ABSTRACT
The broiler industry has incurred significant economic losses due to two muscle myopathies, white striping (WS) and wooden breast (WB), affecting the Pectoralis major (P. major) of commercial broilers. The present study documented macroscopic changes occurring with age/growth in the P. major and P. minor muscles of commercial broilers from day 2 through day 46 (n = 27/day). Distinct myopathic aberrations observed in both breast muscles corresponded to the onset of WB. These distinct morphological changes were used as determinants in developing a ranking system, defining the ontogeny of WB as the following four stages: (1) WS, (2) petechial epimysium haemorrhages, (3) intramuscular haemorrhages and (4) ischaemia. A cumulative logit proportional odds model was used to relate the rank probabilities with the following growth parameters: body weight, P. major and P. minor weight/yield/length/width/depth. The best-fit model included P. major length/width/depth, P. minor width, P. major and P. minor yield as predictors for rank. Increasing P. major depth, P. minor width and P. major yield increased the odds of falling into higher ranks (more severe myopathy). Conversely, increasing P. major length, P. major width and P. minor yield increased the odds of falling into smaller ranks (less severe myopathy). This study describes the macroscopic changes associated with WB ontogeny in the development of a ranking system and the contribution of growth parameters in the determination of rank (WB severity). Results suggest that physical measurements inherent to selection for high-yielding broiler genotypes are contributing to the occurrence and severity of WS and WB.
Introduction
Darwin’s description of natural selection was substantiated, in part, with the knowledge that humans had successfully produced changes, over time, in domestic animals using artificial selection (Citation1859). For the broiler industry, selection has remained focused on maximizing muscle growth for the production of meat (Barbut et al., Citation2008). The Pectoralis major (P. major) and M. supracoracoideus (P. minor) breast muscles are of considerable value to the broiler industry, with the P. major being the predominant meat producer and consequently that of greatest economic value (USDA, Citation2016). As such, selection for increased P. major yield has become a primary genetic focus of the broiler industry, along with improvements in growth rate and feed conversion (Havenstein et al., Citation1994, Citation2003; Zuidhof et al., Citation2014). These improvements have led to significant increases in body weight (BW), P. major yield (Lilburn, Citation1994; Mazzoni et al., Citation2015) and conformational changes in breast muscle (Godfrey & Goodman, Citation1956; Reddish & Lilburn, Citation2004), resulting in commercial strains that are marketed in half the time with nearly twice the BW of their 1950s counterparts (Barbut et al., Citation2008). However, the same factors integral to this success – a large decline in days to market and disproportional increases in breast yield – have contributed to an increase in skeletal muscle myopathies (Petracci et al., Citation2015), such as Deep Pectoral Myopathy (DPM), white striping (WS), wooden breast (WB) and spaghetti meat (SM).
Among the first of these recognized myopathies is DPM, identified as ischaemic necrosis of the P. minor in broiler breeders (Page & Fletcher, Citation1975; Richardson et al., Citation1980; Wight & Siller, Citation1980; Bianchi et al., Citation2006), selected for increased breast muscle growth (Siller, Citation1985). Macro- and microscopic characterizations of WS/WB have striking similarities to what have been described for DPM (Wight et al., Citation1981), including mild to severe oedema (Soglia et al., Citation2016), haemorrhagic/inflammatory lesions (Sihvo et al., Citation2014; Bailey et al., Citation2015; Trocino et al., Citation2015) and ischaemia (Kuttappan et al., Citation2013a; Petracci et al., Citation2013a; Sihvo et al., Citation2014; Zotte et al., Citation2014). Microscopic similarities include polyphasic degeneration, perivascular necrosis and infiltration of connective and fat tissue (Kuttappan et al., Citation2012b; Kuttappan et al., Citation2013b; Kuttappan et al., Citation2013c; Ferreira et al., Citation2014; Mudalal et al., Citation2014; Russo et al., Citation2015; Alnahhas et al., Citation2016; Vignale et al., Citation2017; Zambonelli et al., Citation2016).
P. major breast muscles affected by WS and WB can be easily distinguished from one another by their macroscopic characterizations. WS is characterized by the presence of white striations parallel to muscle fibres (Kuttappan et al., Citation2009; Kuttappan et al., Citation2012b), with varying degrees of haemorrhagic lesions (Kuttappan et al., Citation2017), whereas WB is characterized by a pale, hardened breast muscle with haemorrhagic lesions (Sihvo et al., Citation2014), a condition that was first detected in live broilers, as having palpably stiff breast muscles (Puolanne, Citation2015). When examined histologically, the white striations and haemorrhagic lesions associated with WS and the diffuse, hardened areas associated with WB exhibit overlapping characteristics. Included in these are: polyphasic myodegeneration, perivascular necrosis, interstitial inflammation, perivenular infiltration of T lymphocytes with the infiltration of connective tissue and lipid (Sihvo et al., Citation2014; Velleman & Clark, Citation2015; Abasht et al., Citation2016; Clark & Velleman, Citation2016; Baldi et al., Citation2017).
Lastly, a more recent muscular abnormality to be reported in the P. major of modern broilers has been termed SM, due to the altered structural integrity of the P. major, characterized by poor fibre uniformity (Baldi et al., Citation2017). Baldi et al. (Citation2017) reported an increase in P. major weight, thickness and length in fillets affected by both WS and SM.
In addition to a correlation between WS and SM, there are ample data to support an association etween WS and WB (Sihvo et al., Citation2014, Citation2017; Bowker & Zhuang, Citation2016; Tasoniero et al., Citation2016; Radaelli et al., Citation2017). In addition, the onset and severity of each anomaly are influenced by the same factors: Genotype (high > standard breast yield) (Petracci et al., Citation2013b; Alnahhas et al., Citation2016), gender (males > females) (Lorenzi et al., Citation2014), growth rate (fast > slow) (Kuttappan et al., Citation2012a) diet (high > low energy) (Cruz et al., Citation2017) and P. major weight (heavy > light) (Petracci et al., Citation2014; Mutryn et al., Citation2015; Chatterjee et al., Citation2016). Although these myopathies appear coincidingly with one another, the cause-and-effect relationships underlying their associations are not yet clear.
