ABSTRACT
Bacterial chondronecrosis and osteomyelitis (BCO) is increasingly recognized as a major cause of lameness in commercial broilers chickens worldwide, but the pathogenesis of the condition is incompletely understood. This was a longitudinal study of 20 commercial broiler farms in Victoria, Australia, to investigate the aetiology and pathology of BCO. Thorough postmortem examination was performed on culled and dead birds (n = 325) from 20 different flocks at either 1 week, 4 weeks or 5 weeks of age and samples were analysed by conventional bacteriology, molecular identification of infectious organisms detected, serology and histopathology. BCO occurs throughout the life of broiler flocks at a very high rate, with lesions detected in 28% (95% CI 23–34%) of the mortalities and culls. The condition occurs with similar prevalence in both the femur and tibiotarsus. BCO is an infectious process that appears to result from bacteraemia and haematological spread of bacterial pathogens, especially Escherichia coli, to the bones, with 65.3% bacterial isolates from histologically confirmed BCO identified as E. coli, 11.5% as Staphylococcus and the remainder composed of mixed infections or a range of other minor isolates. We observed that almost all E. coli isolated from cases of BCO are avian pathogenic E. coli, suggesting that preventative measures should be directed at this organism.
Introduction
Lameness is a major economic and welfare issue in commercial broiler bird production worldwide, and it is increasingly being recognized that a large proportion of these lameness cases reflect the condition known as bacterial chondronecrosis and osteomyelitis (BCO), sometimes referred to as femoral head necrosis. The true incidence of this disease is unknown in most countries, but in Northern Ireland the mean incidence of culling due to lameness was 0.52% in male flocks and 0.38% in female flocks, with BCO the most common cause of lameness in broiler birds (McNamee & Smyth, Citation2000). Investigations in Bulgaria also revealed a significant problem, with lameness responsible for up to 15% of mortality in some broiler flocks, and BCO accounting for >90% of these cases (Dinev, Citation2009).
While the pathogenesis of this condition is incompletely understood, BCO is a bacterial osteomyelitis and physitis of the long bones of the hindlimbs, which frequently progresses to osteochondral necrosis and pathological bone fracture, causing significant lameness for the affected bird (McNamee et al., Citation1999b; McNamee & Smyth, Citation2000; Wideman & Prisby, Citation2012; Wideman et al., Citation2012). A range of factors have been proposed to play a role in the development of the condition, including immunosuppression and immunosuppressive disease, vascular impairment within the epiphysis, arthritis, trauma, nutrition and nutritional deficiencies, growth rate, bird breed and hatchery conditions (McNamee & Smyth, Citation2000).
The organism most commonly reported in association with BCO is Staphylococcus aureus. Osteochondral disease associated with S. aureus has been reported in France (Wyers et al., Citation1991) and Bulgaria (Dinev et al., Citation1995), with disease in the latter able to be reproduced experimentally through S. aureus infection (Dinev, Citation1996; Dinev et al., Citation1997). However, other septicaemic pathogens have also been isolated, including coagulase-positive staphylococci other than S. aureus, such as Staphylococcus hyicus, coagulase-negative Staphylococcus such as Staphylococcus xylosus and Staphylococcus simulans, Mycobacterium avium, Salmonella spp., E. coli and Enterococcus (Reece, Citation1992; Thorp et al., Citation1993; McNamee & Smyth, Citation2000; Dinev, Citation2009). More recently, Dinev (Citation2009) reported E. coli was isolated in more than 90% of the bacteriologically tested samples of BCO associated with osteomyelitis, suggesting that pathogens other than Staphylococcus may also play an important role in some cases.
The purpose of this study was to assess the prevalence of BCO in commercial broiler mortalities and culls on 20 commercial broiler farms in Australia and to characterize the infectious agents involved in the development of the disease, as well as assess for correlation between the presence of BCO and other infectious, traumatic and developmental disease.
Materials and methods
Birds and gross examination
Culled birds and mortalities were submitted for assessment from 20 different flocks ranging from 34,000 to 385,000 in size at either 1 week, 4 weeks or 5 weeks of age, based on pilot information received from field veterinarians about the most common ages for outbreaks of BCO. A quarter of the flocks (n = 5) were examined longitudinally at all three age points. Birds in each flock were sourced from one of two separate hatcheries; only one of the hatcheries performed eggshell sterilization via fumigation. All broilers used in this study had received a live infectious bronchitis vaccine by coarse spray in the hatchery while some had also received a live infectious laryngotracheitis vaccine via drinking water during growing period. The breeder flock from which broilers derived had received a series of vaccines against various diseases including infectious bronchitis, infectious bursal disease, Marek’s disease, fowlpox, Newcastle disease, Mycoplasma gallisepticum, Mycoplasma synoviae, avian encephalomyelitis, inclusion body hepatitis, infectious bursal disease, chicken infectious anaemia, fowl cholera and/or egg drop syndrome.
For each submission, all culls or mortalities from a single day were examined, up to a maximum of 20 birds; a total of 325 birds were submitted for necropsy for this study. For each submitted bird, a thorough postmortem examination was performed. The birds were weighed and the skin and mucous membranes examined for any lesions, including external parasitism or trauma (such as foot pad injury). Limbs and joints were examined for swelling or malformation to suggest traumatic injury or arthritis. The vent was assessed for evidence of enteritis or polyuria, and the umbilicus was inspected for evidence of omphalitis. Full internal postmortem examination was performed. Gross evidence of osteomyelitis, dyschondroplasia or bacterial chondronecrosis was recorded. Femoral heads were examined for evidence of damage, cartilage separation or abnormal synovial fluid.
Bacteriology
Bilaterally, the coxofemoral joints were disarticulated and femur and tibiotarsus were separated by removing the surrounding musculature. Femurs and tibiotarsi were cleanly bisected with a sterile knife. One half of the proximal end of each femur and tibiotarsus was swabbed (sterile plain swab with a plastic applicator, Copan, Interpath, VIC, Australia) for microbiological culture, and the other half was used for histopathological examinations (see below). Additional swabs were collected for microbiological assessment from other grossly identified lesions (including swollen joints, swollen foot pads, and any lesions in liver, heart or air sacs). Swabs collected from the birds were inoculated onto sheep blood and MacConkey agars and incubated at 37°C overnight. The plates were examined for the presence of bacterial growth, colony morphology, abundance and purity. Identity of the bacteria was determined using conventional bacteriological and biochemical methods.
