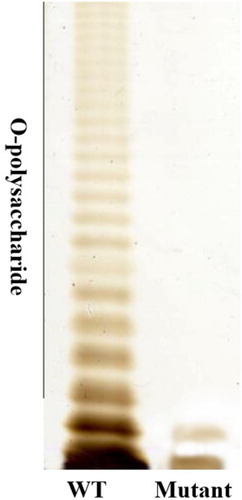
The Salmonella Pullorum S06004ΔwaaL::cat mutant strain exhibited rough colony morphology, while the wild-type formed smooth colonies. The SDS-PAGE analysis showed that wild-type LPS contained a ladder-like pattern with an intact LPS structure, while the mutant strain displayed truncated LPS lacking the O-polysaccharide region ().
Lack of O-polysaccharide increases the sensitivity of the ΔwaaL mutant to egg albumen and serum
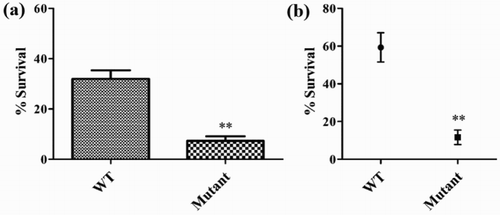
To determine the impact of O-polysaccharide on Salmonella Pullorum survival in egg albumen, the viability of the wild-type strain and O-polysaccharide-deficient mutant was examined following incubation in egg white. Deletion of the waaL gene resulted in a significant reduction in survival (), indicating that O-polysaccharide is essential for the tolerance of Salmonella Pullorum to egg albumen. Moreover, the wild-type strain was highly resistant to killing by normal chicken serum, while the rough mutant exhibited increased serum sensitivity, indicating that the O-polysaccharide of Salmonella Pullorum is required for resistance to serum bactericidal activity ().
Figure 2. Effect of O-polysaccharide on resistance to egg albumen and serum complement. (a) Survival rates of bacteria exposed to egg albumen at 37°C for 24 h. (b) Survival of the Salmonella Pullorum mutant in 50% chicken serum.
The O-polysaccharide-deficient mutant exhibits reduced adherence ability
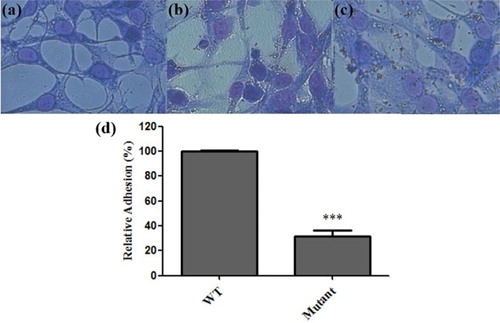
To evaluate the effect of O-polysaccharide deletion on the adherence to cells in vitro, cultured chicken embryo fibroblast DF-1 cells were infected with the waaL mutant and corresponding wild-type strain. The results show that the mutant lacking O-polysaccharide exhibited a 68.33% reduction in the ability to adhere to DF-1 cells compared with the wild-type strain ().
Figure 3. Adhesion of Salmonella Pullorum strains to chicken embryo fibroblast DF-1 cells. Cells were incubated with bacteria grown overnight at a designated multiplicity of infection of 100. (a) Uninfected DF-1 cells were used as the control. (b) DF-1 cells infected with the mutant strain. (c) DF-1 cells infected with the wild-type strain. (d) The adherence of the mutant strain was normalized to that of the wild-type control (***P < 0.001, relative to wild type).
Loss of O-polysaccharide reduces virulence and bacterial colonization in embryos
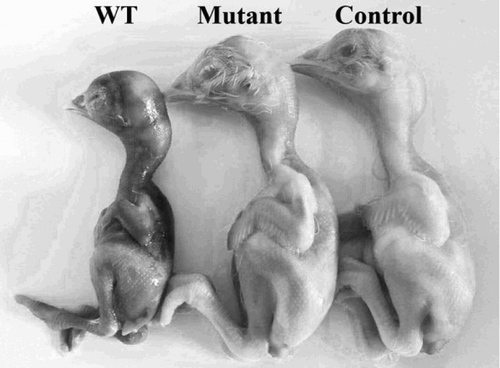
An initial dose–response experiment determined 102 CFU as the optimal dose, exhibiting reliable mortality. This dose was used in subsequent assays. The embryos were candled for 72 consecutive hours post inoculation, and the number of dead embryos was noted. The survival curve for embryos infected with bacteria is presented in . One hundred per cent of embryos inoculated with the wild-type strain were reported dead by the end of the assay, exhibiting extreme lethality compared with the mutant strain and the PBS control, which did not cause embryo death. The bacteriological analysis of the tissues and fluids from embryos at 72 h post inoculation showed that the number of bacterial cells recovered from liver, spleen, allantoic fluid and amniotic fluid was significantly lower in mutant strain-inoculated embryos than in wild-type strain-inoculated embryos (). Macroscopic lesions observed in embryos infected with the wild-type strain included multifocal cutaneous haemorrhage and marked subcutaneous oedema. No significant changes were observed in the mutant strain treatment group or PBS control group ().
