ABSTRACT
In Salmonella, plasmids participate in many pathways involved in virulence, metabolism, and antibiotic resistance. To investigate the function of the ipaJ gene in a multi-copy plasmid pSPI12 prevalent in Salmonella enterica serovar Pullorum (S. Pullorum), we established a method to eliminate the plasmid and constructed the plasmid-cured bacteria C79-13-ΔpSPI12 by using the suicide vector pDM4. Briefly, a 500 bp fragment ipaJU from pSPI12 was cloned into pDM4 and transformed into S. Pullorum C79-13 by conjugative transfer. After homologous recombination, the suicide vector was inserted into pSPI12 to produce pSPI12-pDM4-ipaJU. Induction of the expression of the sacB gene in the suicide vector killed the bacteria harbouring plasmid, while the progeny losing the plasmid survived in the plate with sucrose. The plasmid-cured strain showed extremely decreased ability to infect chicken macrophage HD11 cells and LMH hepatic epithelial cells compared to wild type strain and complementary strain carrying ipaJ. Additionally, IFN-γ mRNA levels were up-regulated in HD11 cells or chicken spleens infected by plasmid-cured strain, but no difference was detected in IL-4 among the three strains. Transforming ipaJ into S. Enteritidis also decreased expression of proinflammatory cytokines in infected macrophages or chicken spleens compared to wild type strain. These results suggest that the ipaJ gene in pSPI12 is involved in S. Pullorum infection and that IpaJ protein modulates immune response.
Introduction
Salmonella enterica serovar Pullorum (S. Pullorum) is a host-restricted serotype of Salmonella that causes pullorum disease in poultry. Although the disease has been eradicated in most developed countries, it has occasionally been reported in the EU and is prevalent in some developing countries, including China and Brazil (Barrow & Freitas Neto, Citation2011). To investigate its pathogenesis, a genomic comparison was performed to identify differences in the genome of S. Pullorum from other closely related serotypes, such as S. Enteritidis and S. Gallinarum (Li et al., Citation2009; Batista et al., Citation2015). Our previous study showed that a small plasmid pSPI12, size 4080 bp, was prevalent in S. Pullorum, and the only putative virulence gene, ipaJ, was involved in infection by the pathogen (Li et al., Citation2014). It is well known that plasmids usually carry genes playing significant roles in bacteria, such as antibiotic resistance, virulence, and metabolic capacity (Trevors, Citation1985, Citation1986; Spengler et al., Citation2006). If a plasmid-cured mutant could be produced, it would be easy to reveal the function of the plasmid by comparing strains with and without the plasmid. To date, many methods have been developed to eradicate plasmids, including physical treatment, chemical induction, and molecular techniques. Because physical and chemical methods have a large impact on the biological characteristics of the host bacteria by damaging chromosomal DNA in the elimination process, the phenotypic changes caused by mutation may interfere with the function analysis of the plasmid (Pickett et al., Citation2005). Therefore, molecular biological programmes, such as plasmid incompatibility and transposon-mediated direct selection techniques using Tn5-sacB or Tn5-rpsL, are more applicable and acceptable than physical and chemical methods (Wang et al., Citation2015). However, it is easy for transposons to produce false positive results, and the engineering plasmid used in plasmid incompatibility method needs to be eliminated during the construction of plasmid-cured strain. In this study, we developed a plasmid-specific curing method that could eliminate plasmids with high copy number in Salmonella using suicide vector pDM4 (Milton et al., Citation1992). Furthermore, we characterized the strain during infection of host cells and immune response.
Materials and methods
Bacterial strains, plasmids, and primers
S. Pullorum C79-13 is a virulent reference strain provided by Chinese Institute of Veterinary Drug Control. pDM4 is a derivative suicide vector of pNQ705 that contains the sacBR genes from Bacillus subtilis, and has been applied to construction of a mutant in Vibrio anguillarum (Milton et al., Citation1992). The bacteria and primers used to construct the plasmid-cured strain are listed in . Bacteria were cultured in ordinary LB medium, and antibiotics or supplements were added to the medium with appropriate concentrations as follows: diaminopimelic acid (DAP, 50 μg/ml), ampicillin (Amp, 100 μg/ml), chloramphenicol (Cm, 25 μg/ml), kanamycin (Km, 50 μg/ml), and sucrose (10%).