Histopathology
Following microbiological sampling, the remaining proximal half of each femur and tibiotarsus was fixed in 10% neutral buffered formalin for histological assessment. The bones were decalcified and the tissues were embedded in paraffin and processed into 3 µm thick sections that were stained with haematoxylin and eosin for histopathological examination. Samples of liver, duodenum, jejunum, ileocaecal junction, yolk sac remnants and representative tissues from other gross lesions observed were similarly collected and processed for histopathological assessment. Bone sections were assessed for the presence of inflammation and/or necrosis, including location; the presence, location and general morphology of bacteria; degeneration of growth plate cartilage, indicated by the presence of non-artefactual separation or clefting of the cartilage, or regions of matrix eosinophilia within the cartilage; presence of a retained cartilage plug within the metaphysis (chondrodystrophy); degenerative changes within growth plate vascular channels; cellular depletion within the bone marrow (bone marrow suppression); lymphoid hyperplasia within the bone marrow (immunological stimulation); synovitis within the adjacent joint (coxofemoral joint for femoral specimens, stifle for tibiotarsal specimens). Histological sections of the duodenum, ileum and colon were assessed for the presence of inflammation and coccidia. Sections of liver and yolk sac remnant/Meckel’s diverticulum (where identified) were assessed for the presence of inflammation or infection. Due to the possibility of postmortem bacterial colonization of the bone, cases of BCO in this study were defined as birds with evidence of inflammation within the proximal tibia or femur, rather than the presence of bacterial organisms alone.
Identification of avian pathogenic E. coli
DNA was extracted from cultures grown on the sheep blood agar plates. Overnight bacterial cultures were harvested and re-suspended in 500 µl of sterile water, boiled for 10 min, pelleted at 16,200 × g for 1 min, and the supernatant (5 µl) was collected for use as template DNA for polymerase chain reaction (PCR) (Johnson & Brown, Citation1996). The sequence of the primers and expected size of the products used to confirm the presence of avian pathogenic E. coli DNA are summarized in . Each PCR was performed in a total volume of 25 µl containing 5 µl template DNA, 4 µl of 1.25 mM of each dNTP mixture (Bio-39025; Bioline, Alexandria, NSW, Australia), 4 µl of 25 mM MgCl2 (A351H; Promega, Auburn, VIC, Australia), 10 µl of 25 µM primer mixture, 1.5 U GoTaq polymerase (Promega, Auburn, VIC, Australia), 5 µl of 5´ GoTaq flexi green buffer (Promega, Auburn, VIC, Australia) and 1 µl of nuclease-free water. The mixture was incubated at 94°C for 2 min, and then subjected to 24 cycles of 94°C for 20 s, 60°C for 10 s and 72°C for 20 s and final extension step of 72°C for 5 min. PCR products were analysed by electrophoresis in a 3% (w/v) agarose gel. The isolates were defined as avian pathogenic E. coli (APEC) if they possessed a combination of any of the siderophore receptor and other virulence genes assessed (Johnson et al., Citation2006; Johnson et al., Citation2008a).
Table 1. Oligonucleotide primer sequences used in identification of APEC.
Serology
Blood samples for assessment of viral antibody titres were collected directly from the heart of the culled birds at each sampling point (approximately weeks 1, 4 and 5). Blood samples were collected into BD Vacutainer® SST™ ΙΙ advance tubes (BD, NSW, Australia) and were allowed to clot for 60 min at room temperature. The blood samples were centrifuged at 1200 × g for 10 min at room temperature. The serum samples were collected into 2 ml self-standing screw cap tubes Neptune™ (Pathtech, VIC, Australia) and stored at – 20°C until use.
Reovirus and infectious bursal disease virus indirect ELISAs
Indirect ELISAs were performed using the commercial IDEXX REO Ab Test (99–09264) and IDEXX IBD Ab Test (99–09260) (IDEXX Laboratories Pty Ltd, Rydalmere, Australia). Briefly, for both tests, all test sera were diluted to 1 : 500 with sample diluent and 100 µl of diluted sera were added to the individual wells of the plates and the plates were incubated at room temperature for 30 min. The contents of the plate were discarded then washed once with wash buffer. Then to each well, 100 µl of prediluted horseradish peroxidase (HRP) anti-chicken conjugate was added and the plates were incubated at room temperature for 30 min. The conjugate was discarded and the plates were washed as described before. A 100 µl 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added to each well and the plates were incubated for 15 min at room temperature under ambient light. The reaction was stopped by adding 100 µl of stop solution and the absorbance of each well at 650 nm was measured using a MULISKAN FC plate reader (Thermo scientific™, VIC, Australia). Results for individual samples were determined by calculating the ratio of sample absorbance compared to positive control. Samples with a ratio above 0.2 were considered positive.
Chicken anaemia virus-blocking ELISA
A blocking ELISA was performed using IDEXX CAV Ab Test (99–08702) (IDEXX Laboratories Pty Ltd). All test sera were diluted 1 in 10 with sample diluent and 100 µl of diluted sera were added to the individual wells of the plate and incubated for 60 min at room temperature. The contents of the plate were discarded then washed once with wash buffer. To each well, 100 µl of prediluted HRP anti-chicken anaemia virus (CAV) conjugate was added and the plates were incubated at room temperature for 30 min. The contents of the plate were discarded then washed with wash buffer. A 100 µl TMB substrate was added to each well and the plates were incubated for 15 min at room temperature under ambient light. The reaction was stopped by adding 100 µl of stop solution and the absorbance of each well at 650 nm was measured using a MULISKAN FC plate reader (Thermo scientific™). Results for individual samples were determined by calculating the ratio of sample absorbance compared to negative ratio. Samples with a ratio below 0.6 were considered positive.
Statistical analyses
The proportion of birds with bacterial isolates from the femur or tibiotarsus (both with and without inflammation) in different flocks and age groups were compared using Fisher’s exact test (IBM SPSS Statistics, version 22). Statistical associations between gross pathology lesions, histological lesions and individual bird-level features and the presence of BCO were quantified using the odds ratio and the null hypothesis that the odds ratio was equal to one was assessed using Fisher’s exact test (IBM SPSS Statistics, version 22). The association between bird age and lesion location was assessed using binary logistic regression analysis and the association between flock size and BCO prevalence assessed using linear regression (IBM SPSS Statistics, version 22). The Mann–Whitney U test was used to test for differences in bodyweight at <2, 2–4 and 5 weeks of age for BCO-positive and BCO-negative birds (IBM SPSS Statistics, version 22). Analysis of serological data was carried out using IBM SPSS Statistics, version 22. Optical densities obtained from chicken infectious anaemia virus (CIAV), Reovirus (ReoV) and infectious bursal disease virus (IBDV) ELISAs were subjected to a one-way ANOVA to statistically test for differences in ELISA readings at different time points. P values ≤0.05 were considered statistically significant.