Not surprisingly, these myopathies have had a negative impact on consumer acceptability (Tasoniero et al., Citation2016; Tijare et al., Citation2016), resulting in the downgrading and condemnation of affected breast fillets. With recent flock incidences (percent of broilers exhibiting WS/WB) reported as high as 90% (Owens, Citation2016), the industry has incurred significant economic losses, with projections estimated to range from $200 million to $1 billion (Owens, Citation2016).
Of greater concern, to the economic implications, is the increasing welfare concern for those broilers exhibiting these myopathies; so the question becomes: at what point does a meat-quality issue become a welfare concern? Although the present study did not investigate welfare parameters, this welfare concern is raised as a direct result of the observations made in the present study that led to its early termination two weeks prior to the proposed end date. At 46 days of age, it was determined unethical to maintain birds on feed for further growth, as the vast majority exhibited severe muscle damage (WS/ WB/ DPM) (details provided in subsequent text).
In an effort to further understand the aetiology and connection between these myopathies and their shared characteristics, the overall objective of the present study was to relate and characterize the muscle development changes that occur with age/growth in the P. major of commercial broilers beginning at day 2 post-hatch through 46 days post-hatch. During the course of the experiment, distinct aberrations in the macroscopic morphology of the P. major and P. minor were observed corresponding to the onset and progression of WB. In the present study, a ranking system was devised to describe the ontogeny of WB and a model was developed to relate the rank probabilities with the physical measurements and growth parameters associated with P. major and P. minor growth during the post-hatch growth period.
Materials and methods
Experimental design
Birds, housing and management
All procedures involving the birds used in the present experiment were approved by The Ohio State University Institutional Animal Care and Use Committee. A mix of 1-day-old male and female commercial broiler chicks (n = 900; Hubbard X Ross 708) was delivered to the Ohio Agricultural Research and Development Center (OARDC, Wooster, OH) Poultry Research Farm. All chicks were wing-banded and randomly placed in nine floor pens with feed and water available for ad libitum consumption.
Sample collection
Broilers (three per pen/n = 27) were randomly sampled on Monday, Wednesday, and Friday of each week from 2 to 46 days of age. Live BW was ascertained. Birds were sacrificed via cervical dislocation, ensuring minimal wing flapping. Immediately following cervical dislocation, the intact P. major breast muscles were exposed and photographed from top and side viewpoints (see below). The subsequent sample procedures were done in a sequential manner directly following photo documentation. At this point, the left P. major and P. minor muscles were immediately filleted and individually weighed. [Weights were later used to calculate P. major and P. minor yield as percentage of live BW. In addition, relative growth was defined as the percent change from the initial weight, set as 9 days of age (Actual-Initial/Initial × 100). Relative growth calculations were made using daily pen weight averages for the three sampled birds and the data represent the average for all pens (n = 9)]. Immediately after weight collection the depth of each muscle was measured at the thickest point in the cranial region of the muscle. At this point, both breast muscles were placed on a ruler grid and fillets were photo documented for a second time for later use in obtaining geometric measurements (length, width) using ImageJ software (Schneider et al., Citation2012). P. major and P. minor length were measured from the longest dimension of the fillet and width from the two points furthest apart from side to side (Kuttappan et al., Citation2013a; Mudalal et al., Citation2014). To maintain consistency, the same individual was responsible for filleting both breast muscles, and obtaining muscle depth, length and width measurements throughout the course of the study.
Photo documentation
Image analysis (Photo documentation of both intact superficial P. major muscles and filleted P. major and P. minor breast muscles) began on day 16, following the initial observation of physical changes to the P. major. All photographs were obtained on an individual bird basis (n = 27 per day). Representative images are shown in . These images were later used for assigning a rank (1–4) to individual birds, representing their degree of progression from WS to WB. Rank 1 was defined by the presence of WS only (WS severity was not measured) (). All birds exhibited some degree of WS following the initial photo documentation, the reason for a lack of control or completely unaffected muscle in the rank system. Rank 2 is defined by the presence of surface (epimysium) petechial haemorrhages (). Rank 3 is defined by intramuscular haemorrhages (bruising) (). Rank 4 is defined by ischaemia (loss of blood supply observed as pale regions (). As WB is defined, in part, by pale regions of the P. major breast muscle (Sihvo et al., Citation2014), rank 4 muscles were considered to have the WB myopathy. Ranks 1–4 were established based on the sequential onset of distinct myopathic abnormalities occurring with increased growth/age. Based on the current literature, it was determined that ranks 1–4 depicted the progression from WS to WB. In support of this conclusion, recent studies have reported an association between haemorrhagic lesions and WS (Kuttappan et al., Citation2017) and numerous studies (see Introduction) have reported varying degrees of haemorrhaging and ischaemia (pale regions) in WB-affected muscles (Sihvo et al., Citation2014; Bailey et al., Citation2015; Trocino et al., Citation2015; Soglia et al., Citation2016).
Figure 1. Rank 1 Pectoralis major breast muscle defined by the presence of white striping (WS) only. WS severity was not measured, although it is worth noting that breast muscles with more apparent WS severity (increased thickness and P. major coverage) exhibited same degree of haemorrhaging or ischaemia and were therefore ranked accordingly.