Table 1. Strains, plasmids and primers used in this study.
Construction of plasmid-cured strain C79-13-ΔpSPI12
A pair of primers (ipaJU-F/R) was designed to amplify a fragment ipaJU with 582 bp from pSPI12 plasmid in S. Pullorum C79-13 (). PCR analysis was conducted using reaction mixtures (total volume 50 μl) containing 25 μl 2× Taq Master Mix for PAGE (Dye Plus) (Vazyme, NanJing, Jiangsu, China), 2 μl forward and reverse primers (10 μM stock), 2 μl of sample, and 25 μl double-distilled H2O (ddH2O). The PCR conditions were as follows: initial denaturation at 94°C for 5 min, 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s, and then final extension at 72°C for 10 min. The fragments were purified after 0.6% agarose gel electrophoresis using an Agarose Gel DNA Extraction Kit (Takara, Kusatsu, Shiga, Japan), and then served as the arm for homologous recombination. Subsequently, the ipaJU fragment and the pDM4 suicide plasmid digested with XbaI were ligated via ClonExpress™ II (Vazyme, USA) to produce pDM4-ipaJU(CmR), which was then further transferred to E. coli DH5α while selecting on agar plates containing Cm (25 μg/ml). The recombinant plasmid pDM4-ipaJU was then transformed into the donor bacteria, E. coli χ7213, by electroporation (200 Ω, 25 μF, 1.8 kV) to generate E. coli χ7213(pDM4-ipaJU).
Conjugative transfer was used to transform the recombinant suicide vector pDM4-ipaJU into C79-13. Briefly, the donor strain E. coli χ7213(pDM4-ipaJU) and the recipient strain C79-13 were cultured in LB at 37°C, with shaking, to OD600 = 0.6. Samples of both strains were then mixed at a ratio of 1:1, washed with LB three times and immobilized on a 0.22 μm membrane filter. The membrane was then incubated on LB agar with DAP at 37°C for 24 h, after which it was immersed in liquid LB and spread on LB agar containing Cm without DAP. Under these conditions, the recombinant plasmid was transformed into C79-13 and homologous recombination caused the insertion of pDM4-ipaJU into pSPI12 to produce pSPI12-pDM4-ipaJU. Single colonies from LB agar were sub-cultured on LB with 10% sucrose and Cm without NaCl at 37°C for 24 h. The bacterial suspension was diluted by 1:10 and spread onto LB agar plates containing 10% sucrose, after which it was cultured at 37°C for 24 h. The clones that could survive in LB agar with sucrose should lose pDM4-pSPI12. The plasmid-cured strain was further identified by PCR to detect genes located in pSPI12 (Fig S1).
Confirmation of C79-13-ΔpSPI12 mutant by plasmid incompatibility
To further verify that the pSPI12 plasmid was cured in the mutant, the pSPI12/KmR plasmid with the KmR cassette inserted into pSPI12 could be transformed into the mutant, but not into the wild type C79-13 with pSPI12 based on the plasmid incompatibility principle (Li et al,. Citation2014). Therefore, the pSPI12/KmR was used to transform the plasmid-cured and wild type strain by electroporation (200 V, 25 mF, 1.8 kV), respectively.
Transformation of pBR322-ipaJ into the plasmid-cured strain and S. Enteritidis P125109
To construct the complementary strain, the ipaJ gene was inserted into pBR322 to give rise to pBR322-ipaJ, which was then transformed into C79-13-ΔpSPI12. For electrotransformation, 250 ng of the desired plasmid was mixed with competent cells, subjected to 1800 V for 4.5 ms, with a resistance of 200 and a capacitance of 25 μF using a Biorad electroporator. In addition, we also transformed the recombinant plasmid into S. Enteritidis P125109 to produce P125109-ipaJ in order to study the role of the ipaJ gene in the closely related serotype of S. Pullorum ().