Results
Prevalence of BCO lesions was similar in all age groups
The prevalence of BCO in the mortalities or culled birds examined is summarized in and . There was marked variation in the prevalence of BCO in culled/mortality birds between flocks, and even between ages in the same flocks. Mean BCO prevalence in culled/mortality birds remained remarkably similar between age groups, with roughly a quarter of all birds displaying histological evidence of disease (). The prevalence did not vary substantially with age, though there was a small non-significant decreasing trend in the 5-week-old birds. Maximum prevalence of BCO in flock culls/mortalities was 50%, and BCO was not identified (0% prevalence) in three flocks sampled at single time points, as well as in two of the longitudinally sampled flocks ().
Table 2. Prevalence of histologically confirmed BCO in necropsied birds.
Table 3. Prevalence of histologically confirmed BCO at different ages in necropsied birds from longitudinally sampled flocks.
E. coli was frequently isolated from the birds
A total of 288 bacterial isolates were recovered from the tissues of 109 birds. The overall summary of isolated bacteria is shown in . E. coli was the most common bacterium isolated from the birds (66.2% of total), followed by Staphylococcus spp. (17%) and other Gram-positive organisms (16.8%). Of birds with positive bone cultures, 41% were positive for the same bacterium in multiple bones. Almost all the E. coli isolates (99%) from bones with inflammation were classified as APEC () although they belonged to a number of different serotypes (results not shown). Of the 154 E. coli isolates from bones, 71 isolates (46% of total) were positive for all the virulence genes examined. In birds with E. coli isolated from multiple bones, the virulence gene distribution was identical at all sites in 63% of the cases. A total of 41 staphylococcal isolates were recovered from bones and six of these isolates (15%) were categorized as coagulase-positive Staphylococcus spp.
Table 4. Total bacteria isolated from all sites.
Table 5. Distribution of the virulence genes among the APEC isolates in the proximal end of the femur or tibia of the birds.
Bacterial isolates from the proximal head of the femur or tibiotarsus bones
Of the birds with positive bacterial culture and histological evidence of inflammation in the bone (n = 52), E. coli was recovered from 34 birds (65.3%), Staphylococcus spp. were isolated from eight birds (11.5%), other bacteria species were isolated from eight birds (15.3%) and a mixed population was recovered from four birds (8%). There was a strong association between the presence of a positive bacteriological culture of the femur and the presence of histologically confirmed BCO in the same leg.
Bacterial isolates from birds with histologically confirmed BCO stratified by age are listed in . There were significantly higher numbers of E. coli isolates than Staphylococcus spp. present in all flocks at all time points (week 1: E. coli, 84% and Staphylococcus spp. 6.4%, P < 0.0001; week 4: E. coli, 68% and Staphylococcus spp. 6.4%, P < 0.0004; week 5: E. coli, 60% and Staphylococcus spp. 6.6%, P < 0.0315). Of birds with positive bone cultures, 55% were positive for the same bacterium in other non-bone tissues, but as cultures were only taken from other tissues when gross lesions were identified, the statistical correlation between bone and tissue isolates could not be assessed.
Table 6. Age distribution of bacterial isolates and histological detection inflammation in the proximal end of the femur or tibia of the birds.
Histological assessment of lesions in bones
Due to the possibility of postmortem bacterial colonization of the bone, cases of BCO in this study were defined as birds with evidence of inflammation within the proximal tibia or femur, rather than the presence of bacterial organisms alone (. With the presence of histological inflammatory lesions as the gold standard for diagnosis of BCO, visual detection of gross lesions in the bone was found to have a good negative predictive value (95.6%) but a poor positive predictive value (61.7%). Of bones with evidence of BCO, 46.4% were femurs and 53.6% were tibias. 29.3% of lesions were present within the growth plate, 25.7% were within the subchondral bone and 45.0% were in both sites.
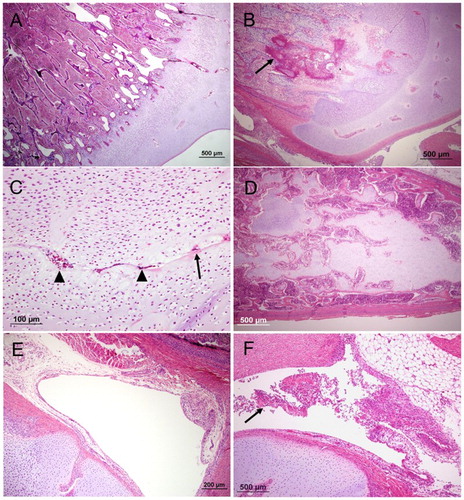
Figure 1. The proximal end of femur or tibiotarsus (haematoxylin and eosin) with different lesions. (A) an example of a typical healthy growth plate; (B), an example of a typical BCO inflammation within the epiphyseal growth plate (arrow); (C), non-artefactual separation at the hypertrophic zone of the growth plate. Note evidence of remodelling at the margins of the cleft (eosinophilic matrix, arrow) and cellular debris within the cleft (arrowheads); (D), large plug of retained cartilage extending down the diaphysis; (E), an example of a typical healthy thin synovium and (F), an example of fibrinoheterophilic synovitis with exudate in joint (arrow) and thickened synovium.
The site of bacterial colonization was assessed during histological review, with colonization classified as arising within cartilaginous vascular channels, within the subchondral metaphysis or within the diaphysis. The majority of the cases (68.6%) displayed bacterial colonization at multiple sites, presumably due to the advanced nature of disease at the time of culling. Of those that displayed colonization at a single site, 15.1% were in the diaphysis, 37.7% were within the subchondral metaphysis and 47.2% were within the vascular channels in the cartilage.
