ABSTRACT
The pathophysiology of heat illnesses in birds has not been well characterized. In this study, we describe the changes in heart rate, respiratory rate, blood biochemistry and histopathological findings in galahs and rock doves after heat exposure under standardized conditions designed to induce heatstroke. Birds in the heat-exposed group were exposed to environmental heat stress and compared to control birds. Both groups of birds were under general anaesthesia throughout the experiment and serial blood collections were performed for biochemical analyses, while organs were collected at the end of the experiment for histopathology. No electromyography traces consistent with the onset of heat cramps were observed in any of the birds. Biochemical changes suggestive of skeletal muscle and hepatocellular injury, including hyperkalaemia and increased serum muscle and hepatic enzyme activities, were often observed in heat-exposed galahs and rock doves at the onset of heatstroke. Microscopic analyses did not reveal any significant cardiac changes, although some lungs had signs of acute congestion. Some heat-exposed rock doves had microscopic changes indicative of necrosis in the pectoral muscle. There were significant hepatic changes in some heat-exposed galahs, but not in rock doves. This suggests that there may be species differences amongst birds in the organs most affected by heatstroke. The observed species differences in the physiological, biochemical and histopathological changes indicate that bird species should be studied separately for clinical syndromes such as heatstroke.
RESEARCH HIGHLIGHTS
Biochemical changes suggestive of skeletal muscle and hepatocellular injury in heat-exposed galahs and rock doves at the onset of heatstroke
No electromyography traces consistent with the onset of heat cramps were observed
Some heat-exposed rock doves had indications of necrosis in the pectoral muscle
There were significant hepatic changes in some heat-exposed galahs
Introduction
An inability of the body to cope with extremely high temperatures will eventually lead to heat illness. Climatic heat waves have been associated with mass mortality events in desert-dwelling birds (McKechnie et al., Citation2012; McKechnie & Wolf, Citation2010), including endangered species (Saunders et al., Citation2011). By the time the birds are found dead after a heatwave, they have presumably gone through the cascade of symptoms associated with, and suffered the effects of, heatstroke. It is therefore difficult to identify pathognomonic changes in wild birds suffering from heat illnesses during climatic heatwaves. Personal communications with avian veterinarians indicate that heat illnesses are extremely rare in clinical avian practice.
While the exact pathophysiology of heat illnesses in birds has not been well characterized, it is better understood in humans. Heat illnesses can be classified as heat syncope, heat cramps, heat exhaustion and heatstroke (Bricknell, Citation1995). Heatstroke in humans was traditionally defined as hot, dry skin accompanied by central nervous abnormalities associated with core body temperatures above 40°C (Bouchama & Knochel, Citation2002). An alternative definition classifying it as a form of hyperthermia, associated with systemic inflammatory response resulting in multi-organ dysfunction predominated by encephalopathy, has also been proposed (Bouchama & Knochel, Citation2002). Heat cramps, muscle spasms that result from exposure to heat, and heat exhaustion, the intermediate heat illness before heatstroke, have not been documented in animals to date. The paucity of reports and studies of heat illnesses in birds other than poultry means that our understanding of the syndrome is largely based on mammalian studies. Heatstroke itself is relatively well-studied and understood in species such as baboons (Bouchama et al., Citation2005), rats (Chen et al., Citation2006), guinea pigs (Dechesne et al., Citation1992) and rabbits (Abdelatif & Modawi, Citation1994; Lin & Lin, Citation1992) serving as experimental models for human heatstroke, and in dogs (Bruchim et al., Citation2009; Oglesbee et al., Citation1999) where it is a relatively common occurrence in veterinary practice (Johnson et al., Citation2006).
One study of baboons suffering from induced heatstroke demonstrated damage to multiple organs including the jejunum, liver, spleen, lung and kidney; this manifested as vascular congestion, haemorrhage, thrombosis, increased inflammatory cells, and disruption of normal cell and tissue architecture (Roberts et al., Citation2008). Histopathological reports for birds dying of heatstroke are uncommon, likely because the quick onset of autolysis under high temperatures precludes any useful post mortem examination unless the carcasses were collected and examined immediately or stored appropriately until examination could be carried out. Overstreet & Rehak (Citation1982) reported that least tern (Sterna albifrons) chicks suspected to be suffering from heatstroke showed microscopic evidence of early ischaemic focal necrosis in the brain, characterized by neuronal degeneration, infiltration of leukocytes and degeneration of the neuropil surrounding some cortical peripheral blood vessels. The splenic sinuses were packed with red blood cells, an indicator of circulatory collapse while small areas of degeneration were found in the liver, kidneys, and intestine (Overstreet & Rehak, Citation1982). However the lungs, heart, and other tissues were not examined. In another report, the pathological changes of a sun conure (Aratinga solstitialis), that was suspected to have suffered heatstroke under general anaesthesia, revealed mild multifocal acute degeneration and contraction band necrosis of the biceps femoris muscle, as well as diffuse moderate acute congestion of the lungs (Hofmeister, Citation2005).