Cell infection assay
The avian macrophage cell line HD11 (Beug et al., Citation1979) and avian hepatocellular carcinoma epithelial cell line LMH (ATCC CRL-2117) (Kawaguchi et al., Citation1987) were used as the phagocyte model and epithelial cell model, respectively (Mu et al., Citation2013), to determine if plasmid pSPI12 could significantly affect the infection ability of S. Pullorum. Briefly, HD11 and LMH cells were suspended in DMEM with 10% foetal bovine serum and then plated with 3 × 105 cells in 24-well plates 12 h prior to infection. The cells were then infected with S. Pullorum at multiplicity of infection = 100:1. After 1 h of incubation with different strains of S. Pullorum at 37°C under 5% CO2, cells were washed three times with sterile PBS buffer and replaced with fresh culture medium supplemented with 100 μg/ml of gentamicin sulphate, then incubated at 37°C under 5% CO2 for 1 h to kill the extracellular bacteria. For invasion ability analysis, HD11 and LMH cells were washed three times with PBS, then subjected to fragmentation of cells for 10 min with 1 ml of Triton X-100 (1% v/v) (Thermo Fisher Scientific, Hudson, NH, USA). Cells not infected by bacteria were used as negative controls. The concentration of intracellular bacteria was determined based on a bacterial coating count. To accomplish this, the lysate was decimal-diluted to 10−4 in triplicate for each strain, after which 100 μl of each dilution was coated onto LB agar plates to determine the number of colonies. The cell infection assay was performed in triplicate and repeated three times.
For detection of cytokines secreted by cells, the infected HD11 cells were cultured in fresh medium with 10 μg/ml of gentamicin sulphate for 4 h. The cells were then washed with PBS and preserved in RNAprotect Cell Reagent (Qiagen, Germany) for extraction of total RNA, which was conducted using an RNA plus Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Extracted RNA was stored at −80°C until qRT-PCR analysis. The cells stimulated by LPS (50 μg/ml) from S. Enteritidis (Sigma, Santa clara, CA, USA) were considered as the positive control for detection of cytokine production.
Animal testing
All of the animal protocols in the study were approved by Yangzhou University’s Institutional Animal Care and Use Committee. Thirty-six 4-day-old specific pathogen free chickens were divided into six groups (six for each group). Each bird was inoculated orally with 109 CFU of the corresponding S. Pullorum strain (C79-13; C79-13-ΔpSPI12; C79-13-ΔpSPI12-pBR322-ipaJ) and S. Enteritidis strain (P125109; P125109-pBR322-ipaJ) in 0.1 ml PBS. The control group was given 0.1 ml of sterile PBS orally. At 7 days post infection, spleens were collected from six birds in each group and prepared for RNA extraction by using RNeasy plus mini kit (Qiagen, Germany).
qRT-PCR analysis of cytokines
The levels of cytokines secreted by HD11 cells and chickens infected by S. Pullorum and S. Enteritidis were measured using qRT-PCR with TaqMan® probes (Tang, Citation2016). Here, IFN-γ, IL-12α, IL-4, IL-6, and IL-1β mRNA levels were measured to evaluate the immune response caused by the ipaJ gene in pSPI12 by S. Pullorum during infection of macrophages and chickens. qRT-PCR for each gene was performed in triplicate and repeated three times.
Statistical analysis
All data were displayed as mean ± standard error of the mean values, unless otherwise specified, and analysed with GraphPad Prism to detect differences among the treated groups (La Jolla, CA, USA). A P value less than 0.05 was considered significant when using one-way analysis of variance.
Results
Construction and confirmation of plasmid-cured strain C79-13-ΔpSPI12
The recombinant suicide plasmid pDM4-ipaJU was successfully constructed and verified by PCR using SalI-up/SacI-down primers to produce an 887 bp fragment, as well as by sequencing analysis. After homologous recombination and induction in 10% sucrose medium, individual clones were analysed by colony PCR, to determine if pSPI12 had been eliminated from the strain, using two pairs of primers (ipaJNB-F/R and ipaJOut-F/R) (, (A,B)). The PCR-identified strain of C7913–ΔpSPI12 mutant was the pSPI12 plasmid-cured strain ((A,B)).