Figure 4. Loss of O-polysaccharide reduces chicken embryo mortality. Survival curves for embryos inoculated with the wild-type strain and isogenic waaL mutant. Results are representative of three individual tests.
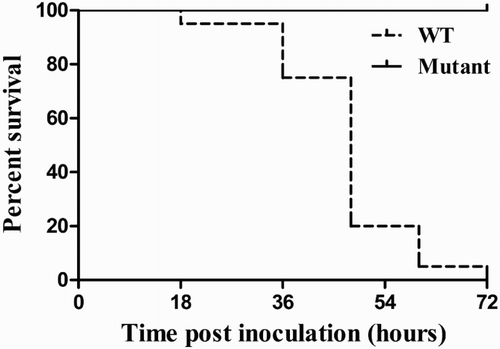
Figure 5. Bacterial load and colonization of the mutant and wild-type strains in chicken embryonic tissues. ALF, allantoic fluid; AMF, amniotic fluid. Statistical significance of differences was evaluated by t-test (*P < 0.5; ***P < 0.001).
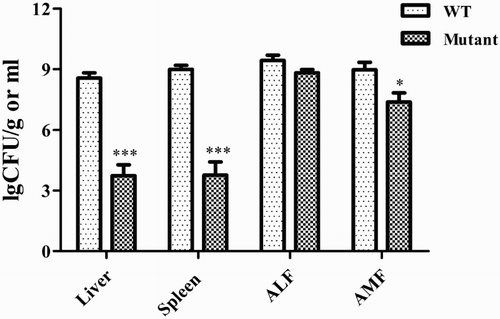
Figure 6. Macroscopic characteristics of embryos infected with Salmonella Pullorum strains. Embryos inoculated with the wild-type strain showed noticeable lesions including multifocal cutaneous haemorrhage and subcutaneous oedema. These changes were not observed in the mutant strain treatment group or PBS control.
Discussion
In chickens, the development of persistent infections is a feature of the systemic disease-causing Salmonella Pullorum. Salmonella Pullorum is thought to persist for a number of months in the spleen, leading to localization in the ovary, and consequently, to vertical transmission of the infection to eggs (Wigley et al., Citation2001). The epidemiology of Salmonella Pullorum infection in poultry is known to be closely associated with infected carriers, which lay infected eggs that lead to vertical transmission (Barrow, Citation2007). However, the factors required for the survival of Salmonella Pullorum in eggs, both during postlay and hatch, remain unclear. The aim of the present study was to investigate the role of O-polysaccharide chains in egg albumen resistance and the colonization of chicken embryos by Salmonella Pullorum.
Most studies aimed at identifying genes involved in the specific response to egg albumen exposure are carried out with Salmonella Enteritidis (Baron et al., Citation2016). Although the mechanisms exploited by Salmonella Enteritidis to survive the hostile antimicrobial environment in egg white are still not completely understood, this specific serotype exhibits superior egg albumen survival compared with other Salmonella serotypes (De Vylder et al., Citation2013). Previous studies searching for genes associated with the survival of Salmonella Enteritidis in chicken egg albumen identified LPS biosynthesis genes (Clavijo et al., Citation2006; Gantois et al., Citation2009; Raspoet et al., Citation2014). LPS plays an important role in bacterial defence mechanisms against antimicrobial substances, such as lysozyme, ovotransferrin and serum. The data in this study show that O-polysaccharide is essential for the survival of Salmonella Pullorum in egg albumen. In addition, the O-polysaccharide mutant exhibited increased sensitivity to killing by chicken serum. O-antigen is the characteristic polysaccharide of the outermost component of the outer membrane of the Gram-negative bacteria cell wall. The presence of O-polysaccharide in LPS molecules may prevent antimicrobial substances from reaching their binding sites in the outer membrane, resulting in enhanced tolerance and survival. Moreover, what other changes in the rough mutant might be responsible for its resistance? Due to the peripheral location in a bacterial cell, outer membrane proteins are active in the process of cell adaptation to fluctuating habitat conditions (Dudek et al., Citation2016). Future studies will evaluate whether the deletion of waaL gene modifies the outer membrane proteins profiles or influences the levels of their expression.