Degeneration of growth plate cartilage was defined as evidence of matrix eosinophilia (indicative of necrosis), or separation or clefting within the cartilage. Separation of zones of the growth plate was very common, but in many cases this appeared to be artefactual. For this study, only cases with additional evidence of remodelling (such as chondrocyte nesting or hyperplasia), debris within the clefts or marginal matrix alterations at the site of separation were considered genuine (. There was no significant variation in prevalence of cartilage degeneration with age (P = 0.08); however, there was a slight but significant increase in prevalence of degeneration in femurs compared to tibiotarsi (15.6% and 11.2%, respectively, P = 0.032). Retained cartilage plugs within the medulla ( were observed significantly more frequently in tibiotarsi than femurs (18.1% of femurs and 23.4% of tibiotarsi, P = 0.031). Plugs predominantly occurred in birds under 2 weeks of age (49.2% of birds) and were rare in older birds (1.4% of birds aged between 2–4 weeks, 2.7% of birds over 4 weeks of age); this difference was statistically significant (P < 0.0001). Histological evidence of vascular degeneration within the growth plate was extremely common (identified in 43.6% of bones), but the degeneration was consistent with progressive replacement by cartilage matrix without evidence of necrosis in areas supplied by the vessel. Early changes in degenerate vessels consisted of fibrin accumulation within cartilage channels, typically in association with pyknotic nuclear debris (. This initial degenerative change was followed by induction of chondrocytic metaplasia of mesenchymal cells within the channel (. Subsequently, these cells generated chondroid matrix which eventually effaced the cartilage channel (. There was a statistically significant increase in the number of birds displaying vascular degeneration at 4 and 5 weeks compared with birds <2 weeks of age (23.8% of birds <2 weeks old versus 53.8% of birds 2–4 weeks old and 60.8% of birds 5 weeks old, P < 0.0001), and a higher prevalence of vascular degeneration was noted in femurs than tibiotarsi (48.9% of femurs versus 38.2% of tibiotarsi, P = 0.025). Lymphoid follicles within the bone marrow were also seen occasionally, consistent with lymphoid hyperplasia. There was significant variation in prevalence of lymphoid follicles with bird age, with birds older than 2 weeks of age significantly more likely to display follicles (0.9%, 9.3% and 6.2% in birds <2 weeks, 2–4 weeks and >4 weeks, respectively, P < 0.0001), and distribution within bones was also uneven, with more follicles in the femur than tibia (6.5% versus 3.7%, P = 0.033). Synovitis observed was predominantly (76.9%) fibrinous, heterophilic or necrotizing (, with the remainder being proliferative or lymphoplasmacytic. Synovitis was significantly more likely to occur in the stifle than the coxofemoral joints (16.5% versus 9.2%, P < 0.0001), but the prevalence of synovitis did not significantly differ with age.
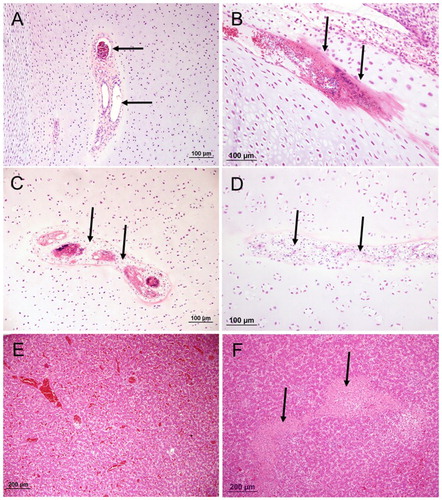
Figure 2. The proximal end and diaphysis of femur or tibiotarsus (haematoxylin and eosin) with different lesions; (A), normal vascular channel containing patent blood vessels (arrows); (B), early-stage vascular regression, cartilage channel displaying fibrin accumulation containing pyknotic nuclear debris (arrows); (C), mid-stage vascular regression; chondrocytic metaplasia of mesenchymal cells (arrows) surrounding degenerate vessels; (D), late-stage vascular regression. Effacement of channel by cartilage matrix; (E), normal liver tissue and (F), typical coalescing regions of parenchymal necrosis in a case of hepatitis (arrow).
Histological assessment of lesions in tissues other than bones
Infection with intestinal coccidia was found in some birds but relatively mild in all cases, and none of the birds displayed evidence of protozoal invasion or inflammation extending beyond the mucosa. Similarly, where detected, other gastrointestinal lesions were only mild in nature, predominantly manifesting as increased numbers of crypts distended by debris (crypt and micro abscesses), sometimes associated with fibrosis. Significant ulcerative or necrotizing gastrointestinal disease was extremely rare (<five cases). Prevalence of enteritis was not significantly different with age (P > 0.05). Hepatitis predominantly presented as multifocal areas of necrosis ( associated with a variable inflammatory cell infiltrate. There was no significant association between the prevalence of hepatitis and age (P > 0.05). Omphalitis and yolk sac infection were grouped together, defined as the gross observation of an unhealed or exudative navel, or histological evidence of inflammation within the yolk sac remnant. Expectedly, there was a significant decrease in prevalence of omphalitis or yolk sac infection with age (44.7% of birds less than 2 weeks, versus 22.1% of birds 2–4 weeks old and 6.8% of birds over 4 weeks old, P < 0.0001).
Association between BCO and other lesions
The co-prevalence of other diseases in birds with BCO is summarized in . Almost 50% of birds with BCO displayed lesions in multiple bones. Birds with evidence of hepatitis were over eight times more likely to have concurrent BCO than unaffected birds, and over 60% of birds with hepatitis had concurrent BCO. Birds with synovitis were also greater than 10 times more likely to display BCO than those without BCO lesions. The presence of cartilage abnormalities, either growth plate cartilage defects or retained cartilage plugs within the medulla, was also strongly correlated with the occurrence of BCO. Non-significant association trends were noted with vascular channel regression (P = 0.08) and concurrent respiratory disease (P = 0.07), though the calculated correlation was slight.
Table 7. Association between BCO and other lesions.
No significant association was noted between BCO and omphalitis/yolk sac infections, skin scratches, tibial malformation (either within the same limb or the contralateral limb), presence of coccidia or enteritis, lymphoid hyperplasia within bone marrow or marrow depletion (see ). While no significant correlation was noted with serositis and BCO, there was a trend towards statistically significant association in serositis cases where E. coli was isolated (P = 0.064), in comparison with Staphylococcus (P = 0.3) (data not shown).
Association between BCO and other bird factors
BCO was identified in 26.6% of male birds and 32.86% of female birds; this difference was not statistically significant (P = 0.355, odds ratio 0.742). The association of BCO with bodyweight is summarized in . There was a significant correlation between BCO and lower bodyweight in birds under 5 weeks of age, but this association did not hold for 5-week-old birds. There was no statistically significant association between BCO and the hatchery of origin (P = 0.273). This holds true even for exclusive assessment of birds <2 weeks of age (data not shown, P = 0.233).
Table 8. Correlation between BCO and bodyweight at different ages.