Figure 1. Identification of C79-13-ΔpSPI12 and C79-13-ΔpSPI12-pSPI12/ KmR by PCR. (A) Six mutant clones were selected for the identification of pSPI12 by PCR with ipaJNB-F/R. Lanes 1–6: S. Pullorum C79-13-ΔpSPI12 strains; Lane 7: wild type strain C79-13, which was used as a positive control for pSPI12. (B) PCR identification of C79-13-ΔpSPI12 by ipaJOut-F/R. Lanes 1–4: S. Pullorum C79-13-ΔpSPI12 strains; Lane 5: C79-13. (C) PCR identification of pSPI12/KmR plasmid transformed into C79-13-ΔpSPI12 by primers ipaJNB-F/ R. The plasmid pSPI12/KmR was transformed into C79-13-ΔpSPI12 to produce C79-13-ΔpSPI12-pSPI12/KmR strain. Lane 1: S. Pullorum C79-13-ΔpSPI12-pSPI12/KmR strain; Lane 2: S. Pullorum C79-13-ΔpSPI12 strain.
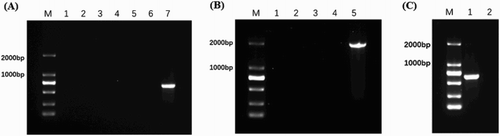
As shown in (C), the C79-13-ΔpSPI12 mutant obtaining pSPI12/KmR plasmid was verified by ipaJNB-F/ipaJNB-R (pSPI12/KmR plasmid can be amplified to produce an approximately 2000 bp fragment). Whole genome sequencing was also used to demonstrate that the pSPI12 was deleted from the mutant.
Decreased invasion ability of C79-13-ΔpSPI12 to cells
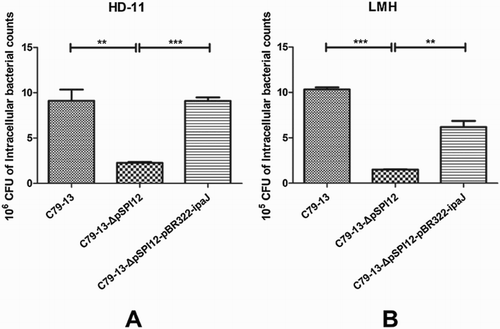
Both epithelial cells and macrophages were used to compare the invasion ability of three strains C79-13, C79-13-ΔpSPI12, and C79-13-ΔpSPI12-pBR322-ipaJ (Table 2). As shown in , the average number of wild type and complementary strain invading into HD11 cells was 9.12 × 106 and 9.11 × 106 CFU respectively, which was significantly higher than that of mutant strain (2.27 × 106 CFU). Although the invasion ability of S. Pullorum to epithelial cells was lower than that of macrophages, the average number of C79-13-ΔpSPI12 invading into LMH cells was 1.51 × 105 CFU - less than C79-13 (1.03 × 106 CFU) and complementary strain (6.2 × 105 CFU), respectively. The results showed that the invasion ability of C79-13-ΔpSPI12 to epithelial cells and macrophages was greatly reduced, compared to the wild type and the complementary strain.
Figure 2. Invasion ability of three S. Pullorum strains in infected HD11 and LMH cells. The ability of S. Pullorum to invade HD-11 (A) and LMH (B) cells was compared among C79-13, C79-13-ΔpSPI12, and C79-13-ΔpSPI12-pBR322-ipaJ strains. Each experiment was repeated three times. * indicates a significant difference, **P < 0.01, ***P < 0.001. The initial number of bacteria used to infect cells: C79-13 (1.82 × 108 CFU), C79-13-ΔpSPI12 (1.70 × 108 CFU), and C79-13-ΔpSPI12-pBR322-ipaJ (1.48 × 108 CFU).
