ABSTRACT
The pathogenicity of a variant goose parvovirus (GPV), isolated from short beak and dwarfism syndrome of Pekin ducks (strain Cherry Valley), was investigated in embryonating goose eggs and goslings. The virus was easily grown in GPV antibody-free goose embryos and caused high mortality and severe lesions of goose embryos, indicating that the variant GPV has good adaptation and high pathogenicity to embryonated goose eggs similar to the classical GPV. Like the third egg-passage virus (strain H) of a classical GPV, the third egg-passage virus (strain JS1) of the variant GPV caused Derzsy’s disease in 2-day-old goslings with high mortality. The findings suggest that the variant GPV strain, which had specifically adapted to Pekin ducks, still retained high pathogenicity for its original host. The mortality (73.3–80%) caused by the first and third egg-passages of the variant GPV was somewhat lower than that (93.3%) caused by the third passage virus of the classical GPV, reflecting the higher pathogenicity of the classical GPV for its original host. These findings are likely to reinforce the importance of surveillance for parvoviruses in different waterfowl species and stimulate further study to elucidate the impact of mutations in the GPV genome on its pathogenicity to goslings and ducks.
Introduction
Waterfowl parvoviruses (WPVs) are currently classified within the species Anseriform dependoparvovirus 1 in the genus Dependoparvovirus of the subfamily Parvovirinae of the family Parvoviridae (http://www.ictvonline.org/virusTaxonomy.asp). Based on molecular analysis and virus neutralization tests, WPVs are divided into two groups: the goose parvovirus (GPV)-related group and the Muscovy duck parvovirus (MDPV)-related group (Le Gall-Reculé & Jestin, Citation1994; Zádori et al., Citation1995; Chang et al., Citation2000; Glávits et al., Citation2005; Poonia et al., Citation2006). Within the GPV-related group, four monophyletic subgroups are recognized, namely, the Hungarian virulent strains, the vaccine and low pathogenic strains, the West-European strains, and the Asian strains (Tatár-Kis et al., Citation2004).
GPV can cause a severe disease, called Derzsy’s disease, which affects young geese and Muscovy ducks. In susceptible birds less than 1-week-old, mortality may reach 100%. In susceptible birds 1–3 weeks of age, high mortality (10–60%) may also occur (Fang, Citation1962; Derzsy, Citation1967; Schettler, Citation1971; Hoekstra et al., Citation1973; Fang et al., Citation1981; Jestin et al., Citation1991; Glávits et al., Citation2005). Therefore, the disease has serious economic importance in regions with intensive goose and Muscovy duck production. Multiple pathologic features have been described by different authors for GPV infection in goslings, such as ascites, hydropericardium, hepatitis, myocarditis, and enteritis (Gough, Citation1991; Glávits et al., Citation2005; Palya, Citation2013).
GPV can also cause short beak and dwarfism syndrome (SBDS) (Palya et al., Citation2009). The disease was originally recognized in mule ducks in France in 1971/1972, and subsequently found in Poland in 1995 (Palya et al., Citation2009). In recent years, SBDS affecting Pekin duck (strain Cherry Valley) has been documented in China (Chen et al., Citation2015; Chen et al., Citation2016; Li et al., Citation2016; Yu et al., Citation2016). According to the description of Palya (Citation2013), the clinical signs of SBDS in mule ducks are growth retardation, short beak, and the shortening of the leg bones, with a low morbidity rate, and, usually, no gross pathological changes are found in the internal organs of affected ducks.
On the basis of phylogentic analysis of partial capsid-encoding region, the GPVs isolated from SBDS of mule ducks in France and Cherry Valley Pekin ducks in China belong to the West-European lineage of the GPV-related group (Palya et al., Citation2009; Chen et al., Citation2015; Yu et al., Citation2016; Ning et al., Citation2017). This may reflect a genetic difference between the GPV isolates of SBDS and the classical GPV isolates of Derzsy’s disease. In a recent work, a GPV isolate (QH15) from SBDS of Pekin ducks was shown to share a relatively low antigenic relatedness index (58%) with a classical GPV isolate (GY13) (Yu et al., Citation2016). This suggests that the GPVs isolated from SBDS share a partial antigenic relationship with the classical GPV strain and can be regarded as variant GPVs. Previous work by Palya et al. (Citation2009) showed that SBDS in mule ducks can be reproduced with the mule duck isolate but not with classical GPV strain of Derzsy’s disease, indicating the variant and classical GPVs are distinct in terms of pathogenicity in mule ducks. It has been well documented previously that the classical GPV cannot cause disease in Pekin ducks (Hoekstra et al., Citation1973; Gough, Citation1991; Palya, Citation2013). Emergence of SBDS in Pekin ducks in China suggests that the variant and classical GPVs are distinct in pathogenicty in Pekin ducks. These findings have raised a concern about the pathogenicity of the variant GPV in its original host. Such information would help us to better understand the relationship of the variant GPV with the classical GPV and to control GPV infection in goslings.