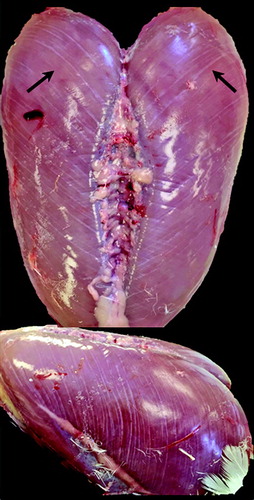
Figure 2. Rank 2 Pectoralis major breast muscle defined by surface (epimysium) haemorrhaging near the sternal apex.

Figure 3. Rank 3 Pectoralis major breast muscle defined by intramuscular haemorrhaging (bruising) near the sternal apex.
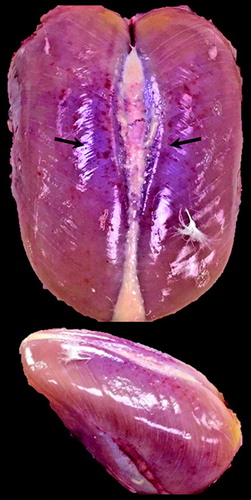
Figure 4. Rank 4 Pectoralis major breast muscle defined by ischaemia (extreme paleness) near the periphery regions of the muscle.
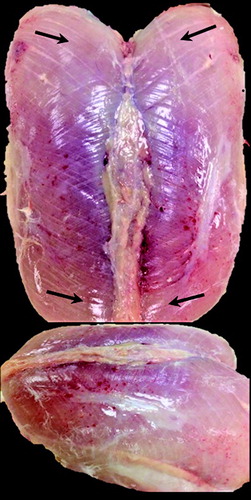
Once P. major myopathic abnormalities were observed during the sampling process, palpable detection of live birds was conducted to assess the presence of WB based on the established tactile method of WB detection reported in the recent literature (i.e. hardness of the breasts in live birds; Sihvo et al., Citation2014). The present study outlines the sequential myopathic aberrations associated with growth occurring in the P. major, to elucidate the underlying morphological changes occurring at earlier ages, prior to the point at which WB can be detected in live birds. Therefore, the present study reports the first day in which WB was first detected using these same tactile methods (Sihvo et al., Citation2014), but did not intend to use this method to characterize WB severity, as the first-day detection was not conducted until day 42.
Statistical analyses
The initial exploratory analysis of the data investigated rank (P. major myopathy progression) differences on growth parameters, using a linear mixed effects model. In particular, the PROC MIXED of SAS v. 9.4 was used to test for differences in growth parameters among ranks, using a model with fixed effect of rank and random effect of pen. The second step of the data analysis focused on understanding the contributions of specific growth parameters in the determination of ranks. A generalized linear mixed model was used to examine the effect of growth parameters on the rank probabilities. A cumulative logit link function was used in a proportional odds model (Stroup, Citation2013) with a random intercept for pens and fixed regression coefficients for growth parameters. The selection of growth parameters to be used as subsequent predictors was conducted using the following sequential steps: (i) all possible models were created with all possible combinations of the independent variables (P. major length, P. major width, P. major depth, BW, P. major weight, P. minor weight, P. minor length, P. minor width, P. minor depth, P. major yield and P. minor yield); (ii) models for which the variance inflation factor was greater than 10 were discarded; (iii) all remaining models were fitted to the data; (iv) the 10 models with the smallest Akaike Information Criterion (AIC) were selected for further examination and (v) the model with the smallest AIC was used for biological inference. Both the ordinal R package (Christensen, Citation2015) and the PROC GLIMMIX procedure of SAS v. 9.4 were used for model selection and fitting.
Results
Age- and growth-associated myopathic aberrations
Based on the progressive changes and abnormal clinical presentation observed during P. major post-hatch growth, the present study developed an objective ranking system which defined the onset and progression of WB. Relative growth rate and relative growth changes in the weight of the P. major and P. minor are presented in . The distinct myopathic changes associated with age and relative growth are indicated on the day in which they were initially observed (), starting with the onset of WS on day 16. On day 18, was the initial onset of SM and surface (epimysium) petechial haemorrhages followed by deep intramuscular haemorrhaging (bruising) on day 21.
Figure 5. Relative growth rate and relative growth changes in P. major and P. minor breast muscles ([Actual weight−initial weight (day 9)]/initial weight (day 9) × 100). Grossly observed myopathic changes associated with age and relative growth are indicated above the day in which they were initially observed. WS = White striping WB = Wooden breast DPM = Deep pectoral myopathy.
![Figure 5. Relative growth rate and relative growth changes in P. major and P. minor breast muscles ([Actual weight−initial weight (day 9)]/initial weight (day 9) × 100). Grossly observed myopathic changes associated with age and relative growth are indicated above the day in which they were initially observed. WS = White striping WB = Wooden breast DPM = Deep pectoral myopathy.](/cms/asset/34e04e3e-7146-41f5-b1df-cba8491080db/cavp_a_1356908_f0005_b.gif)
SM myopathy has been previously shown to be associated with WS (Baldi et al., Citation2017). In the present study, the initial onset of SM occurred after (day 18) the initial onset of WS (day 16) and coincided with the initial onset of epimysium haemorrhages. Throughout the remainder of the study and after a thorough study of the numerous photographs over the course of the experiment, it became clear that the occurrence of the SM myopathy was highly variable among birds and often difficult to detect visually in the photographs. In contrast, epimysium haemorrhaging progressed in a consistent pattern with growth/age. Given the nature of the study, and the aim to objectively define ranks, for these reasons, SM was not included as a rank determinant. However, the timing of SM onset, relative to WS and WB, could perhaps prove useful in subsequent studies.