Modulation of immune response by IpaJ plasmid pSPI12
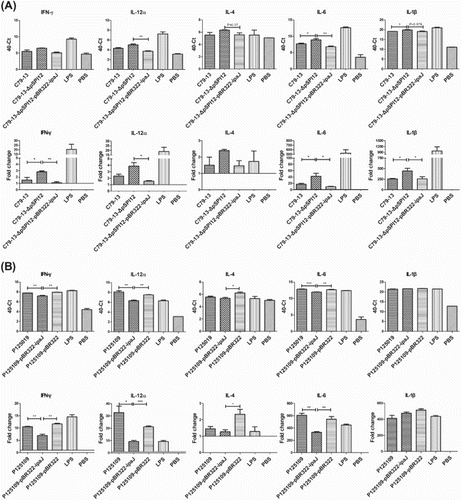
A TaqMan® probe-based qRT-PCR assay was used to detect the T cell-associated and proinflammatory cytokines expression of HD11 cells infected by C79-13, C79-13-ΔpSPI12, and C79-13-ΔpSPI12-pBR322-ipaJ, respectively. As shown in (A), the mRNA levels of IFN-γ, IL-6, and IL-1β in the C79-13-ΔpSPI12 group were higher than that in the wild type and complementary strain group. Transformation of the ipaJ gene into the mutant caused decreased transcription of IFN-γ and IL-12α, as well as that of the wild type strain. In addition, cytokines were also detected in HD11 cells infected by S. Enteritidis P125109 and P125109-pBR322-ipaJ ((B)). Interestingly, transformation of ipaJ into P12109 caused a significantly decreased transcription of IFN-γ, IL-12α, and IL-6 when compared to the wild type strain, but no difference was detected in levels of IL-4 and IL-1β mRNA between strains.
Figure 3. Detection of cytokine mRNA levels in HD11 cells infected by S. Pullorum or S. Enteritidis, using qRT-PCR. TaqMan probe-based qRT-PCR was used to detect the mRNA level of IFN-γ, IL-12α, IL-4, IL-6, and IL-1β in HD-11 cells infected by S. Pullorum (C79-13, C79-13-ΔpSPI12, and C79-13-ΔpSPI12-pBR322-ipaJ) (A) and S. Enteritidis (P125109, P125109-ipaJ, P125109-pBR322) (B), respectively, * indicates a significant difference, *P < 0.05, **P < 0.01, ***P < 0.001.
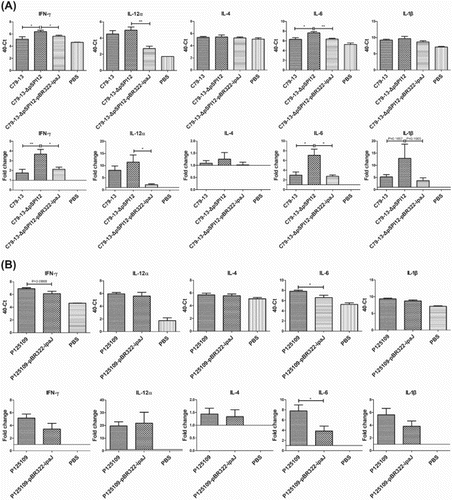
qRT-PCR assay was used to detect the cytokine expression in the spleen of chickens infected by C79-13, C79-13-ΔpSPI12, and C79-13-ΔpSPI12-pBR322-ipaJ, respectively. As shown in (C), the mRNA levels of IFN-γ and IL-6 in the C79-13-ΔpSPI12 group were obviously higher than that in the wild type and complementary strain groups, but no difference was detected in the IL-12α and IL-1β mRNA levels. In addition, cytokines were also detected in the spleen of chickens infected by S. Enteritidis P125109 or P125109-pBR322-ipaJ ((D)). Interestingly, transformation of ipaJ into P125109 caused decreased transcription of IL-6 when compared to the wild type strain, but no difference was detected in IL-4 and IL-12α mRNA levels between strains. Furthermore, the transcription of IFN-γ and IL-1β in the group infected by P125109-pBR322-ipaJ was reduced when compared to the wild type group.