The chicken embryo lethality assay is widely used to predict virulence based on embryo death because it is relatively reliable and simple (Seo et al., Citation2013; Borst et al., Citation2014). However, chicken embryo infection models applicable for Salmonella Pullorum are seldom investigated, and the interaction between Salmonella Pullorum and the developing embryo is unclear. Herein, we established a chicken embryo infection model for evaluating the virulence and colonizing ability of Salmonella Pullorum. Embryos inoculated with the wild-type strain demonstrated 100% mortality within 72 h, while the survival of all embryos treated with the mutant strain lacking O-polysaccharide was similar to that of PBS control. The bacterial load of the mutant strain was significantly lower than that of the wild-type strain, indicating a correlation between invasion or replication capability and embryo lethality. Furthermore, successful colonization of the host relies on the ability to adhere to host cells and tissues. The O-polysaccharide-deficient mutant showed reduced adherence ability to chicken embryo fibroblast DF-1 cells. These results suggest that the O-polysaccharide is involved in invasion, colonization and adherence of Salmonella Pullorum in embryonic tissues, and contributes to systemic infection and death of chicken embryos.
Previous studies have shown that members of the chicken Toll-like receptor family are expressed abundantly in embryos during embryonic development, suggesting that the Toll-like receptor-mediated immune system probably plays a role in the recognition of Salmonella, and in initiation of the immune response (Michailidis et al., Citation2010). Identification of Salmonella Pullorum characteristics that allow this pathogen to survive in the developing embryo is important for not only understanding the innate immunity responses associated with this pathogen, but will also contribute to the development of more rational vaccines to protect laying hens and embryos. The results of this study confirm that the chicken embryo infection model is significant and reliable and that it could be used for future research.
In conclusion, we determined that O-polysaccharide has a specific function in the survival of Salmonella Pullorum in egg albumen, and in its tolerance to the bactericidal activity of the complement system. Additionally, we provide evidence of the contribution of O-polysaccharide to colonization and virulence in chicken embryos.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baron, F., Nau, F., Guérin-Dubiard, C., Bonnassie, S., Gautier, M., Andrews, S.C. & Jan, S. (2016). Egg white versus Salmonella Enteritidis! A harsh medium meets a resilient pathogen. Food Microbiology, 53, 82–93. doi: 10.1016/j.fm.2015.09.009
- Barrow, P.A. (2007). Salmonella infections: immune and non-immune protection with vaccines. Avian Pathology, 36, 1–13. doi: 10.1080/03079450601113167
- Berchieri Jr., A., Murphy, C.K., Marston, K. & Barrow, P.A. (2001). Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens; effect of bacterial and host genetic background. Avian Pathology, 30, 229–239.
- Borst, L.B., Suyemoto, M.M., Keelara, S., Dunningan, S.E., Guy, J.S. & Barnes, H.J. (2014). A chicken embryo lethality assay for pathogenic Enterococcus cecorum. Avian Diseases, 58, 244–248. doi: 10.1637/10687-101113-Reg.1
- Clavijo, R.I., Loui, C., Andersen, G.L., Riley, L.W. & Lu, S. (2006). Identification of genes associated with survival of Salmonella enterica serovar Enteritidis in chicken egg albumen. Applied and Environmental Microbiology, 72, 1055–1064. doi: 10.1128/AEM.72.2.1055-1064.2006
- Coward, C., Sait, L., Cogan, T., Humphrey, T.J. & Maskell, D.J. (2013). O-antigen repeat number in Salmonella enterica serovar Enteritidis is important for egg contamination, colonisation of the chicken reproductive tract and survival in egg albumen. FEMS Microbiology Letters, 343, 169–176. doi: 10.1111/1574-6968.12143
- Datsenko, K.A. & Wanner, B.L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences, 97, 6640–6645. doi: 10.1073/pnas.120163297