Lastly, the first documented case of WB (i.e. rank 4) was noted on day 23 and was characterized as a P. major muscle that was extremely pale in colour with a ridge-like bulge on the caudal end. More severe cases of WB were observed from day 30 onwards and included prominent changes in muscle shape (firm and flat with a prominent ridge on the caudal end), interstitial viscous fluid accumulation and severe haemorrhaging (surface and intramuscular). In the most severe cases, affected muscles were completely void of vascularization throughout the entire P. major muscle with a thick, viscous yellow interstitial exudate surrounding the muscle. Other characteristically severe cases included breast muscles that were nearly completely covered with thick white striations (WS), displacing the surrounding epimysium with a thick collagenous layer, some with extreme haemorrhaging, where the white striation coverage was largely red in colour due to the extent of petechial haemorrhaging associated with the WS.
The most common method reported amongst the literature for detecting WB is via palpable detection of stiff breast muscles in live birds. As such, the present study aimed to determine the day in which live detection was first possible. Using tactile methods, WB was first detected in live broilers on day 42. As mentioned previously in Materials and methods, it was not the intention of the present study to utilize this method as a means to detect WB severity, as the majority of birds by this age exhibited mild to severe myopathies (rank 3 and 4). Rather, the intention was to determine the age at which live detection was possible for relative comparisons to the underlying aetiology of myopathic progression occurring in the P. major reported herein.
As shown in , the P. minor and Gastrocnemius muscles underwent similar myopathic changes during later stages of growth, starting with the initial observation of WS on day 32. The first reported case of DPM in the P. minor was on day 35. Lastly, on day 46 the P. minor exhibited WB-like abnormalities (i.e. rank 4), as a grossly pale muscle with a ridge-like bulge at the caudal region.
The muscle changes leading to the onset of WB occurred in a cumulative manner paralleling their progression with age/growth. Birds exhibiting surface epimysium haemorrhages (rank 2) also exhibited varying degrees of WS (rank 1). Furthermore, muscles exhibiting deep intramuscular haemorrhages (rank 3) also exhibited surface haemorrhages (rank 2) and WS (rank 1). Lastly, ischaemic muscles (rank 4) exhibited intramuscular (rank 3) and surface (rank 2) haemorrhages, as well as varying degrees of WS (rank 1). Given this progressive pattern, these distinct changes were used as markers for each rank. Representative images from each rank group are shown in .
Image quality rank determinants
Rank 1 is classified by the presence of WS. On day 16, faint white striations were observed in the cranial region of the P. major, present beneath the surrounding fascia (epimysium). By day 21, striations were easily seen on the P. major surface as part of the surrounding epimysium. Throughout the remainder of the study, WS was no longer limited to the cranial region of the P. major but had infiltrated the entire P. major muscle. This progressive infiltration coincided with an increase in WS thickness (). These data agree with authors who have grossly classified the severity of WS as an increase in striation number and size (Kuttappan et al., Citation2012b), initially limited to the cranial region and subsequently developing caudally in the P. major (Kuttappan et al., Citation2013c).
Rank 2 is classified by the presence of surface (epimysium) haemorrhages which was initially observed on day 18. Epimysial haemorrhages were initially small and focalized near the sternal apex, where visible stretching of the epimysial sheath could be seen. Throughout the course of the study, haemorrhages increased in size and dispersed throughout the P. major muscle (). These data agree with studies that have reported P. major surface haemorrhages associated with varying severity of WB (Sihvo et al., Citation2014; Bailey et al., Citation2015).
Rank 3 is classified by the presence of deep intramuscular haemorrhaging (bruising), initially observed on day 21 (). Similar to the progression described for rank 2, intramuscular haemorrhages were initially present near the sternum apex, dispersing distally throughout the P. major with age/growth. This coincided with an increase in colour intensity (red and purple). This is in agreement with studies that have characterized WB by the presence of large haemorrhagic foci (Trocino et al., Citation2015).
Rank 4 is classified by the presence of extreme paleness (ischaemia), initially observed on day 23, coinciding with the first documented case of WB (). Ischaemia was initially present in the peripheral regions of the P. major muscle at earlier ages, later diffusing throughout the entire muscle. Although not used as a determinant for rank 4 classification, it should be noted that in conjunction with the onset of ischaemia, there were observable changes in shape, which included a flat, ridge-like bulge on the caudal end of the P. major. These data are in agreement with the current literature, which partially defines WB by the presence of a pale ridge-like bulge (Sihvo et al., Citation2014; Trocino et al., Citation2015; Soglia et al., Citation2016). It is also reported to be lighter in colour when compared to normal breast fillets (Kuttappan et al., Citation2013a; Petracci et al., Citation2013a).
Image rank frequency and growth parameters by day
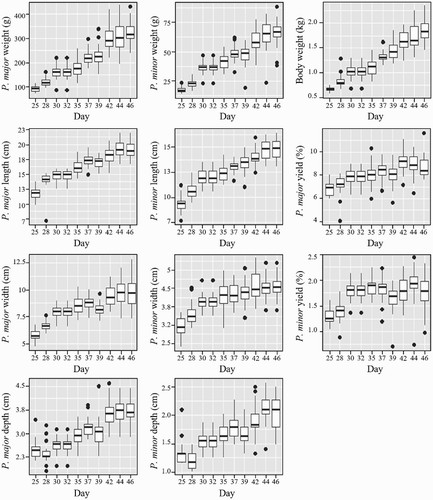
As previously mentioned, the occurrence and severity of WS/WB is positively correlated with increased growth rate (Kuttappan et al., Citation2012a). As such, rank frequency, as a percentage of total birds, was calculated on each day to show the distribution of rank (1–4) with age and growth (). A clear trend from lower to higher ranking (less to more severe myopathy) can be observed with age/growth from day 18 through day 46 (). In addition, individual growth data (without rank separation) were plotted by day, to show growth trends over time ().
Figure 6. Image rank (1–4) frequency by day. Rank percentage is based on total birds for each respective day.