Figure 4. Detection of cytokine mRNA levels in spleen of chickens infected by S. Pullorum or S. Enteritidis, using qRT-PCR. TaqMan probe-based qRT-PCR was used to detect the mRNA level of IFN-γ, IL-12α, IL-4, IL-6, and IL-1β in spleen of chickens infected by S. Pullorum (C79-13, C79-13-ΔpSPI12, and C79-13-ΔpSPI12-pBR322-ipaJ) (A) and S. Enteritidis (P125109, P125109-ipaJ) (B), respectively, * indicates a significant difference, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
S. Pullorum is the host-restricted serotype of Salmonella, which is a common cause of disease in poultry. The pathogen can not only infect young chicks through horizontal transfer, but it can also transmit to the offspring by vertical transfer (Barrow & Freitas Neto, Citation2011). Although the pullorum disease has reportedly been eradicated in developed countries, it still poses a great threat to developing countries (Barrow & Freitas Neto, Citation2011). However, the host-restriction and immune response induced by the pathogen remain unclear. Comparative genomic analysis revealed that the high number of genes converted to pseudogenes may be the reason for its host-restriction (Batista et al., Citation2015), but we found that some specific genes carried by S. Pullorum may be another cause for its pathogenesis (Li et al., Citation2009; Li et al., Citation2014). In our previous study, we detected a specific plasmid, pSPI12, that was prevalent in S. Pullorum isolates (Li et al., Citation2014). Interestingly, the plasmid is small (about 4 kb) with only one putative virulence-related gene, ipaJ, which was also detected in Shigella flexneri; however, the homology between both IpaJ proteins was less than 50% (Li et al., Citation2014). Insertion mutation of the ipaJ gene caused decreased infection ability of S. Pullorum to chicken kidney cells (Li et al., Citation2014). To analyse the function of the ipaJ gene, we attempted to construct a plasmid-cured strain. In this study, we reported a new method for curing native multi-copy plasmids in Salmonella by pDM4 suicide vector (Figure S1). Briefly, a specific fragment from the plasmid was cloned into pDM4 vector, which was then transformed into the wild type strain. After homologous recombination, the suicide vector was inserted into the plasmid. Upon induction by sucrose, the sacB gene in pDM4 was expressed to produce levansucrase, which could hydrolyse sucrose and glucose to fructose, resulting in the production of a toxic fructan that kills the bacteria having the pDM4-containing plasmid, while only bacteria that lost the plasmid could survive in sucrose (Thomas, Citation2014). We also constructed the complementary strain with only the ipaJ gene transformed into the mutant strain.
As an invasion plasmid antigen (Ipa), IpaJ participates in the invasion of Shigella to host cells and dispersion of bacteria among the cells (Buysse et al., Citation1997). Here, the plasmid-cured S. Pullorum without ipaJ showed greatly decreased ability to invade the avian epithelial cell line (LMH) and macrophages (HD11), which confirmed its participation in the infection process. However, because SPI-1 has been confirmed to play important roles in Salmonella invasion into host cells, future studies should be conducted to determine if IpaJ acts as an effector of SPI-1.
Chickens that recover from pullorum disease or adult chickens infected by S. Pullorum can still carry the pathogen without evidence of infection; however, the bacteria are not eradicated by the host immune response in these chickens. The Th2 response is reportedly induced by S. Pullorum to kill the bacteria outside of cells, while the Th1 response to kill bacteria inside the macrophages is not adequately induced by S. Pullorum (Chappell et al., Citation2009). Unlike S. Pullorum, S. Enteritidis not only induced a Th2 response, but also a strong Th1 response. Therefore, it is speculated that some proteins in S. Pullorum may prevent induction of Th1 response or S. Pullorum loses the genes involved in inducing Th1 response. IFN-γ and IL-4 are the hallmark Th1 and Th2 cytokines, respectively. IFN-γ was originally defined as produced only by CD4+ Th1 lymphocytes, CD8+ cytotoxic lymphocytes, and NK cells and is involved in the activation of macrophages for nitric oxide production and promoting intracellular killing of Salmonella (Schroder et al., Citation2004). However, macrophages and dendritic cells can also produce IFN-γ and are regulated by IFN-γ in an autocrine fashion, which led to the idea of autocrine myeloid-cell activation in innate immunity (Rothfuchs et al., Citation2004; Puddu et al., Citation2005; Bogdan & Schleicher, Citation2006). Additionally, IL-2 induces expression and secretion of IFN-γ in murine peritoneal macrophages, demonstrating the immune regulatory function of IFN-γ and IL-4 on chicken macrophage effector function (He et al., Citation2011). Chicken IL-12α was shown to induce IFN-γ production and promote the proliferation of chicken splenocytes, which was similar to its activity in driving inflammatory responses in mammals (Degen, et al., Citation2004). In poultry, invasion by S. Typhimurium and S. Enteritidis induces strong inflammatory responses by a rapid increase in IL-1β, IL-6, and CXCLi2 transcription, notably high levels of IL1-β, and up-regulated the expression of IL-6 mRNA by 8–10 fold, but this was not evident during S. Pullorum infection (Chappell et al., Citation2009; Salisbury et al., Citation2014). In this study, we detected that S. Pullorum without the ipaJ gene showed increased IFN-γ mRNA levels and inflammatory responses compared to wild type strain and complementary strain with ipaJ gene. Interestingly, transformation of ipaJ into S. Enteritidis induced decreased IFN-γ mRNA levels and inflammatory responses compared to wild type strain. Therefore, we speculate that IpaJ acted as an inhibitor to induction of avian IFN-γ mRNA levels and inflammatory responses. However, the molecular mechanism of IpaJ action needs to be further studied in the future.