In the present study, we describe the changes in heart rate, respiratory rate, blood biochemistry and histopathological findings in galahs (Eolophus roseicapilla) and rock doves (Columba livia) after heat exposure under general anaesthesia in standardized conditions designed to induce heatstroke.
Materials and methods
Birds
Eight galahs and eight rock doves were obtained from private bird breeders in Adelaide from 2016 to 2017 and immediately transported to the University of Adelaide Roseworthy Campus (Roseworthy, South Australia) to be subjected to the experimental protocol. All experiments were conducted according to the Australian code for the care and use of animals for scientific purposes, and approved by the University of Adelaide Animal Ethics Committee (S-2015-189).
Experimental protocol
Each bird was randomly assigned to the heat-exposed or control group, such that there were four birds of each species in each group. The birds were anaesthetised using isoflurane in 100% oxygen delivered via facemask connected to a non-rebreathing circuit, and anaesthesia was maintained at surgical depth this way throughout each procedure. Birds in the heat-exposed group were exposed to environmental heat stress using three Thermotex Infrared Heating Mats (Thermotex Therapy Systems Ltd, Calgary, Alberta, Canada) each set at the maximum 55°C. Birds in the control group were placed on the same heat mats, but with each set at 45°C to maintain a constant normal body temperature (Tb) throughout general anaesthesia.
After each bird was anaesthetised, blood was collected via the jugular vein using an insulin syringe (Time 1). Birds in the heat-exposed group also had blood collected when their body temperatures reached 41.5°C (Time 2), 43.0°C (Time 3) and after heatstroke had been induced (Time 4). Heatstroke was deemed to have occurred when the bird exhibited an exponential increase in body temperature with a concurrent increase in heart rate followed by a rapid decrease in heart rate. Birds in the control group had blood collected at 20 min (Time 2), 35 min (Time 3) and 60 min (Time 4) after the first blood collection. A volume of 0.2 ml of blood was collected at each time point and all blood samples were collected into lithium heparin blood tubes. The blood samples were then centrifuged at 7000 × g for 10 min, and the plasma subsequently collected and stored at −80°C until analysis.
Birds in the heat-exposed group were euthanized once heatstroke had been induced whereas birds in the control group were euthanized after collection of the last blood sample; all birds were euthanized with 100 mg/kg of sodium pentobarbitone administered intracoelomically. Each bird’s brain, eyes, pectoral muscle, heart, gastrointestinal tract, liver, lungs and kidneys were collected and stored in 10% neutral buffered formalin.
Physiological parameters
The heart rate, electromyograph (EMG), electrocardiograph (ECG) and cloacal temperature were monitored and recorded throughout the experimental protocol using PowerLab with LabChart 8 (AD Instruments, Colorado Springs, USA). The pectoral muscle of one of the heat-exposed rock doves was stimulated using a nerve stimulator to simulate EMG traces that might be expected during a heat cramp. This was done to produce waveforms that could be used to identify muscle cramps associated with heat exposure for the rest of the birds in the experiment. The heart rate was obtained using the ECG function, and the respiration rate using the cyclic measurement rate detection function with smoothing at 500 milliseconds on the EMG data in LabChart 8. Heart and respiration rate data were averaged in 10 s blocks and then ranked by average cloacal temperature over the same 10 s block. The summarized heart rate and respiration rate data were then grouped into 0.25°C cloacal temperature increments (bins) and the average plotted against the median cloacal temperature. Data are presented as mean ± SEM.