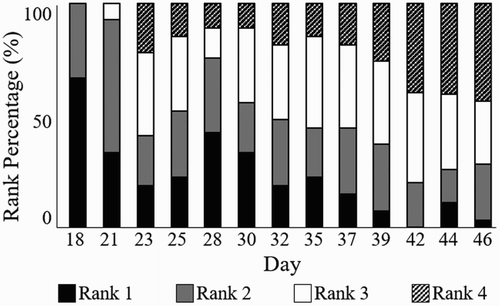
Figure 7. Boxplots display individual growth parameter data over age, starting on day 25 through day 46. Boxes indicate the range of data values between the first and third quartiles (25th and 75th percentiles); intra-quartile range (IQR). Whiskers extending from each box indicate range of data outside the IQR, ending with minimum and maximum data points. Within each box, lines (▬) represent day averages and circles (●) represent outlier data points.
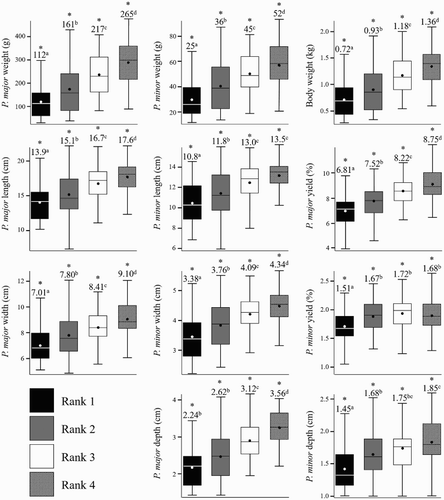
The overall objective of the present study was to relate the rank probabilities (stage/severity of WB) with physical measurements associated with P. major and P. minor muscle growth and development. In order to ensure differences in growth among the ranks, multiple least square mean comparisons for growth parameters were performed with respect to rank group (1–4). Significant differences (P < 0.001) were shown for all growth parameters investigated among ranks. The variation in BW and muscle data (P. major and P. minor weight, yield, length, width, depth) among ranks 1–4 are presented in as individual boxplots. Mean rank values are denoted above respective rank boxes and differing superscripts signifying least square mean separation are denoted between each sequential rank (P < 0.001).
Figure 8. Boxplots show descriptive growth parameter data for individual ranks 1–4. Boxes indicated data values falling within the Intra-quartile range (IQR) between the first and third quartiles (25th and 75th percentiles). Whiskers extending from each box indicate range of data outside the IQR, ending with minimum and maximum data points of each growth parameter. Within each box, lines (▬) indicate rank median and circles (●) indicate rank means. Mean values are denoted above their respective rank boxes and differing superscripts indicate significant differences in least square means among ranks (* = P < 0.001).
Cumulative logit model
In order to understand the contributions of specific growth parameters in the determination of ranks (WB ontogeny), a cumulative logit mixed model was developed to determine the role of one or more growth attributes as predictors for the probability of breast fillets being assigned to the four ranks. The 10 best (i.e. smallest AIC) cumulative logit mixed models with proportional odds are presented in , with intercepts, parameter estimates and standard errors given for all 10 best-fit models. Within each model, odds ratios were determined for individual predictors while holding all other predictors at a constant value. The model which best fit the data (Model 1) included the following growth parameters as predictors for rank: P. major length, P. major width, P. major depth, P. minor width, P. major yield and P. minor yield. For the best fit model (Model 1), the odds ratios showed that increasing P. major depth, P. minor width and P. major yield increased the odds of the muscle falling into a higher rank (i.e. more severe myopathy) versus a lower rank (less severe myopathy). In other words, increasing these muscle factors increased the likelihood of them having a higher rank (i.e. increased progression and severity of WB). Conversely, increased values for P. major length, P. major width and P. minor yield increased the odds of these muscles falling into lower ranks (less severe myopathy) versus a higher rank (more severe myopathy). In other words, increasing these muscle factors decreased the likelihood of them having a higher rank.
Table 1. The 10 best (i.e. smallest AIC) cumulative logit mixed models with proportional odd estimates for growth parameters determined to be predictors for myopathy rank.
In considering the top 10 best models shown in , P. major depth, P. major yield and P. minor yield were included as predictors for rank in all of the 10 best-fit models. P. major width was present in 8 of the 10 best models and P. major length was present in five of the 10 best models. P. minor width was present in seven of the 10 best models.
Discussion
The present study characterized the macroscopic phenotypic muscle traits of the P. major and P. minor corresponding to the ontogeny of WB in commercial broilers and developed a rank score system based on these distinct traits. It is generally accepted that during the onset of WB there is an inflammatory response, the cause of which is still unknown. In the present study, the first abnormality observed during post-hatch growth was the appearance of WS (fat/connective tissue) on day 16. As the muscle increased in size with age, the WS striations were observed close to the surface of the P. major and ultimately became part of the surrounding epimysium (rank 1). The primary damage resulting from this was surface haemorrhaging present on the epimysium only (rank 2). With advancing age and increased muscle growth, intramuscular haemorrhages (rank 3) were observed in regions with prominent surface haemorrhaging (near the sternal apex). It is hypothesized that the continued increase in the degree and depth of the haemorrhages further decreased intramuscular blood flow, leading to ischaemia (rank 4).
A cumulative logit model was developed to try and understand the morphological components of muscle growth that contributed most to the ranking system described in this report. The model that best fit the data showed that an increase in P. major depth and P. major yield increased the odds of that breast muscle exhibiting a more severe stage of WB (i.e. higher rank). Alternatively, the predictors P. major width and P. major length were shown to be inversely related to the severity of WB. These data are in agreement with studies that reported an association between WS and WB and P. major depth (Kuttappan et al., Citation2013a; Mudalal et al., Citation2014) as well as P. major yield (Petracci et al., Citation2013b; Alnahhas et al., Citation2016). Among these reports, Kuttappan et al. (Citation2013b) measured P. major length, width and depth and reported an association between the occurrence of WS and P. major depth, only (Kuttappan et al., Citation2013a; Kuttappan et al., Citation2017). Furthermore, Mudalal et al. (Citation2014) reported significantly greater P. major depth values in WS/WB-affected fillets, with no differences in fillet length or width (Mudalal et al., Citation2014).