In the present study, we describe the isolation and pathogenicity of a variant GPV strain (JS1), from SBDS of Pekin ducks, in embryonating goose eggs and 2-day-old goslings.
Materials and methods
Ethics statement
Experiments involving birds were conducted following the guidance and under approval of the Animal Welfare and Ethical Censor Committee at China Agricultural University and Beijing Administration Committee of Laboratory Animals (Approval ID SYXK [Jing] 2015-0028).
Virus isolation and virus growth
The liver tissue used for virus isolation was collected in 2015 in Jiangsu province of China from a 15-day-old Pekin duck (strain Cherry Valley) exhibiting signs typical of SBDS. The sample was processed as described previously (Liu et al., Citation2016). A volume of 0.2 ml of resulting filtrate was inoculated onto the chorioallantoic membrane (CAM) of GPV antibody-free 10-day-old embryonating goose eggs. Embryos were incubated at 38°C for 14 days. The viscera (heart, liver, spleen, etc.) and allantoic fluids of embryos which died postinoculation (PI) were harvested and clarified as described previously (Wang et al., Citation2013). The resulting filtrate was harvested for an additional two passages. The H isolate of classical GPV was kindly provided by Mr J. Wang (ZhongshengTiaozhan Bioengineering, Tianjin, China). The virus was originally isolated in 2002 from Derzsy’s disease of goslings. For the purpose of this study, the virus was passaged three times through 10-day-old goose embryos as described above.
The third passages of the Pekin duck- (designated strain JS1) and goose- (strain H) origin viruses were used for the bird experiments; the first passages (designated JS1f and Hf, respectively) of the two GPV strains were also used in laboratory infection experiments.
Electron microscopy
Viral filtrate was mixed with an equal volume of chloroform and shaken vigorously for 15 min, followed by centrifugation at 12,000g for 20 min. The supernatant was harvested and further treated with chloroform. Following centrifugation at 150,000g for 3 h at 4°C, the resulting pellet was resuspended in TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0) to 5% of the original volume. Suspension containing viral particles was placed on carbon-coated copper grids, subjected to negative staining, and examined using an H-7500 electron microscope (Hitachi, Tokyo, Japan).
Agar gel precipitin test
The agar gel precipitin (AGP) tests were performed as described previously (Gough, Citation1984). The antigens specific to JS1 and H were produced as clarified allantoic fluids of goose embryos which died from infection with strains JS1 and H, respectively. The clarified allantoic fluid of uninfected goose embryos was used as a control. Antisera against JS1 and H were prepared in BALB/c mice by inoculation of inactivated JS1 and H four times at 1- to 2-week intervals, respectively. Antisera were collected 2 weeks after the final inoculation, and inactivated at 56°C for 30 min.
Polymerase chain reaction
Viral DNA was extracted from 250 μl of clarified samples using the phenol/chloroform/isoamyl alcohol (25:24:1) method. The extracted DNA was subjected to polymerase chain reaction (PCR) assays with previously reported primer pairs, which amplified DNA fragments of 493 and 539 bp from the VP1 and VP3 regions of the WPV genome, respectively (Chang et al., Citation2000; Tatár-Kis et al., Citation2004). The PCR assay consisted of 5 μl of DNA, 20 pmol of each upstream and downstream primer, 12.5 μl of 2× Taq Master Mix (Vazyme, Nanjing, China), and 3.5 μl of ddH2O. Cycling conditions were as follows: 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 40 s, and a final extension step at 72°C for 10 min. The amplification products were examined by using 1.2% agarose gel electrophoresis.
Cloning, sequencing, and sequence analysis
PCR products amplified from the JS1 and H isolates were purified using an E.Z.N.A. Gel Extraction Kit (Omega, Doraville, GA, USA), and then cloned into pGEM-T Easy Vector (Promega, Madison, WI, USA), according to the manufacturer’s instructions. The recombinant plasmid was transformed into the competent Escherichia coli (strain DH10b). After identification, positive transformants were sequenced commercially (Taihe, Beijing, China).