Serological examination of the flocks showed elevated CIAV antibodies later in a bird’s life
Four out of five longitudinally examined flocks in this study had minimal CIAV antibodies in week 1 but had significantly elevated antibodies at weeks 4 and 5 of age (). ReoV antibodies remained relatively low at all time points examined for all flocks in this study, though three out of five flocks showed a very mild increase in ReoV antibodies in week 5. With IBDV antibodies, four out of five flocks had moderate to high antibody titres in week 1 but showed significantly reduced antibody titres at weeks 4 and 5 of age.
Table 9. Average (SD) of CIAV, IBDV and ReoV ELISA antibodies in broiler flocks examined in this study.
When serological titres for all flocks were combined and analysed (), CIAV antibodies were found to be low in week 1 but significantly elevated in weeks 4 (P < 0.0001) and 5 (P < 0.0001). ReoV antibodies were also low in week 1, showed no increase in week 4 and only a very mild but significant increase in week 5 (P = 0.001). In contrast, IBD antibodies were relatively strong in week 1, but significantly reduced in week 4 (P = 0.000) and then only mildly elevated in week 5 (P = 0.040).
There was no correlation between the CIAV titre and inflammation in the bones, using either individual bird (correlation coefficient = 0.184) or flock average data (0.202). With the view that CIAV antibodies take few weeks to develop, this analysis was repeated only for older flocks (over 3 weeks); however correlation was still very poor (–0.025).
Discussion
In this study a strong correlation was found between the presence of chondronecrosis and osteomyelitis lesions and isolation of bacteria from bone, supporting the notion that that this process is primarily due to bacterial infection (BCO). E. coli was by far the most frequently isolated bacterium from all the sites that were swabbed, including bones with BCO. Staphylococcal species, in contrast, were rarely isolated, and the majority of the Staphylococcus isolated were coagulase-negative. Previous studies have most commonly associated BCO with S. aureus infection (McNamee et al., Citation1998; Wideman & Prisby, Citation2012), to the extent that this is the generally accepted perception on the matter (Andreasen, Citation2013). Only one paper has previously noted E. coli as the main organism isolated from BCO-associated osteomyelitis (Dinev, Citation2009). The results of this study clearly indicate that E. coli is the major pathogen in BCO within the Australian broiler industry, with S. aureus accounting for a small minority of the cases. Moreover, in almost half of the cases where E. coli was isolated the lesions were identified exclusively in the bone, suggesting that the organism may have a specific tropism for this site. The observation of a higher proportion of bacterial colonization arising within or adjacent to the growth plate is consistent with the known predilection for bacterial osteomyelitis to develop at this site, purportedly reflecting the vascular anatomy in this location. Physeal vessels are fenestrated and loop acutely, with substantial slowing of blood flow in this region, providing excellent conditions for attachment and establishment of bacterial emboli (Craig et al., Citation2016).
Avian pathogenic E. coli is a significant pathogen in poultry, responsible for causing massive economic losses through septicaemia (colisepticaemia) and various localized forms of infections including airsacculitis, pericarditis, perihepatitis, cellulitis, omphalitis, salpingitis, peritonitis and osteomyelitis (Barnes et al., Citation2008). APEC is a subset of extra-intestinal pathogenic E. coli, a pathotypic category which also includes human uropathogenic E. coli (Russo & Johnson, Citation2003). However, APEC strains possess additional virulence genes, including the CoIV plasmid-associated genes omT, hlyF, iss, iutA and iroN, (Johnson et al., Citation2008a) that potentiate their pathogenicity in poultry (Kaper et al., Citation2004; Johnson et al., Citation2008a). Due to a lack of definitive classification criteria, there is currently no single reliable method for identification and characterization of APEC strains due to their high diversity (Mokady et al., Citation2005; Moulin-Schouleur et al., Citation2007); however, ColV plasmid-associated virulence genes have been proposed as potential markers for differentiation and identification (Rodriguez-Siek et al., Citation2005). In this study, E. coli isolates were categorized as APEC if they were positive for combination of any of the siderophore receptor and other virulence genes assessed. The majority of the E. coli isolates from the bones possessed all five genes, and the rest of the isolates carried at least one virulence gene. Some studies suggest that the APEC population may be divided into a number of subpopulations based on their pathology and virulence factors, essentially classified as different pathotypes (Maturana et al., Citation2011; Hussein et al., Citation2013). However, in approximately one-third of the cases from this study, E. coli isolates from different sites in the same bird displayed different virulence genes, suggesting that the genetic profile of APEC organisms may be quite fluid.
There was a marked variation in the prevalence of BCO in culled/dead birds between individual flocks in this study, consistent with the sporadic and highly variable nature of the disease. Despite this, the mean prevalence of BCO in culled/dead birds was remarkably similar in all age groups and also overall, ranging between 22% and 29% of culled or dead birds, supporting the notion that BCO is a major cause of wastage in Australian commercial broiler birds. These prevalence figures are nearly double those reported for UK broiler flocks, where 13.7% of dead or culled birds displayed histological evidence of BCO (McNamee & Smyth, Citation2000). Moreover, these figures do not capture the number of subclinically affected birds which survive to slaughter, and so total prevalence in flocks is almost certainly higher than the figures reported in this study. It is also important to note that BCO was more frequently identified within the proximal tibiotarsus than the femur, demonstrating that the outdated term “femoral head necrosis” is no longer a suitable descriptor for this disease.
The consistent prevalence across all age groups is another interesting finding, as previous studies have predominantly recognized BCO in older birds (Nairn & Watson, Citation1972; McNamee et al., Citation1999a; Dinev, Citation2009). The reason for this variation from previous studies is unclear; it could reflect a focus on older birds in previous research, or it may represent an epidemiological peculiarity of Australian broiler flocks. Nevertheless, it is clear that BCO is a more significant disease process in younger birds than has previously been recognized, and many birds that have previously been classified as non-specific early mortalities may have been cases of unrecognized BCO.
Of all bone lesions, growth plate cartilage abnormalities were found to be strongly associated with the development of BCO. Separation, clefting or ischaemia of the cartilage would appear to provide a suitable local environment for subsequent bacterial colonization. These findings account for the increased incidence of BCO observed with the wire-floor experimental model developed by Wideman (Wideman et al., Citation2012). In this model, birds are thought to be more susceptible to BCO due to the degeneration of epiphyseal cartilage induced by increased shearing forces within the limbs. The wire flooring used in this model is unlikely to provide an accurate representation of the field environment, but it is quite possible that such lesions are induced at a lesser level by flooring issues in broiler sheds. However, it is interesting to note that there was no significant correlation between femoral head necrosis and tibiotarsal malformation. If sustained alteration to limb forces predisposed to development of BCO, a statistical association between these diseases would be expected. This lack of association instead suggests that cartilage defects are induced by sudden or dynamic shifts in the forces applied to limbs, such as from slippage or uneven flooring. Irrespective of the strong association between BCO and cartilage lesions, it is important to note that 25% of birds displayed inflammatory lesions located exclusively within the subchondral bone, and so defects within the cartilage may not be necessary for the development of BCO in all cases.