The current data further support a hypothesis that by increasing P. major length and width, the odds of falling in a higher rank (more severe myopathy) are actually decreased. The contrasting relationships between selected phenotypic muscle measurements and the severity of WB confirms the fact that physical measurements which are inherent to selection for high-yielding broiler genotypes are contributing to the occurrence and severity of the observed myopathies.
The growth of the P. minor as a predictor for rank is also of interest, particularly due to the inverse relationship between P. major and P. minor yield and rank. The best fit model for predicting rank indicated that an increase in P. minor yield would decrease the odds of a higher rank (more severe WB) whereas increasing P. major yield increased the odds of higher rank. These data, in conjunction with the physical measurement data, suggest that alternate selection strategies for optimizing growth rather than maximizing it might be considered when trying to genetically minimize these growth-associated muscle anomalies.
It has been hypothesized that in poultry selected for meat production, growth of the epimysium can no longer keep pace with underlying muscle growth, leading to the onset of muscle damage (Swatland, Citation1990; Kranen et al., Citation2000) Observations from the present study provide evidence in support of that hypothesis – that the breast muscle has outgrown its surrounding epimysium. It is likely that accelerated muscle growth reduces the interstitial space between the P. major muscle and its surrounding epimysium, limiting subfascial movement in response to normal physiologic stimuli (movement, wing flapping, etc.), leading to muscle damage. In the P. minor, the consequences of such fascial inelasticity (DPM) result in a rise in intramuscular pressure (IMP), ischaemia, hypoxia and eventual necrosis (Martindale et al., Citation1979), of which, the onset of ischaemia is prevented experimentally by cutting the surrounding epimysium (fasciotomy) (Martindale et al., Citation1979).
DPM in the P. minor exhibits striking similarities in appearance and potential growth limitations by the surrounding epimysium to WB in the P. major. The most obvious difference was the grossly observed yellow/green necrotic muscle associated with DPM in the P. minor. When consideration is given to the differences in confounding factors between the P. minor (surrounding epimysium, sternum and overlaying P. major muscle) and P. major (surrounding epimysium), it is possible that WB represents simply a slower progression of DPM that is now manifest in the P. major as a result of continued selection for increased muscle mass. There are thus significantly less pressure-inducing factors confounding the P. major, preventing it from exhibiting necrosis phenotypically analogous to DPM in the P. minor.
DPM occurs as a result of increased IMP, limited by the space within the surrounding epimysium. In the present study, an increase in P. major depth and P. major yield was the primary predictor for WB progression or rank, suggesting that an increase in IMP may be a contributing factor in the progression of WB. Furthermore, several studies have reported the spatial morphological effect on the degree of WS/WB severity. In particular, the anterior cranial end of the P. major has been reported to be more severely affected by these conditions (Bowker & Zhuang, Citation2016; Clark & Velleman, Citation2016). In addition, WB is initially palpable in the anterior region of the P. major in less-severe cases, but progresses throughout the entire fillet in severe cases (Bailey et al., Citation2015; Soglia et al., Citation2016). Soglia et al. (Citation2016) reported a clear and gradual decrease in histopathological lesions from the P. major surface towards the inside of the P. major.
It is important to consider the repercussions of long-term changes resulting from 50 years of focused quantitative trait selection for increased breast muscle mass (yield). The substantial economic losses resulting from recent muscle myopathies provide a unique opportunity for the industry to re-evaluate current selection strategies and aim towards alternative sustainable solutions, focused on optimizing growth rather than maximizing it. Such considerations are critical to ensuring a sustainable future for the broiler industry, on an economic, environmental and social level.
Acknowledgements
Grateful acknowledgements are given to the following OARDC Chicken Research Facilities staff:Keith Patterson, Jordan Welsh and Jarrod Snell. Their support and enthusiasm during frequent and time-consuming data collections made this research possible.
References
- Abasht, B., Mutryn, M.F., Michalek, R.D. & Lee, W.R. (2016). Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE, 11, 1–16. doi: 10.1371/journal.pone.0153750
- Alnahhas, N., Berri, C., Chabault, M., Chartrin, P., Boulay, M., Bourin, M.C. & Le Bihan-Duval, E. (2016). Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genetics, 17, 61. doi: 10.1186/s12863-016-0369-2
- Bailey, R.A., Watson, K.A., Bilgili, S.F. & Avendano, S. (2015). The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poultry Science, 94, 2870–2879. doi: 10.3382/ps/pev304
- Baldi, G., Soglia, F., Mazzoni, M., Sirri, F., Canonico, L., Babini, E., Laghi, L., Cavani, C. & Petracci, M. (2017). Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal, 17, 1–10. doi: 10.1017/S1751731117001069
- Barbut, S., Sosnicki, A.A., Lonergan, S.M., Knapp, T., Ciobanu, D.C., Gatcliffe, L.J., Huff-Lonergan, E. & Wilson, E.W. (2008). Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Science, 79, 46–63. doi: 10.1016/j.meatsci.2007.07.031
- Bianchi, M., Petracci, M., Franchini, A. & Cavani, C. (2006). The occurrence of deep pectoral myopathy in roaster chickens. Poultry Science, 85, 1843–1846. doi: 10.1093/ps/85.10.1843
- Bowker, B. & Zhuang, H. (2016). Impact of white striping on functionality attributes of broiler breast meat. Poultry Science, 95, 1957–1965. doi: 10.3382/ps/pew115
- Chatterjee, D., Zhuang, H., Bowker, B.C., Rincon, A.M. & Sanchez-Brambila, G. (2016). Instrumental texture characteristics of broiler pectoralis major with the wooden breast condition. Poultry Science, 95, 2449–2454. doi: 10.3382/ps/pew204
- Christensen, R.H.B. (2015). Ordinal – Regression Models for Ordinal Data. R package version 2015. 6–28.