Sequence similarity searches were performed in GenBank using the BLASTx program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Alignments of nucleotide sequences were performed with the ClustalW 1.83 program (http://www.genome.jp/tools/clustalw/). Based on alignments of the partial VP1 and VP3 nucleotide sequences, phylogenetic trees were constructed using the neighbour-joining method implemented in MEGA 6.0, with the Tamura 3-parameter model of nucleotide substitutions (Tamura et al., Citation2013).
Experiment design
Forty-five 2-day-old goslings, which were derived from non-immune goose breeders that had no antibody to GPV, were divided into three groups (15 birds/group). The challenged groups were inoculated intramuscularly with strains JS1 and H at the dose of 5 × 106 egg lethal dose (ELD50) per bird, respectively, and the mock-infected control group was injected with 0.5 ml of PBS. The birds were reared in isolators and monitored daily for 35 days. Dead goslings were immediately examined for gross pathological changes of the internal organs including heart, liver, spleen, kidney, bursa of Fabricius, intestine, and pancreas. All these organs were collected from three dead goslings from each of the challenged groups at 10 days PI for histopathological examinations. Meanwhile, three dead goslings were randomly selected from each of the challenged groups for calculation of the body weight/liver ratio. Three goslings were randomly selected from the control group and used for comparison. Liver and spleen were sampled from all dead goslings for detection of parvoviruses using the WPV VP3-based PCR as described above. Both clinical signs and gross lesions were divided into 5-grade severities of no change, very mild, mild, moderate, and marked, giving scores 0, 1, 2, 3, and 4, respectively ( and ).
Table 1. Clinical signs in goslings infected with GPV strains at two days of age.
Table 2. Gross lesions of dead goslings following infection with GPV strains at two days of age.
The challenge experiments were repeated to investigate the pathogenicity of strain JS1f in goslings, using strain Hf as a positive control. The viral titre was adjusted to a level similar to that used in the first experiment. The experiments were performed as described above. The birds were monitored daily for 35 days.
Histopathology
The tissues were fixed in 4% neutral formalin at room temperature for 48 h, embedded in paraffin, and cut into 5-μm-thick sections. After deparaffinization, the sections were stained with haematoxylin and eosin (H&E). Microscopic lesions were observed under an Olympus microscope (Olympus, Tokyo, Japan). The tissue sections were evaluated by a veterinary pathologist. For each tissue, microscopic lesions were divided into 5-grade severities of no change, very mild, mild, moderate, and marked, giving scores 0, 1, 2, 3, and 4, respectively ().
Table 3. Microscopic lesions of dead goslings 10 days after infection with GPV strains.
Nucleotide sequence accession numbers
The VP1 and VP3 sequences of strain H have been deposited into GenBank, under accession numbers of KY768911 and KY768912, respectively. The VP1 and VP3 sequences of strain JS1 determined in this study were identical to those in the recently sequenced JS1 genome sequence (accession numbers KT935531). For comparison, the nucleotide sequences of previously reported classical and variant GPV strains were retrieved from GenBank. Their accession numbers are shown in the phylogenetic trees.
Statistical analysis
The body weight/liver ratios were calculated as mean ± standard deviation (SD). A student’s t-test was employed to analyse the significance of differences in clinical signs and gross and microscopic lesion scores as well as the body weight/liver ratios. The analysis was performed using the GraphPad Prism software (version 6.01; GraphPad Software Inc., San Diego, CA, USA). Differences were considered statistically significant at a value of P < 0.05.
Results
Virus isolation
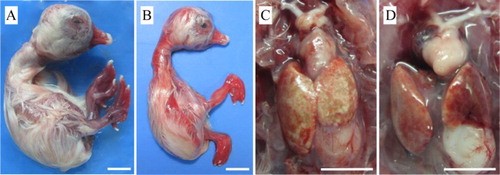
Inoculation of the liver sample collected from SBDS of Pekin ducks caused 57.14% mortality of goose embryos within 10–14 days PI. On two subsequent passages, all goose embryos died within 5–8 days PI. Gross lesions in dead embryos were observed beginning with the first passage. The goose embryos appeared oedematous and stunted with severely haemorrhagic skin of feet and the surface of beaks ((A,B)). Besides haemorrhages, the digits of most of dead embryos were curled dorsally ((B)). In embryos that died within 5–8 days PI, haemorrhages on the skin of the whole body were observed ((B)). On necropsy, lesions were commonly found in the liver, which was ochre colour with pale patches. The heart was generally pale, but the degree of paleness varied ((C,D)). Using strain H as inoculum, all infected goose embryos died within 3–6 days PI. The dead embryos displayed the same gross lesions as described above.