Supplemental Material
Download Zip (653.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Qiuchun Li http://orcid.org/0000-0003-0923-9758
Additional information
Funding
References
- Barrow, P.A. & Freitas Neto, O.C. (2011). Pullorum disease and fowl typhoid – new thoughts on old diseases: a review. Avian Pathology, 40, 1–13. doi: 10.1080/03079457.2010.542575
- Batista, D.F., Freitas Neto, O.C., Barrow, P.A., Oliveira, M.T., Almeida, A.M., Ferraudo, A.S. & Berchieri, A. Jr. (2015). Identification and characterization of regions of difference between the Salmonella Gallinarum biovar Gallinarum and the Salmonella Gallinarum biovar Pullorum genomes. Infection Genetics & Evolution, 30, 74–81. doi: 10.1016/j.meegid.2014.12.007
- Beug, H., von Kirchbach, A., Döderlein, G., Conscience, J.F. & Graf, T. (1979). Chicken hematopoietic cells trans-formed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell, 18, 375–390. doi: 10.1016/0092-8674(79)90057-6
- Bogdan, C. & Schleicher, U. (2006). Production of interferon-γ by myeloid cells – fact or fancy? Trends in Immunology, 27, 282–290. doi: 10.1016/j.it.2006.04.004
- Buysse, J.M., Dunyak, D.S., Hartman, A.B. & Venkatesan, M.M. (1997). Identification and molecular characterization of a 27 kDa Shigella flexneri invasion plasmid antigen, IpaJ. Microbial Pathogenesis, 23, 357–369. doi: 10.1006/mpat.1997.0164
- Chappell, L., Kaiser, P., Barrow, P., Jones, M.A., Johnston, C. & Wigley, P. (2009). The immunobiology of avian systemic salmonellosis. Veterinary Immunology & Immunopathology, 128, 53–59. doi: 10.1016/j.vetimm.2008.10.295
- Degen, W.G., Van Daal, N., van Zuilekom, H.I., Burnside, J. & Schijns, V.E. (2004). Identification and molecular cloning of functional chicken IL-12. The Journal of Immunology, 172, 4371–4380. doi: 10.4049/jimmunol.172.7.4371
- Geng, S.Z., Jiao, X.A., Pan, Z.M., Chen, X.J., Zhang, X.M. & Chen, X. (2014). An improved method to knock out the asd gene of Salmonella enterica serovar Pullorum. Journal of Biomedicine & Biotechnology, 2009, 646380.
- He, H., Genovese, K.J. & Kogut, M.H. (2011). Modulation of chicken macrophage effector function by T(H)1/T(H)2 cytokines. Cytokine, 53, 363–369. doi: 10.1016/j.cyto.2010.12.009
- Kang, H.Y., Srinivasan, J. & Curtiss, R. (2002). Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infection and Immunity, 70, 1739–1749. doi: 10.1128/IAI.70.4.1739-1749.2002
- Kawaguchi, T., Nomura, K., Hirayama, Y. & Kitagawa, T. (1987). Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Research, 47, 4460–4464.