- Clark, C.L. & Velleman, S.G. (2016). Spatial influence on breast muscle morphological structure, myofiber size and gene expression associated with the wooden breast myopathy in broilers. Poultry Science, 95, 2930–2945. doi: 10.3382/ps/pew243
- Cruz, R.F.A., Vieira, S.L., Kindlein L., Kipper M., Cemin H.S. & Rauber S.M. (2017). Occurrence of white striping and wooden breast in broilers fed grower and finisher diets with increasing lysine levels. Poultry Science, 96, 501–510. doi: 10.3382/ps/pew310
- Darwin, C. (1859). On the Origin of Species By Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray.
- Ferreira, T.Z., Casagrande, R.A., Vieira, S.L., Driemerier, D. & Kindlein, L. (2014). An investigation of a reported case of white striping in broilers. The Journal of Applied Poultry Research, 23, 748–753. doi: 10.3382/japr.2013-00847
- Godfrey, G.F. & Goodman, B.L. (1956). Genetic variation and covariation in broiler body weight and breast width. Poultry Science, 35, 47–50. doi: 10.3382/ps.0350047
- Havenstein, G.B., Ferket, P.R. & Qureshi, M.A. (2003). Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poultry Science, 82, 1500–1508. doi: 10.1093/ps/82.10.1500
- Havenstein, G.B., Ferket, P.R., Scheideler, S.E. & Larson, B.T. (1994). Growth, livability, and feed conversion of 1957 vs. 1991 broilers when fed “typical” 1957 and 1991 broiler diets. Poultry Science, 73, 1785–1794. doi: 10.3382/ps.0731785
- Kranen, R.W., Lambooy, E., Veerkamp, C.H., Kuppevelt, T.H. & Veerkamp, J.H. (2000). Histological characterization of hemorrhages in muscle of broiler chickens. Poultry Science, 79, 110–116. doi: 10.1093/ps/79.1.110
- Kuttappan, V.A., Brewer, V.B., Apple, J.K., Waldroup, P.W. & Owens C.M. (2012a). Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poultry Science, 91, 2677–2685. doi: 10.3382/ps.2012-02259
- Kuttappan, V.A., Lee, Y.S., Erf, G.F., Meullenet, J.-F.C., McKee, S.R. & Owens, C.M. (2012b). Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poultry Science, 91, 1240–1247.
- Kuttappan, V.A., Brewer, V.B., Clark, F.D., McKee, S.R., Meullenet, J.F., Emmert, J.L. & Owens, C.M. (2009). Effect of white striping on the histological and meat quality characteristics of broiler fillets. Poultry Science, 88, 136–137.
- Kuttappan, V.A., Brewer, V.B., Mauromoustakos, A., McKee, S.R., Emmert, J.L., Meullenet, J.F. & Owens, C.M. (2013a). Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poultry Science, 92, 811–819. doi: 10.3382/ps.2012-02506
- Kuttappan, V.A., Huff, G.R., Huff, W.E., Hargis, B.M., Apple, J.K., Coon, C. & Owens, C.M. (2013b). Comparison of hematologic and serologic profiles of broiler birds with normal and severe degrees of white striping in breast fillets. Poultry Science, 92, 339–345. doi: 10.3382/ps.2012-02647
- Kuttappan, V.A., Shivaprasad, H.L., Shaw, D.P., Valentine, B.A., Hargis, B.M., Clark, F.D., McKee, S.R. & Owens, C.M. (2013c). Pathological changes associated with white striping in broiler breast muscles. Poultry Science, 92, 331–338.
- Kuttappan, V.A., Owens, C.M., Coon, C., Hargis, B.M. & Vazquez- Añon, M. (2017). Incidence of broiler breast myopathies at 2 different ages and its impact on selected raw meat quality parameters. Poultry Science, 96 https://doi.org/10.3382/ps/pex072.
- Lilburn, M.S. (1994). Skeletal growth of commercial poultry species. Poultry Science, 73, 897–903. doi: 10.3382/ps.0730897
- Lorenzi, M., Mudalal, S., Cavani, C. & Petracci, M. (2014). Incidence of white striping under commercial conditions in medium and heavy broiler chickens in Italy. The Journal of Applied Poultry Research, 23, 754–758. doi: 10.3382/japr.2014-00968
- Martindale, L., Siller, W.G. & Wight, P.A.L. (1979). Effects of subfascial pressure in experimental deep pectoral myopathy of the fowl: an angiographic study. Avian Pathology, 8, 425–436. doi: 10.1080/03079457908418369
- Mazzoni, M., Petracci, M., Meluzzi, A., Cavani, C., Clavenzani, P. & Sirri, F. (2015). Relationship between pectoralis major muscle histology and quality traits of chicken meat. Poultry Science, 94, 123–130. doi: 10.3382/ps/peu043
- Mudalal, S., Babini, E., Cavani, C. & Petracci, M. (2014). Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poultry Science, 93, 2108–2116. doi: 10.3382/ps.2014-03911
- Mutryn, M.F., Brannick, E.M., Fu, W., Lee, W.R. & Abasht, B. (2015). Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics, 16, 399. doi: 10.1186/s12864-015-1623-0
- Owens, C. (2016). Wooden breast syndrome in young broilers. AMSA Educational webinar.
- Page, R.K. & Fletcher, O.J. (1975). Case report – myopathy of the deep pectoral muscle in broiler breeder hens. Avian Diseases, 19, 814–821. doi: 10.2307/1589195
- Petracci, M., Mudalal, S., Babini, E. & Cavani, C. (2014). Effect of white striping on chemical composition and nutritional value of chicken breast meat. Italian Journal of Animal Sciences, 13, 179–183.