Figure 1. Gross lesions in goose embryos which died from infection with the JS1 GPV isolate. (A) 22-day-old embryo with oedema, subcutaneous haemorrhages, and haemorrhagic feet and beak. (B) 20-day-old embryo showing retardation, subcutaneous haemorrhages, and digits curled dorsally. (C) 14-day-old embryo with an ochre-coloured liver showing patches of necrosis. (D) 16-day-old embryo with a pale heart. Bar = 10 mm.
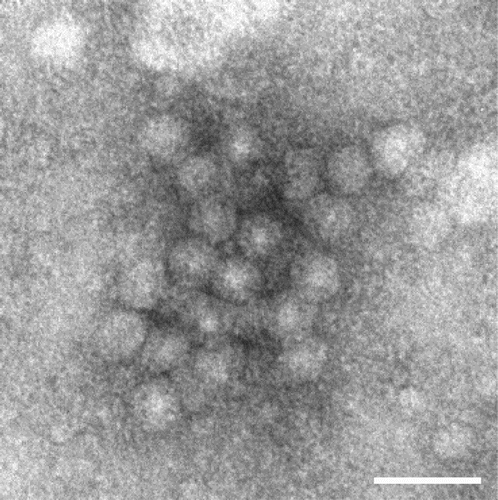
Morphological and serological identification of the GPV isolate
Examination of a concentrated preparation from the JS1 culture in an electron microscope revealed aggregates of parvovirus-like virus particles with a diameter of approximately 20 nm (). No other virus-like particles were observed. In the AGP tests, both JS1 and H strains reacted with homologous and heterologous antisera. No reactions were observed using the control antigen.
Molecular identification of JS1 and H isolates
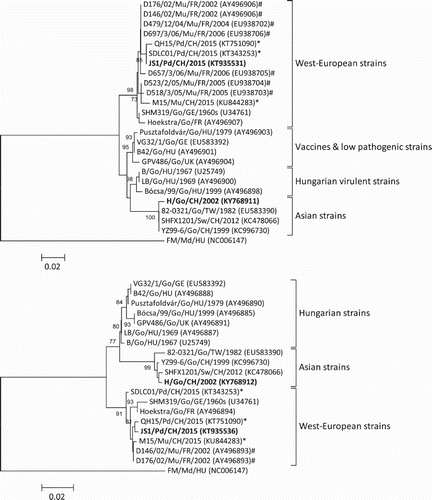
DNA fragments with expected lengths were amplified from DNA templates extracted from the JS1 and H isolates. Following cloning and sequencing of the PCR products, the 493- and 539-bp sequences were identified by BLASTP analysis in GenBank as encoding GPV-specific VP1 and VP3 proteins, respectively. Phylogenetic analysis on the basis of the partial VP1(443 nt) and VP3 (495 nt) nucleotide sequence (excluding the primer sequences) revealed that strains JS1 and H fell into the West-European lineage and the Asian lineage of the GPV-related group, respectively ().The JS1 virus was closely related to previously reported GPV variants isolated from SBDS of Pekin ducks in China (Chen et al., Citation2015; Chen et al., Citation2016; Yu et al., Citation2016) and mule ducks in France (Tatár-Kis et al.,Citation2004; Palya et al., Citation2009), whereas strain H was grouped together with previously isolated classical GPV strains in Asia (Shien et al., Citation2008; Wang et al., Citation2014).
Figure 3. Neighbour-joining trees based on alignments of the 443 nt VP1 (A) and 495 nt VP3 (B) nucleotide sequences of goose paroviruses. Numbers on the branches indicate bootstrap percentages obtained using 1000 replicates (only values of 70% and above are shown). The FM isolate of MDPV was included as an outgroup. Lineages in panels (A) and (B) are according to Tatár-Kis et al. (Citation2004). GenBank accession numbers of the sequences are indicated in parentheses. For each virus strain, bird species (Pd, Pekin duck; Go, goose; Md, Muscovy duck; Mu, mule duck; Sw, swan), origin (CH, mainland China; GE, Germany; HU, Hungary; FR, France, TW, Taiwan; UK, United Kingdom), and year of sampling or isolation (if available) are shown. *GPVs isolated from SBDS of Pekin ducks and Muscovy ducks in China (Chen et al., Citation2015; Chen et al., Citation2016; Yu et al., Citation2016). #GPVs from SBDS of mule ducks in France (Tatár-Kis et al., Citation2004; Palya et al., Citation2009). Bold letters, strains determined in this study.