- Li, Q., Hu, Y., Xu, Y., Chen, J., Fang, L., Liu, Z. & Jiao, X. (2014). A gene knock-in method used to purify plasmid pSPI12 from Salmonella enterica serovar Pullorum and characterization of IpaJ. Journal of Microbiological Methods, 98, 128–133. doi: 10.1016/j.mimet.2014.01.011
- Li, Q., Xu, Y. & Jiao, X. (2009). Identification of Salmonella pullorum genomic sequences using suppression subtractive hybridization. Journal of Microbiology & Biotechnology, 19, 898–903. doi: 10.4014/jmb.0812.694
- Milton, D.L., Norqvist, A. & Wolfwatz, H. (1992). Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. Journal of Bacteriology, 174, 7235–7244. doi: 10.1128/jb.174.22.7235-7244.1992
- Mu, X., Huan, H., Xu, H., Gao, Q., Xiong, L., Gao, R. & Liu, X. (2013). The transfer-messenger RNA-small protein B system plays a role in avian pathogenic Escherichia coli pathogenicity. Journal of Bacteriology, 195, 5064–5071. doi: 10.1128/JB.00628-13
- Puddu, P., Carollo, M., Pietraforte, I., Spadaro, F., Tombesi, M., Ramoni, C., Belardelli, F., & Gessani, S. (2005). IL-2 induces expression and secretion of IFN-γ in murine peritoneal macrophages. Journal of Leukocyte Biology, 78, 686–695. doi: 10.1189/jlb.0105035
- Pickett, M.A., Everson, J.S., Pead, P.J. & Clarke, I.N. (2005). The plasmids of Chlamydia trachomatis and Chlamydophila pneumoniae (N16): accurate determination of copy number and the paradoxical effect of plasmid-curing agents. Microbiology, 151, 893–903. doi: 10.1099/mic.0.27625-0
- Rothfuchs, A.G., Kreuger, M.R., Wigzell, H. & Rottenberg, M.E. (2004). Macrophages, CD4+ or CD8+ cells are each sufficient for protection against Chlamydia pneumoniae infection through their ability to secrete IFN-γ. The Journal of Immunology, 172, 2407–2415. doi: 10.4049/jimmunol.172.4.2407
- Salisbury, A.M., Leeming, G., Nikolaou, G., Kipar, A. & Wigley, P. (2014). Salmonella Virchow infection of the chicken elicits cellular and humoral systemic and mucosal responses, but limited protection to homologous or heterologous re-challenge. Frontiers in Veterinary Science, 1, 6. doi: 10.3389/fvets.2014.00006
- Schroder, K., Hertzog, P. J, Ravasi, T., & Hume, D. A. (2004). Interferon-γ: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology, 75, 163–189. doi: 10.1189/jlb.0603252
- Spengler, G., Molnár, A., Schelz, Z., Amaral, L., Sharples, D. & Molnár, J. (2006). The mechanism of plasmid curing in bacteria. Current Drug Targets, 7, 823–841. doi: 10.2174/138945006777709601
- Tang, Y. (2016). Immune modulation of Salmonella enterica serotype Pullorum in the chicken. The degree of Doctor of Philosophy, University of Nottingham.
- Thomas, C.M. (2014). Plasmid curing. US. Patent 8877502.
- Trevors, J.T. (1985). Bacterial plasmid isolation and purification. Journal of Microbiological Methods, 3, 259–271. doi: 10.1016/0167-7012(85)90008-9
- Trevors, J.T. (1986). Plasmid curing in bacteria. FEMS Microbiology Letters, 32, 149–157. doi: 10.1111/j.1574-6968.1986.tb01189.x
- Wang, D., Gao, Z., Wang, H., Feng, E., Zhu, L., Liu, X. & Wang H. (2015). Curing both virulent mega-plasmids from Bacillus anthracis wild-type strain A16 simultaneously using plasmid incompatibility. Journal of Microbiology & Biotechnology, 25, 1614–1620. doi: 10.4014/jmb.1503.03083