- Petracci, M., Mudalal, S., Bonfiglio, A. & Cavani, C. (2013a). Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poultry Science, 92, 1670–1675. doi: 10.3382/ps.2012-03001
- Petracci, M., Sirri, F., Mazzoni, M. & Meluzzi, A. (2013b). Comparison of breast muscle traits and meat quality characteristics in 2 commercial broiler chicken hybrids. Poultry Science, 92, 2438–2447. doi: 10.3382/ps.2013-03087
- Petracci, M., Soglia, F., Sirri, F., Zambonelli, P., Davoli, R. & Cavani, C. (2015). Broiler Pectoralis major muscle affected by emerging abnormalities: composition and technological traits. Italian Journal of Animal Science, 14, 363–374.
- Puolanne E. (2015). Wooden breast – a meat quality defect. Poultry Science Association Annual Meeting, 94(E-Suppl. 1), 70.
- Radaelli, G., Piccirillo, A., Birolo, M., Bertotto, D., Gratta, F., Ballarin, C., Vascellari, M., Xiccato, G. & Trocino, A. (2017). Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poultry Science, 96, 309–319. doi: 10.3382/ps/pew270
- Reddish, J.M. & Lilburn, M.S. (2004). A comparison of growth and development patterns in diverse genotypes of broilers. 1. Male broiler growth 1. Poultry Science, 83, 1067–1071. doi: 10.1093/ps/83.7.1067
- Richardson, J.A., Burgener, J., Winterfield, R.W. & Dhillon, A.S. (1980). Deep pectoral myopathy in seven-week-old broiler chickens. Avian Diseases, 24, 1054–1059. doi: 10.2307/1589983
- Russo, E., Drigo, M., Longoni, C., Pezzotti, R., Fasoli, P. & Recordati, C. (2015). Evaluation of white striping prevalence and predisposing factors in broilers at slaughter. Poultry Science, 94, 1843–1848. doi: 10.3382/ps/pev172
- Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. doi: 10.1038/nmeth.2089
- Sihvo, H.-K., Immonen, K. & Puolanne, E. (2014). Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Veterinary Pathology, 51, 619–623. doi: 10.1177/0300985813497488
- Sihvo, H.-K., Linden, J., Airas, N., Immonen, K., Valaka, J. & Puolanne, E. (2017). Wooden breast myodegeneration of Pectoralis major muscle over the growth period of broilers. Veterinary Pathology, 54, 119–128. doi: 10.1177/0300985816658099
- Siller, W.G. (1985). Deep pectoral myopathy: a penalty of successful selection for muscle growth. Poultry Science, 64, 1591–1595. doi: 10.3382/ps.0641591
- Soglia, F., Mudalal, S., Babini, E., Di Nunzio, M., Mazzoni, M., Sirri, F., Cavani, C. & Petracci, M. (2016). Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poultry Science, 95, 651–659. doi: 10.3382/ps/pev353
- Stroup, W.W. (2013) Generalized Linear Mixed Models: Modern Concepts, Methods and Applications. Boca Raton: CRC Press.
- Swatland H.J. (1990). A fibre-optic probe for muscle composition in poultry. Journal of Food Science and Technology, 23, 239–241.
- Tasoniero, G., Cullere, M., Cecchinato, M., Puolanne, E. & Dalle Zotte A. (2016). Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by white striping and wooden breast myopathies. Poultry Science, 95, 2707–2714. doi: 10.3382/ps/pew215
- Tijare, V., Yang, F.L., Kuttappan, V.A., Alvarado, C.Z., Coon, C.N. & Owens, C.M. (2016). Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poultry Science, 95, 2167–2173. doi: 10.3382/ps/pew129
- Trocino, A., Piccirillo, A., Birolo, M., Radaelli, G., Bertotto, D., Filiou, E., Petracci, M. & Xiccato, G. (2015). Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poultry Science, 94, 2996–3004. doi: 10.3382/ps/pev296
- USDA. (2016). Broiler Market News Report. Des Moines, IA: Livestock Poultry Grain Market News Service, 49.
- Velleman, S.G. & Clark, D.L. (2015). Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Diseases, 59, 410–418. doi: 10.1637/11097-042015-Reg.1
- Vignale, K., Caldas, J.V., England, J.A., Boonsinchai, N., Magnuson, A., Dridi, S., Owens, C.M., Coon, C.N. & Pollock, E.D. (2017). Effect of white striping myopathy on breast muscle (Pectoralis major) protein turnover and gene expression in broilers. Poultry Science, 96, 886–893.
- Wight, P.A., Siller W.G. & Martindale L. (1981). The sequence of pathological events in deep pectoral myopathy of broiler. Avian Pathology, 10, 57–76. doi: 10.1080/03079458108418458
- Wight, P.A.L. & Siller, W.G. (1980). Pathology of deep pectoral myopathy of broilers. Veterinary Pathology, 17, 29–39. doi: 10.1177/030098588001700103
- Zambonelli, P., Zappaterra, M., Soglia F, Petracci, M., Sirri, F., Cavani, C. & Davoli, R. (2016). Detection of differentially expressed genes in broiler pectoralis major muscle affected by White Striping – wooden Breast myopathies. Poultry Science, 95, 2771–2785. doi: 10.3382/ps/pew268
- Zotte, A.D., Cecchinato, M., Quartesan, A., Bradanovic, J., Tasoniero, G. & Puolanne E. (2014). How does “wooden breast” myodegeneration affect poultry meat quality. Archivos Latinoamericanos de Producción Animal, 22, 476–479.
- Zuidhof, M.J., Schneider, B.L., Carney, V.L., Korver, D.R. & Robinson, F.E. (2014). Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poultry Science, 93, 2970–2298. doi: 10.3382/ps.2014-04291