Clinical signs
Similar clinical signs typical of GPV infection were detected in both JS1- and H-infected groups (). The first clinical signs were anorexia, polydipsia, and weakness, which appeared at 2 days PI. Other consistent signs caused by the two viruses included nasal and ocular discharge with associated headshaking, profuse yellow-white diarrhoea, conjunctivitis, and feather clutter. Sick goslings developed convulsions and paralysis of their legs prior to death. At death the tip of the bills was cyanotic. For these clinical signs, there was no significant difference between the challenged groups (P > 0.05).
There were also differences in clinical signs between the challenged groups (). Loss of down and marked reddening around cloaca were observed in the JS1-infected group after 15 days PI, but not in the H-infected group. Although clinical signs of dark-coloured and dehydrated feet were seen in both challenged groups, the number of geese showing such clinical signs in the H-infected group was greater than those in the JS1-infected group (P < 0.05).
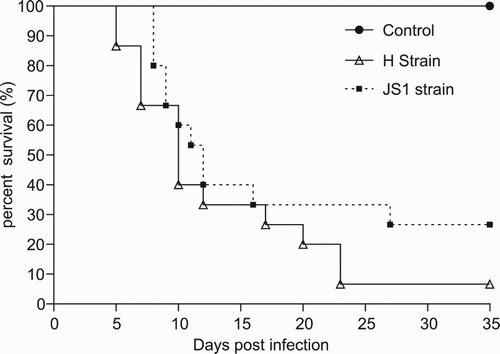
In JS1- and H-infected groups, death occurred within 8–27 and 5–23 days PI, with mortalities of 73.3% (11/15) and 93.3% (14/15), respectively ().
Figure 4. Survival curve of geese after infection with GPVs. (●) Control group inoculated with PBS. (▪) Group inoculated with the JS1 isolate. (△) Group inoculated with the H isolate.
Infection of strains JS1f and Hf at 2 days of age resulted in the appearance of clinical signs as described above. In the JS1f- and Hf-infected groups, deaths occurred within 2–15 and 4–17 days PI, with 80% (12/15) and 93.3% (14/15) mortality, respectively.
In all experiments, survivors showed profound growth retardation. No clinical signs and deaths were found in the control group.
Gross lesions
In both JS1- and H-infected infected groups, most dead goslings exhibited sloughing of the intestinal mucous membrane and thinned wall of the small intestine ((A–C); ). Other dead goslings displayed fibrinous exudate on the surface of the mucous membrane of the small intestine ((B); ). There was no significant difference in intestinal lesions between JS1- and H-infected groups (P > 0.05; ). Analysis of the body weight/liver ratio revealed significant difference among the challenged groups and the controls (JS1: 17.83 ± 0.53; H: 12.74 ± 0.48; control: 21.57 ± 1.14) (P < 0.05), indicating that both JS1 and H viruses caused liver dystrophy and that the H virus caused more serious liver dystrophy than the JS1 virus ((D–F)).
Figure 5. Gross lesions in geese which died from experimental infection with the JS1 or H GPV strains. A, D (right), and G (right) – JS1-infected group. B, E, and F – H-infected group. (A) Small intestine with thinned wall. (B) Small intestine with fibrinous exudate on the mucous surface. (C) Small intestine from the control group. (D) Liver dystrophy. Left, control. (E) Atrophied liver with fibrinous exudate on the surface. (F) Congested liver with fibrinous exudate on the surface. (G) Tongue and oral cavity with fibrinous pseudomembrane. Left, control. Bar = 20 mm.
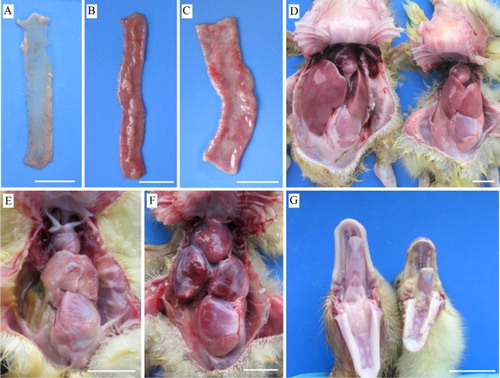
Significantly different gross lesions were also observed in the two challenged groups (P < 0.05; ). In the JS1-infected group, five of 11 dead birds had a fibrinous pseudomembrane covering the tongue and oral cavity ((G)). In the H-infected group, all dead geese displayed fibrinous perihepatitis, and six of 14 dead goslings had congested liver ((E,F)).