Biochemistry
A biochemistry panel including sodium, potassium, chloride, calcium, phosphorus, total protein, albumin, globulin, uric acid, aspartate aminotransferase (AST), glutamate dehydrogenase (GLDH), creatinine kinase (CK), glucose and cholesterol was performed on each blood sample using a Beckman-Coulter AU480 Chemistry Analyzer (Beckman-Coulter Diagnostics, Lane Cove, NSW, Australia). All samples were diluted 1:4 using distilled water to achieve sufficient volumes to run the full panel. Despite the dilution, the blood samples from two galahs and two rock doves (one control and one heat-exposed for each species) were still not of sufficient volume to include GLDH, albumin and globulin in the analyses.
Histopathology
Formalin-fixed samples of the organs collected were trimmed and embedded in paraffin blocks, sectioned at 3–4 μm, mounted and stained with haematoxylin & eosin for routine microscopic examination. The slides were examined at the Veterinary Diagnostic Laboratory, University of Adelaide, Roseworthy, South Australia using a BX53 microscope (Olympus, Japan), and photomicrographs were captured using Labsens (Olympus, Japan).
Statistics
For both species and all parameters included in the biochemical analyses, species baseline 95% reference intervals (RI) and 90% confidence intervals (CI) specific to this study population were calculated using the results of all blood samples in the control groups and only Time 1 and Time 2 blood samples from birds in heat-exposed groups. Analyses were conducted using the Reference Value Advisor (RefVal) version 2.1 add-in for Microsoft Excel (Geffré et al., Citation2011). RI and CI were determined for each biochemical analyte following guidelines for generation of de novo reference intervals as published by the American Society of Veterinary Clinical Pathology (Friedrichs et al., Citation2012). All analytes, except GLDH, had sample sizes 20 ≤ n < 40 and had Gaussian distributions. Outlier values for all analytes were identified by RefVal using Dixon’s and Tukey’s range tests, and manually removed if attributable to determinable reasons such as poor sample quality or analytic error. CI for the reference limits were calculated using nonparametric bootstrap methods for all analytes (except GLDH). Because n = 10 for GLDH, a table of ascending values, mean, median values and a histogram were reported, but RIs were not determined due to the uncertainty of limits based on the small sample size (Friedrichs, et al., Citation2012).
Broken-stick linear regression models fitted in the R package segmented (Muggeo, Citation2008) were used to identify inflection points for the relationships between the heart rate and Tb, as well as respiration rate and Tb, for individuals of each species in the heat-exposed group, using the ranked 10 s block averaged data.
Results
Cloacal temperature
The mean cloacal temperature in heat-exposed galahs at the start of the experiments was 38.70 ± 1.33°C, increasing at a rate of 0.0025 ± 0.0004°C min−1, and lethal Tb was reached in 63.92 ± 30.25 min. The mean cloacal temperature in control galahs at the start of the experiments was 39.64 ± 0.55°C and decreased at a rate of 0.0002 ± 0.0006°C min−1. The mean cloacal temperature in heat-exposed rock doves at the start of the experiments was 41.77 ± 2.09°C, increasing at a rate of 0.0018 ± 0.0006°C min−1, and lethal Tb was reached in 92.38 ± 45.45 min. The mean cloacal temperature in control rock doves at the start of the experiments was 41.27 ± 0.12°C and decreased at a rate of 0.0009 ± 0.0009°C min−1.
Heart rate
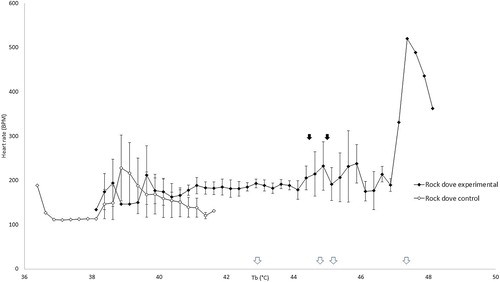
The heart rate of control galahs remained between 207 and 341 beats per minute (BPM) throughout the experiment () and control rock doves remained between 111 and 229 BPM ().
Figure 1. Heart rate in control galahs remained stable with increasing cloacal temperatures, but increased in heat-exposed galahs at higher cloacal temperatures initially before decreasing rapidly. The mean inflection points of these changes are indicated by the black arrows.