- De Vylder, J., Raspoet, R., Dewulf, J., Haesebrouck, F., Ducatelle, R. & Van Immerseel, F. (2013). Salmonella Enteritidis is superior in egg white survival compared with other Salmonella serotypes. Poultry Science, 92, 842–845. doi: 10.3382/ps.2012-02668
- Dudek, B., Krzyżewska, E., Kapczyńska, K., Rybka, J., Pawlak, A., Korzekwa, K., Klausa, E. & Bugla-Płoskońska, G. (2016). Proteomic analysis of outer membrane proteins from Salmonella Enteritidis strains with different sensitivity to human serum. PLoS One, 11, e0164069. doi: 10.1371/journal.pone.0164069
- El-Malah, S.S., Yang, Z., Hu, M., Li, Q., Pan, Z. & Jiao, X. (2014). Vibrio parahaemolyticus strengthens their virulence through modulation of cellular reactive oxygen species in vitro. Frontiers in Cellular and Infection Microbiology, 4, 168. doi: 10.3389/fcimb.2014.00168
- Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F. & Van Immerseel, F. (2009). The Salmonella Enteritidis lipopolysaccharide biosynthesis gene rfbH is required for survival in egg albumen. Zoonoses and Public Health, 56, 145–149. doi: 10.1111/j.1863-2378.2008.01195.x
- Guard-Bouldin, J., Gast, R.K., Humphrey, T.J., Henzler, D.J., Morales, C. & Coles, K. (2004). Subpopulation characteristics of egg-contaminating Salmonella enterica serovar Enteritidis as defined by the lipopolysaccharide O chain. Applied and Environmental Microbiology, 70, 2756–2763. doi: 10.1128/AEM.70.5.2756-2763.2004
- Guo, R., Geng, S., Jiao, H., Pan, Z., Chen, X. & Jiao, X. (2016). Evaluation of protective efficacy of a novel inactivated Salmonella Pullorum ghost vaccine against virulent challenge in chickens. Veterinary Immunology and Immunopathology, 173, 27–33. doi: 10.1016/j.vetimm.2016.03.015
- Han, Y., Han, X., Wang, S., Meng, Q., Zhang, Y., Ding, C. & Yu, S. (2014). The waaL gene is involved in lipopolysaccharide synthesis and plays a role on the bacterial pathogenesis of avian pathogenic Escherichia coli. Veterinary Microbiology, 172, 486–491. doi: 10.1016/j.vetmic.2014.05.029
- Liu, B., Knirel, Y.A., Feng, L., Perepelov, A.V., Senchenkova, S.N., Reeves, P.R. & Wang, L. (2014). Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiology Reviews, 38, 56–89. doi: 10.1111/1574-6976.12034
- Michailidis, G., Theodoridis, A. & Avdi, M. (2010). Transcriptional profiling of Toll-like receptors in chicken embryos and in the ovary during sexual maturation and in response to Salmonella enteritidis infection. Animal Reproduction Science, 122, 294–302. doi: 10.1016/j.anireprosci.2010.09.004
- Raspoet, R., Shearer, N., Appia-Ayme, C., Haesebrouck, F., Ducatelle, R., Thompson, A. & Van Immerseel, F. (2014). A genome-wide screen identifies Salmonella Enteritidis lipopolysaccharide biosynthesis and the HtrA heat shock protein as crucial factors involved in egg white persistence at chicken body temperature. Poultry Science, 93, 1263–1269. doi: 10.3382/ps.2013-03711
- Seo, H.S., Cha, S.Y., Kang, M. & Jang, H.K. (2013). Chicken embryo lethality assay for determining the virulence of Riemerella anatipestifer isolates. Avian Pathology, 42, 387–392. doi: 10.1080/03079457.2013.816654
- Shah, D.H., Zhou, X., Kim, H.Y., Call, D.R. & Guard, J. (2012). Transposon mutagenesis of Salmonella enterica serovar Enteritidis identifies genes that contribute to invasiveness in human and chicken cells and survival in egg albumen. Infection and Immunity, 80, 4203–4215. doi: 10.1128/IAI.00790-12
- Szmolka, A., Wiener, Z., Matulova, M.E., Varmuzova, K. & Rychlik, I. (2015). Gene expression profiles of chicken embryo fibroblasts in response to Salmonella Enteritidis infection. PLoS One, 10, e0127708. doi: 10.1371/journal.pone.0127708
- Wigley, P., Berchieri, A. Jr., Page, K.L., Smith, A.L. & Barrow, P.A. (2001). Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infection and Immunity, 69, 7873–7879. doi: 10.1128/IAI.69.12.7873-7879.2001
- Wigley, P., Hulme, S.D., Powers, C., Beal, R.K., Berchieri Jr., A., Smith, A. & Barrow, P. (2005). Infection of the reproductive tract and eggs with Salmonella enterica serovar Pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infection and Immunity, 73, 2986–2990. doi: 10.1128/IAI.73.5.2986-2990.2005
- Wigley, P., Jones, M.A. & Barrow, P.A. (2002). Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian Pathology, 31, 501–506. doi: 10.1080/0307945021000005879