No gross lesions were observed in the uninfected control group.
Microscopic lesions
The goslings which died 10 days PI with the JS1 and H strains showed similar microscopic lesions in duodenum, heart, and liver (P > 0.05; ). Missing villi, degeneration and necrosis of epithelial cells (enterocytes), and exposed lamina propria of duodenal villi were observed in the duodenum ((A–C)). Cardiac lesions included thickening of epicardium, necrosis and loss of striation of myocardial fibres, and infiltration of inflammatory cells and lymphocytes ((D–F)). In the liver, degeneration and necrosis of hepatocytes, hepatic sinusoidal dilatation, and infiltration of inflammatory cells and lymphocytes occurred ((G,H)). Other lesions caused by strains JS1 and H included necrosis of pancreatic acinar cells, degeneration and necrosis of renal tubular epithelial cells, and necrosis and detachment of mucous epithelial cells and depletion of lymphocytes of lymphoid follicles in bursa of Fabricius ().
Figure 6. Microscopic lesions in 12-day-old goslings which died from infection with the JS1 and H isolates of GPV. Top panel, JS1-infected group; middle panel, H-infected group; bottom panel, uninfected control group. (A and B) Duodenum with haemorrhages (black arrow), villi missing, degeneration, and necrosis of epithelial cells (solid triangle). (C) Small intestine from an uninfected gosling. (D and E) Heart with thickened epicardium (solid triangle), infiltration of lymphocytes (open triangle). (F) Heart from an uninfected gosling. (G and H) Liver with degeneration and necrosis of hepatocytes (black arrow), and infiltration of lymphocytes (solid triangle). (I) Liver from an uninfected gosling.
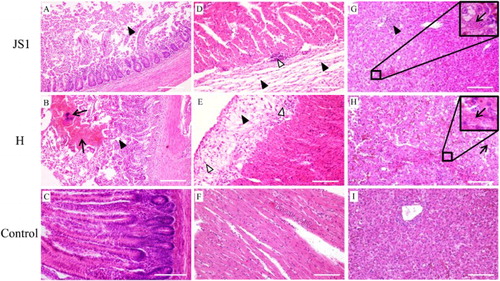
The challenged groups also showed different microscopic lesions (P < 0.05; ). Splenic haemorrhage and congestion and renal interstitial congestion were also seen in JS1-infected goslings, but not in H-infected birds. Duodenal haemorrhage and splenic lymphocyte depletion and infiltration of mononuclear macrophages were observed in H-infected groups, but not in JS1-infected groups.
No microscopic lesions were found in the uninfected control group.
PCR detection
Using previously reported parvovirus PCR assay (Chang et al., Citation2000), all samples collected from JS1- and H-infected groups tested positive, while the samples collected from the control group tested negative.
Discussion
The present paper describes the isolation and characterization of the JS1 virus from SBDS of Pekin ducks in China. On the basis of virion morphology, serological test, and molecular analysis, the JS1 virus can be identified as a GPV. Based on phylogenetic analysis of the partial VP1 and VP3 sequences, this work has also shown that JS1 belongs to the variant GPV, and that strain H can be confirmed as an isolate of the classical GPV.
It has been well documented that GPV can be easily isolated from Derzsy’s disease of goslings following inoculation of suitable post mortem specimens into embryonated goose eggs (Hoekstra et al., Citation1973; Gough et al., Citation1981). We showed that the JS1 virus from SBDS of Pekin ducks was also readily isolated in GPV antibody-free goose embryos, suggesting that the variant and classical GPV strains share similar adaptation to goose embryos. Although the original material collected from Pekin duck caused a lower mortality compared with those caused by clinical samples containing the classical GPV (Hoekstra et al., Citation1973), on subsequent two passages 100% mortality occurred within 5–8 days PI. In addition, the isolated virus caused severe embryo lesions typical of GPV infection (Hoekstra et al., Citation1973). These results suggest that the variant and classical GPV strains share similarly high pathogenicity in embryonated goose eggs.