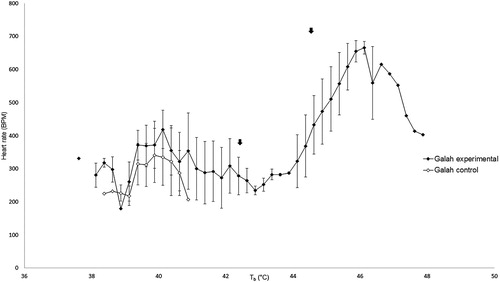
Figure 2. Heart rate in control rock doves remained stable with increasing cloacal temperatures, but increased in heat-exposed rock doves at higher cloacal temperatures initially before decreasing rapidly. The mean inflection points of these changes are indicated by the black arrows. The white arrows along the x-axis indicate the cloacal temperature at which peak heart rate was reached for each individual heat-exposed rock dove.
The mean Tb inflection point at which the heart rate of heat-exposed galahs started increasing above the range of the control galahs was 43.63 ± 0.71°C. Past this point, heart rate increased with Tb until a maximum of 656 ± 45 BPM. The heart rate began to decrease after the second mean Tb inflection point of 46.09 ± 0.16°C (). We were only able to perform the analysis for Tb inflection points for two heat-exposed galahs as the other two died just as their Tb was reaching the lower inflection point. The peak heart rates for the two heat-exposed galahs that showed a rapid increase and then decrease in heart rate occurred at 46.13 and 45.88°C.
The mean Tb inflection point at which the heart rate of heat-exposed rock doves started increasing above the range of the control rock doves was 44.59 ± 0.93°C. Past this point, heart rate increased with Tb until a maximum of 520 BPM. The heart rate began to decrease after the second Tb inflection point of 45.16 ± 0.91°C (). The same pattern of increased and then decreased heart rate was observed in all the rock doves in the heat-exposed group. The peak heart rates for individual heat-exposed rock doves occurred at 47.38, 44.88, 42.88 and 45.63°C.
Respiration rate
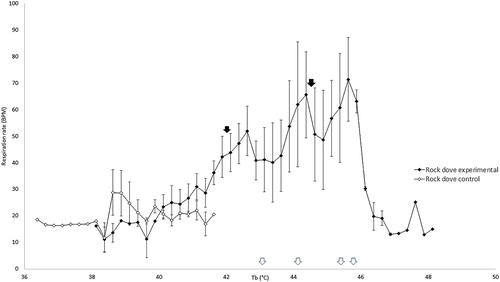
The respiration rate of control galahs remained between 13 and 30 breaths per minute (BrPM) throughout the experiment () and for control rock doves remained between 12 and 29 BrPM ().
Figure 3. Respiration rate in control galahs remained stable with increasing cloacal temperatures, but increased in heat-exposed galahs at higher cloacal temperatures initially before decreasing. The mean inflection points of these changes are indicated by the black arrows.
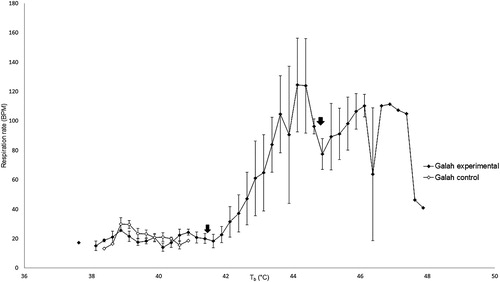
Figure 4. Respiration rate in control rock doves remained stable with increasing cloacal temperatures, but increased in heat-exposed rock doves at higher cloacal temperatures initially before decreasing. The mean inflection points of these changes are indicated by the black arrows. The white arrows along the x-axis indicate the cloacal temperature at which peak respiration rate was reached for each individual heat-exposed rock dove.
The mean Tb inflection point at which the respiration rate of heat-exposed galahs started increasing above the range of the control galahs was 41.34 ± 0.02°C. Past this point, respiration rate increased with Tb until a maximum of 124 ± 45 BrPM. The respiration rate began to decrease after the second mean Tb inflection point of 44.70 ± 1.05°C ().
The mean Tb inflection point at which the respiration rate of heat-exposed rock doves started increasing above the range of the control rock doves was 41.94 ± 0.68°C. Past this point, respiration rate increased with Tb until a maximum of 71 ± 22 BrPM. The respiration rate began to decrease after the second mean Tb inflection point of 44.33 ± 0.63°C ().
EMG
There was no evidence of heat cramps in the muscles, i.e. no EMG traces consistent with traces induced by muscle stimulation were observed, in either species in either control and heat-exposed groups.