Retention of a cartilage plug within the tibial or femoral medulla was also strongly correlated with the occurrence of BCO. Cartilage plugs are relatively avascular islands of retained cartilage which presumably provide a suitable environment for bacterial colonization, similar to sites of separation, clefting or ischaemia within the growth plate. While some of these cartilage plugs were of sufficient size to warrant a diagnosis of clinical dyschondroplasia, the majority were smaller islands of retained cartilage consistent with subclinical manifestations of the disease. Nevertheless, based on this study it appears that even mild cartilage retention and delayed maturation can produce a suitable local environment for development of BCO, and so reducing the prevalence of dyschondroplastic lesions may provide the additional benefit of reduction in prevalence of BCO. Dyschondroplasia in broiler birds is a complex multifactorial disease, and development of the condition has been associated with a range of factors, including nutritional imbalances (calcium:phosphate ratio, hypovitaminosis D), bird breed and dietary mycotoxins (Leach & Monsonego-Ornan, Citation2007; Khan et al., Citation2010). Nutritional issues in particular are a factor that can be addressed readily on broiler farms, and so supplying a diet that minimizes dyschondroplasia (e.g. cholecalciferol supplementation, optimizing dietary calcium and phosphate concentrations) may be cost-effective means of reducing the incidence of BCO by minimizing the occurrence of retained cartilage.
Vascular degeneration within the growth plate has been proposed as a predisposing factor in the pathogenesis of BCO (Wideman & Prisby, Citation2012). The ordered regression of vessels and cartilage canals without evidence of ischaemia that was observed in birds in this study is consistent with the process of chondrification, a physiological remodelling and reduction of the vascular supply to the cartilage during normal development. This age-related process is well documented in horses and pigs (Ytrehus et al., Citation2004), and though the change has previously been suggested to predispose to development of osteochrondrosis dissecans in these species, studies have failed to document an association. In the present study, there was a slight non-significant trend of correlation between BCO and vascular regression, and it is possible that chondrification sites may provide a suitable site for initiation of BCO in some instances.
Synovitis was strongly associated with the presence of BCO, but to a large extent this correlation is likely to reflect local extension of inflammation through the articular surface. Almost half of bones with BCO displayed evidence of synovitis within the adjacent joint, and the majority of these were fibrinoheterophilic, consistent with a bacterial aetiology.
The correlation between the occurrence of omphalitis or yolk sac infection and BCO was very poor, suggesting that latent neonatal infection is not a major source for bacterial dissemination in this disease. This interpretation is further supported by the observation that hatchery of origin and eggshell disinfection had no effect on the prevalence of BCO in this study.
Experimental models of BCO have induced the disease through both respiratory (McNamee et al., Citation1999b) and gastrointestinal (Al-Rubaye et al., Citation2015) bacterial exposure, but this study was not able to determine a common route of entry for bacteria involved in BCO under field conditions. Scarcity of severe enteric lesions in the sampled birds, particularly those with defects extending beyond the mucosa, means that the intestine cannot be entirely excluded as a potential source of bacteraemia. Nevertheless, it appears that the presence of enteritis is not a necessary condition for the development of BCO. Similarly, no statistical association was noted between the presence of BCO and concurrent skin injuries or respiratory disease. However, the strong correlation between hepatitis and BCO, and frequent identification of concurrent infection in multiple bones implies bacteraemic dissemination. Overall, these findings suggest that BCO may not result from bacteraemia caused by deficiencies in a particular mucosal or skin barrier, but instead is more likely to reflect opportunistic infection following low-level endemic bacteraemia. This is consistent with recent investigations (Jiang et al., Citation2015) which detected a range of bacterial organisms within the epiphyses of normal chickens via 16S rRNA sequencing, and also identification of a range of bacteria (including proteobacteria) within the blood of broiler chickens (Mandal et al., Citation2016). It appears likely that low-level bacteraemia is inevitable in broilers, but successful bacterial colonization of bone is dependent on favourable local environmental and host immune factors. If this is the case, improving immunological resistance to bacterial colonization though vaccination against common pathogens or ensuring appropriate protection from other immunosuppressive disease may prove to be effective in reducing the prevalence of the disease. Alternatively, shifting the microbiological flora towards non-pathogenic organisms, such as through the use of probiotics, is another approach that may limit development of BCO.
The presence of BCO in younger birds that were culled or died was significantly associated with decreased bodyweight. This is consistent with previously reported research (Emslie et al., Citation1983), where decreased weight has been used as a predictor for the presence of BCO. It has previously been suggested that this decrease in bodyweight reflects reduced nutritional and water intake secondary to lameness. However, in this study the birds assessed were all culls or mortalities, and therefore illness is likely to have reduced food and water intake in all of the birds to some degree prior to death. It is therefore possible that this trend towards decreased weight is a genuine association rather than a secondary effect. Irrespective, these findings provide further confirmation that development of BCO is not directly correlated with heavier birds, and so restriction of weight gain may not reduce the prevalence of BCO.
The flocks included in this study all derived from parents that were vaccinated against IBDV and CIAV, but were not themselves vaccinated against either virus. The birds examined appeared to carry ample maternal antibodies to IBDV and showed only a modest elevation of IBDV antibodies in weeks 4 and 5; therefore there was very little evidence of field IBDV infection early in a chick’s life. Previous work by McNamee identifying Staphylococcus as the major pathogen in BCO also detected IBDV in a number of the birds examined (McNamee et al., Citation1998), and this distinction may help explain the difference in pathogen profile within our flock. IBDV infection has been demonstrated to increase colonization of bone by S. aureus in experimental models (McNamee et al., Citation1999b). In contrast, infection with IBDV increases septicaemic mortality in birds inoculated with E. coli (Nakamura et al., Citation1990). It is possible that non-IBDV-infected birds are more likely to have sufficient immunological competence to prevent E. coli septicaemia, but not to eliminate infection entirely, predisposing to the development of less fulminant infections such as BCO. Thus, the authors speculate that immunosuppression caused by IBDV infection may increase susceptibility to Staphylococcus-induced BCO, while simultaneously increasing the likelihood of fatal E. coli septicaemia rather than E. coli-induced BCO.