So far, there have been a number of reports describing the clinical features and pathological changes of Derzsy’s disease (Fang, Citation1962; Derzsy, Citation1967; Schettler, Citation1971; Hoekstra et al., Citation1973; Fang et al., Citation1981; Gough, Citation1991; Jestin et al., Citation1991; Glávits et al., Citation2005; Palya, Citation2013). In the present work, we focused on the pathogenicity of the variant GPV for goslings, employing the H isolate of the classical GPV as a control. We showed that both JS1 and H strains resulted in acute infections in 2-day-old goslings. The course of the disease accords with previous reports of the acute form of Derzsy’s disease (Gough, Citation1991; Palya, Citation2013). Like the H virus, the JS1 isolate also caused clinical signs and gross pathological changes typical of Derzsy’s disease. Pale myocardium and dilatation of the heart were considered characteristic gross lesions in acute cases of geese (Gough, Citation1991; Palya, Citation2013). In the present study, cardiac lesions appeared to be less obvious. Nevertheless, histopathological examinations revealed the presence of necrosis and loss of striation of myocardial fibres, which were regarded as consistent microscopic lesions in geese induced by GPVs (Gough, Citation1991; Palya, Citation2013). Previously reported microscopic lesions in other organs were also found in the present study (e.g. degeneration and necrosis of hepatocytes). Together these findings demonstrate that Derzsy’s disease of goslings can be repeated by experimental infection with the variant GPV strain JS1 from SBDS of Pekin ducks. In particular, both the first and third passages of the JS1 GPV strain caused high mortalities in 2-day-old goslings. It is therefore suggested that the variant GPV strain, which had specifically adapted to Pekin ducks, still retains the high pathogenicity in its original host.
Taken together, it is noteworthy that the variant GPV strain from SBDS of Pekin ducks exhibits high pathogenicity in goslings. Due to lack of continuous monitoring, it is unclear about the current prevalence of the variant GPV strains in goose populations. Surveillance for GPV in different waterfowl species may enhance our understanding of the molecular epidemiology and ecology of GPV, which may be of help to the control of GPV infections in waterfowl.
Acknowledgments
We thank J. Wang of ZhongshengTiaozhan Bioengineering, Tianjin, China for providing the H strain of GPV.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chang, P.C., Shien, J.H., Wang, M.S. & Shieh, H.K. (2000). Phylogenetic analysis of parvoviruses isolated in Taiwan from ducks and geese. Avian Pathology, 29, 45–49. doi: 10.1080/03079450094270
- Chen, H., Dou, Y., Tang, Y., Zhang, Z., Zheng, X., Niu, X., Yang, J., Yu, X. & Diao, Y. (2015). Isolation and genomic characterization of a duck-origin GPV-related parvovirus from cherry valley ducklings in China. PLoS One, 10, e0140284. doi: 10.1371/journal.pone.0140284
- Chen, S., Wang, S., Cheng, X., Xiao, S., Zhu, X., Lin, F., Wu, N., Wang, J., Huang, M., Zheng, M., Chen, S. & Yu, F. (2016). Isolation and characterization of a distinct duck-origin goose parvovirus causing an outbreak of duckling short beak and dwarfism syndrome in China. Archives of Virology, 161, 2407–2416. doi: 10.1007/s00705-016-2926-4
- Derzsy, D. (1967). A viral disease of goslings. I. Epidemiological, clinical, pathological and aetiological studies. Acta Veterinaria Academiae Scientiarum Hungaricae, 17, 443–448.
- Fang, D. (1962). Recommendation of gosling plague. China Animal Husbandry and Veterinary Medicine, 8, 19–20. (in Chinese).
- Fang, D., Wang, Y., Zheng, Y., Zhou, Y., Jiang, M. & Dong, G. (1981). Studies on the aetiology and specific control of gosling plague. Scientia Agricultura Sinica, 4, 1–8. (in Chinese).
- Glávits, R., Zolnai, A., Szabó, E., Ivanics, E., Zarka, P., Mató, T. & Palya, V. (2005). Comparative pathological studies on domestic geese (Anser anserdomestica) and Muscovy ducks (Cairina moschata) experimentally infected with parvovirus strains of goose and Muscovy duck origin. Acta Veterinaria Hungarica, 53, 73–89. doi: 10.1556/AVet.53.2005.1.8
- Gough, R.E. (1984). Application of the agar gel precipitin and virus neutralisation tests to the serological study of goose parvovirus. Avian Pathology, 13, 501–509. doi: 10.1080/03079458408418551
- Gough, R.E. (1991). Goose parvovirus infection. In B.W. Calnek, H.J. Barnes, C.W. Beard, W.M. Reid, & H.W. Yoder Jr (Eds.), Diseases of poultry 9th edn (pp. 684–690). Ames: Iowa State University Press.
- Gough, R.E., Spackman, D. & Collins, M.S. (1981). Isolation and characterisation of a parvovirus from goslings. Veterinary Record, 108, 399–400. doi: 10.1136/vr.108.18.399
- Hoekstra, J., Smit, T. & Van Brakel, C. (1973). Observations on host range and control of goose virus hepatitis. Avian Pathology, 2, 169–178. doi: 10.1080/03079457309353794
- Jestin, V., Le Bras, M., Cherbonnel, M., Le Gall-Recule, G. & Bennejean, G. (1991). Demonstration of very pathogenic parvoviruses (Derzsy disease virus) in Muscovy duck farm. Recueil de Medecine Veterinaire (France), 167, 849–857.
- Le Gall-Reculé, G. & Jestin, V. (1994). Biochemical and genomic characterization of Muscovy duck parvovirus. Archives of Virology, 139, 121–131. doi: 10.1007/BF01309459
- Li, C.F., Li, Q., Chen, Z.Y. & Liu, G.Q. (2016). Novel duck parvovirus identified in Cherry Valley ducks (Anas platyrhynchos domesticus), China. Infection, Genetics and Evolution, 44, 278–280. doi: 10.1016/j.meegid.2016.07.020
- Liu, N., Jiang, M., Wang, M., Wang, F., Zhang, B. & Zhang, D. (2016). Isolation and detection of duck astrovirus CPH: implications for epidemiology and pathogenicity. Avian Pathology, 45, 221–227. doi: 10.1080/03079457.2016.1143549
- Ning, K., Liang, T., Wang, M., Dong, Y., Qu, S. & Zhang, D. (2017). Genetic detection and characterization of goose parvovirus: implications for epidemiology and pathogenicity in Cherry Valley Pekin ducks. Infection, Genetics and Evolution, 51, 101–103. doi: 10.1016/j.meegid.2017.03.024
- Palya, V.J. (2013). Parvovirus infections of waterfowl. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V. Nair (Eds.), Diseases of poultry 13th edn (pp. 444–451). Ames, IA: John Wiley & Sons, Inc.
- Palya, V., Zolnai, A., Benyeda, Z., Kovács, E., Kardi, V. & Mató, T. (2009). Short beak and dwarfism syndrome of mule duck is caused by a distinct lineage of goose parvovirus. Avian Pathology, 38, 175–180. doi: 10.1080/03079450902737839
- Poonia, B., Dunn, P.A., Lu, H., Jarosinski, K.W. & Schat, K.A. (2006). Isolation and molecular characterization of a new Muscovy duck parvovirus from Muscovy ducks in the USA. Avian Pathology, 35, 435–441. doi: 10.1080/03079450601009563
- Schettler, C. H. (1971). Virus hepatitis of geese. II. Host range of goose hepatitis virus. Avian Diseases, 15, 809–823. doi: 10.2307/1588871
- Shien, J.H., Wang, Y.S., Chen, C.H., Shieh, H.K., Hu, C.C. & Chang, P.C. (2008). Identification of sequence changes in live attenuated goose parvovirus vaccine strains developed in Asia and Europe. Avian Pathology, 37, 499–505. doi: 10.1080/03079450802356979
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). Mega6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. doi: 10.1093/molbev/mst197
- Tatár-Kis, T., Mató, T., Markos, B. & Palya, V. (2004). Phylogenetic analysis of Hungarian goose parvovirus isolates and vaccine strains. Avian Pathology, 33, 438–444. doi: 10.1080/03079450410001724067
- Wang, D., Shi, J., Yuan, Y., Zheng, L. & Zhang, D. (2013). Complete sequence of a reovirus associated with necrotic focus formation in the liver and spleen of Muscovy ducklings. Veterinary Microbiology, 166, 109–122. doi: 10.1016/j.vetmic.2013.05.022
- Wang, J., Duan, J., Meng, X., Gong, J., Jiang, Z. & Zhu, G. (2014). Cloning of the genome of a goose parvovirus vaccine strain SYG61v and rescue of infectious virions from recombinant plasmid in embryonated goose eggs. Journal of Virological Methods, 200, 41–46. doi: 10.1016/j.jviromet.2014.02.014
- Yu, K., Ma, X., Sheng, Z., Qi, L., Liu, C., Wang, D., Huang, B., Li, F. & Song, M. (2016). Identification of goose-origin parvovirus as a cause of newly emerging beak atrophy and dwarfism syndrome in ducklings. Journal of Clinical Microbiology, 54, 1999–2007. doi: 10.1128/JCM.03244-15
- Zádori, Z., Stefancsik, R., Rauch, T. & Kisary, J. (1995). Analysis of the complete nucleotide sequences of goose and Muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2. Virology, 212, 562–573. doi: 10.1006/viro.1995.1514