There was also no evidence of ReoV maternal antibodies, or indication of serious field ReoV exposure later in a chick’s life. In contrast, it was apparent – based on the low titres in young birds and the subsequent titre increase in older birds – that chicks carry only minimal maternal antibodies against CIAV and they are usually exposed to a field challenge with CIAV early in life. CIAV infection has been used in BCO models to increase susceptibility to the condition (McNamee et al., Citation1999b), and corticosteroid administration has also been demonstrated to increase the risk of BCO, presumably through immunosuppression (Wideman & Pevner, Citation2012). However, despite these suggestions that CIAV infection may predispose to BCO, we were unable to demonstrate a correlation between CIAV titres and the presence of BCO lesions. It is possible that better indications of an association may be obtained through specific viral testing (PCR), rather than assessing for an association with immunity through antibody titres.
Due to the restriction of analysis to culled and dead birds in the present study, the overall prevalence of BCO and related diseases in the flocks cannot be ascertained. While the prevalence of lesions such as tibial dyschondroplasia has been reported previously in some flock populations (Sanotra et al., Citation2003), for many of the changes assessed in this study, including vascular regression, cartilage defects and synovitis the prevalence in normal birds is unknown. This study identifies several factors that are associated with development of BCO, but further research in disease prevention will hinge on understanding the prevalence of such factors within the normal broiler population.
This is the first comprehensive study of bacteriological agents and histopathology associated with BCO in broiler chickens in Victoria, Australia, and demonstrates that the disease has distinct regional characteristics compared with other populations. In contrast to other studies of this disease, we found that BCO was prevalent in culled or dead birds of all ages, and was identified in approximately one-quarter of broiler culls and mortalities. BCO appears to be an infectious process that occurs as a result of bacteraemia and haematological spread of bacterial pathogens – especially E. coli – to the bones. We observed that almost all E. coli isolated from cases of BCO are APEC, and thus conclude that further research should be performed to develop preventative measures against this organism.
Acknowledgements
The authors would like to thank field veterinarians and poultry servicemen for facilitating submission of the specimens for the duration of this study, and Faye Docherty and Paul Benham for their assistance in histopathological processing.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Anthony Neal Chamings http://orcid.org/0000-0002-7762-4757
Mark Stevenson http://orcid.org/0000-0003-1890-9784
Marc Serge Marenda http://orcid.org/0000-0001-6778-1110
Amir H. Noormohammadi http://orcid.org/0000-0003-3459-2692
Andrew Stent http://orcid.org/0000-0002-4432-8185
Additional information
Funding
References
- Al-Rubaye, A.A., Couger, M.B., Ojha, S., Pummill, J.F., Koon, J.A., Wideman Jr, R.F. & Rhoads, D.D. (2015). Genome analysis of Staphylococcus agnetis, an agent of lameness in broiler chickens. PloS One, 10, e0143336. doi: 10.1371/journal.pone.0143336
- Andreasen, C.B. (2013). Staphylococcosis. In D.E. Swayne, J.R. Glison, L.R. McDougald, L.K. Nolan, D.L. Suarez, & V. Nair (Eds.), Diseases of Poultry 13th ed. (pp. 1394). Ames: John Wiley & Sons.
- Barnes, H.J., Nolan, L.K. & Vaillancourt, J.F. (2008). Colibacillosis. In Y.M. Saif, A.M. Fadly, J.R. Glison, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.), Diseases of Poultry 12th ed. (pp. 691–732). Ames, IA: Blackwell publishing.
- Craig, L.E., Dittmer, K.E. & Thompson, K.G. (2016). Bones and joints. In M.G. Maxie (Ed.), Jubb, Kennedy and Palmer’s Pathology of Domestic Animals 6th ed. (pp. 98–103). St. Louis, MO: Elsevier.
- Dinev, I. (1996). Studies on staphylococcal infection in fowls. II. Veterinary Medicine, 2, 90–93.
- Dinev, I. (2009). Clinical and morphological investigations on the prevalence of lameness associated with femoral head necrosis in broilers. British Poultry Science, 50, 284–290. doi: 10.1080/00071660902942783
- Dinev, I., Lutzkanov, M., Stoikov, D. & Grozeva, V. (1995). Clinical-epizootological and histological investigations in staphylococcal infection in birds. 1. In Proceedings of the 6th National Conference of the Union of the Bulgarian Scientists (pp. 137—144.). Stara Zagora.
- Dinev, I., Lutzkanov, M., Stoikov, D. & Grozeva, V. (1997). Investigation on staphylococcal infection in birds. III. Veterinary Science, 1–2, 346–349.
- Emslie, K.R., Ozanne, N.R. & Nade, S.M. (1983). Acute haematogenous osteomyelitis: an experimental model. The Journal of Pathology, 141, 157–167. doi: 10.1002/path.1711410206
- Hussein, A.H., Ghanem, I.A., Eid, A.A., Ali, M.A., Sherwood, J.S., Li, G., Nolan, L.K. & Logue, C.M. (2013). Molecular and phenotypic characterization of Escherichia coli isolated from broiler chicken flocks in Egypt. Avian Diseases, 57, 602–611. doi: 10.1637/10503-012513-Reg.1
- Jiang, T., Mandal, R.K., Wideman, R.F.J., Khatiwara, A., Pevzner, I. & Kwon, Y.M. (2015). Molecular survey of bacterial communities associated with bacterial chondronecrosis with osteomyelitis (BCO) in broilers. PloS One, 10, e0124403. doi: 10.1371/journal.pone.0124403
- Johnson, J.R. & Brown, J.J. (1996). A novel multiply primed polymerase chain reaction assay for identification of variant papG genes encoding the Gal(α1–4)Gal-binding PapG adhesins of Escherichia coli. Journal of Infectious Diseases, 173, 920–926. doi: 10.1093/infdis/173.4.920
- Johnson, T.J., Siek, K.E., Johnson, S.J. & Nolan, L.K. (2006). DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. Journal of Bacteriology, 188, 745–758. doi: 10.1128/JB.188.2.745-758.2006
- Johnson, T.J., Wannemuehler, Y. M., Doetkott, C., Johnson, S., Rosenberger, S. & Nolan, L.K. (2008a). Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. Journal of Clinical Microbiology, 46, 3987–3996. doi: 10.1128/JCM.00816-08
- Johnson, T.J., Wannemuehler, Y.M. & Nolan, L.K. (2008b). Evolution of the iss gene in Escherichia coli. Applied Environmental Microbiology, 74, 2360–2369. doi: 10.1128/AEM.02634-07
- Kaper, J.B., Nataro, J.P. & Mobley, H.L. (2004). Pathogenic Escherichia coli. Nature Reviews Microbiology, 2, 123–140. doi: 10.1038/nrmicro818
- Khan, S.H., Shahid, R., Mian, A.A., Sardar, R. & Anjum, M.A. (2010). Effect of the level of cholecalciferol supplementation of broiler diets on the performance and tibial dyschondroplasia. Journal of Animal Physiology and Animal Nutrition, 94, 584–593. doi: 10.1111/j.1439-0396.2009.00943.x
- Leach, R.M., Jr. & Monsonego-Ornan, E. (2007). Tibial dyschondroplasia 40 years later. Poultry Science, 86, 2053–2058. doi: 10.1093/ps/86.10.2053
- Mandal, R.K., Jiang, T., Al-Rubaye, A.A., Rhoads, D.D., Wideman, R.F., Zhao, J., Pevzner, I. & Kwon, Y.M. (2016). An investigation into blood microbiota and its potential association with bacterial chondronecrosis with osteomyelitis (BCO) in broilers. Scientific Reports, 6, 25882. doi: 10.1038/srep25882
- Maturana, V.G., de Pace, F., Carlos, C., Mistretta Pires, M., Amabile de Campos, T., Nakazato, G., Guedes Stheling, E., Logue, C.M., Nolan, L.K. & Dias da Silveira, W. (2011). Subpathotypes of avian pathogenic Escherichia coli (APEC) exist as defined by their syndromes and virulence traits. Open Microbiology Journal, 5, 55–64. doi: 10.2174/1874285801105010055
- McNamee, P.T., McCullagh, J.J., O’Hagan, J., Spratt-Davidson, S., Mulholland, E.J., Ball, H.J. & Smyth, J.A. (1999a). A longitudinal study of leg weakness in five commercial broiler flocks. In Proceedings of the 48th Western Poultry Disease Conference, Vancouver, BC.
- McNamee, P.T., McCullagh, J.J., Rodgers, J.D., Thorp, B.H., Ball, H.J., Connor, T.J., McConaghy, D. & Smyth, J.A. (1999b). Development of an experimental model of bacterial chondronecrosis with osteomyelitis in broilers following exposure to Staphylococcus aureus by aerosol, and inoculation with chicken anaemia and infectious bursal disease viruses. Avian Pathology, 28, 26–35. doi: 10.1080/03079459995019
- McNamee, P.T. & Smyth, J.A. (2000). Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: a review. Avian Pathology, 29, 477–495. doi: 10.1080/030794500750047243
- McNamee, P.T., Thorp, J.J., Ball, B.H., Graham, H.J., McCullough, D., McConaghy, S.J. & Smyth, D.J.A. (1998). Study of leg weakness in two commercial broiler flocks. Veterinary Record (United Kingdom), 143, 131–135. doi: 10.1136/vr.143.5.131
- Mokady, D., Ghopna, U. & Ron, E.Z. (2005). Extensive gene diversity in septecemic Escherichia coli strains. Journal of Clinical Microbiology, 43, 66–73. doi: 10.1128/JCM.43.1.66-73.2005
- Morales, C., M. D. Lee, C. Hofacre & Maurer, J.J. (2004). Detection of a novel virulence gene and a Salmonella virulence homologue among Escherichia coli isolated from broiler chickens. Foodborne Pathogens and Disease, 1, 160–165. doi: 10.1089/fpd.2004.1.160
- Moulin-Schouleur, M., Reperant, M., Laurent, S., Bree, A., Mignon-Grasteau, S., Germon, P., Rasschaert, D. & Schouler, C. (2007). Extraintestinal pathogenic Escherichia coli strains of avian and human origin: link between phylogenetic relationships and common virulence patterns. Journal of Clinical Microbiology, 45, 3366–3376. doi: 10.1128/JCM.00037-07
- Nairn, M.E. & Watson, A.R. (1972). Leg weakness of poultry – a clinical and pathological characterisation. Australian Veterinary Journal, 48, 645–656. doi: 10.1111/j.1751-0813.1972.tb09237.x
- Nakamura, K., Yuasa, N., Abe, H. & Narita, M. (1990). Effect of infectious bursal disease virus on infections produced by Escherichia coli of high and low virulence in chickens. Avian Pathology, 19, 713–721. doi: 10.1080/03079459008418726
- Reece, R.L. (1992). The role of infectious agents in leg abnormalities in growing birds. In C.C. Whitehead (Ed.), Bone Biology and Skeletal Disorders in Poultry (pp. 231–263). Abingdon: Carfax Publishing Ltd.
- Rodriguez-Siek, K.E., Giddings, C.W., Doetkott, C., Johnson, T.J., Fakhr, M.K. & Nolan, L.K. (2005). Comparison of Escherichia coli isolated implicated in human urinary tract infection and avian colibacillosis. Microbiology, 151, 2097–2110. doi: 10.1099/mic.0.27499-0
- Russo, T. & Johnson, J.R. (2003). Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infection, 5, 449–456. doi: 10.1016/S1286-4579(03)00049-2
- Sanotra, G.S., Berg, C. & Lund, J.D. (2003). A comparison between leg problems in Danish and Swedish broiler production. Animal Welfare, 12, 677–683.
- Thorp, B.H., Whitehead, C.C., Dick, L., Bradbury, J.M., Jones, R.C. & Wood, A. (1993). Proximal femoral degeneration in growing broiler fowl. Avian Pathology, 22, 325–342. doi: 10.1080/03079459308418924
- Wideman, R.F.J., Hamal, K.R., Stark, J.M., Blankenship, J. & Lester, H. (2012). A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poultry Science, 91, 870–883. doi: 10.3382/ps.2011-01907
- Wideman, R.F.J. & Pevner, I. (2012). Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poultry Science, 10, 2464–2474. doi: 10.3382/ps.2012-02386
- Wideman, R.F.J. & Prisby, R.D. (2012). Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Frontiers in Endocrinology, 3, 183.
- Wyers, M., Cherel, Y. & Plassiart, G. (1991). Late clinical expression of lameness related to associated osteomyelitis and tibial dyschondroplasia in male breeding turkeys. Avian Diseases, 35, 408–414. doi: 10.2307/1591199
- Ytrehus, B., Carlson, C.S., Lundeheim, N., Mathisen, L., Reinholt, F.P., Teige, J. & Ekman, S. (2004). Vascularisation and osteochondrosis of the epiphyseal growth cartilage of the distal femur in pigs – development with age, growth rate, weight and joint shape. Bone, 34, 454–465. doi: 10.1016/j.bone.2003.07.011