Biochemistry
The reference intervals of the measured biochemical parameters for control galahs and rock doves in this study are summarized in and , except for GLDH, which is summarized in as the sample size was insufficient to determine reference intervals for this parameter. The blood biochemistry results are summarized in and . At the final time point and compared to galahs in the control group, galahs in the heat-exposed group had elevated potassium, phosphorus, total protein, AST and GLDH; whereas chloride and calcium were decreased. However, no such differences were found in rock doves in the control and heat-exposed groups. Rock doves in both control and heat-exposed groups had elevated potassium, AST and CK at the final time point.
Figure 5. Summary of the mean blood chemistry values in galahs, with each parameter plotted against time points 1–4. The units for sodium, potassium, chloride, calcium, phosphorus, glucose and cholesterol were mmol/l; total protein g/l; uric acid µmol/l; AST and CK IU/l. The SD values for each parameter were included in of the supplemental materials section. Values outside of the reference intervals are indicated by *.
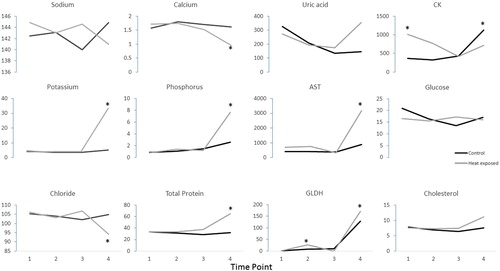
Figure 6. Summary of the mean blood chemistry values in rock doves, with each parameter plotted against time points 1–4. The units for sodium, potassium, chloride, calcium, phosphorus, glucose and cholesterol were mmol/l; total protein g/l; uric acid µmol/l; AST and CK IU/l. The SD values for each parameter were included in of the supplemental materials section. Values outside of the reference intervals are indicated by *.
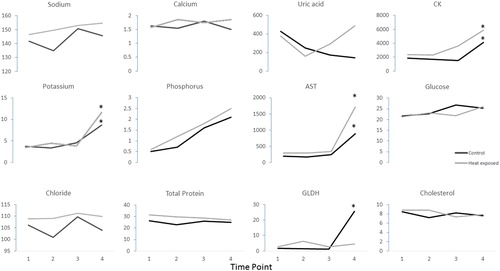
Table 1. Descriptive statistics and reference intervals for galahs.
Table 2. Descriptive statistics and reference intervals for rock doves.
Table 3. Descriptive statistics and table of ascending values for GLDH in galahs and rock doves (SD = standard deviation).
Table 4. Summary of blood results in galahs. Values outside of the reference intervals are in bold.
None of the blood samples analysed were affected by significant haemolysis. There were obvious differences between galahs and rock doves in the biochemistry changes associated with the onset of heatstroke. In the heat-exposed group, 4/4 (100%) of the galahs developed hyperkalaemia and hyperphosphataemia and 2/4 (50%) had elevated AST and CK. 2/2 (100%) had elevated GLDH, along with elevations in AST. 3/4 (75%) had hypocalcaemia and elevated total protein and individual birds were also identified with hyponatraemia or hypernatraemia, hypochloraemia, hypoglycaemia and elevated cholesterol.
Similar to galahs, many of the rock doves in the heat-exposed group [3/4 (75%)] developed hyperkalaemia and elevated CK, and 2/4 (50%) developed elevated AST, 1/2 (50%) had elevated GLDH, while 1/4 (25%) had elevated uric acid.
One control galah had elevations in GLDH and AST and hepatocellular degeneration was observed histologically. These values were therefore excluded from generation of reference intervals.
Gross post mortem findings
There were no obvious macroscopic lesions found on post mortem evaluation of any of the birds.
Histopathology
There was moderate diffuse congestion and intra-airway haemorrhage with endothelial hypertrophy of the lungs in two out of the four heat-exposed galahs. There was also congestion of the brain, heart and small intestines of these two galahs. In the liver of galah 5 (heat-exposed group), there was also congestion, mild endothelial hypertrophy, and scattered single cell necrosis and random foci of hepatocellular degeneration.
Histopathological changes were observed in one heat-exposed rock dove; these included diffuse congestion and mild haemorrhage of the lungs, and scattered rare myofiber swelling with loss of striations, indicative of acute degeneration in the pectoral muscle (). Rock dove 8 (control group) had mild to moderate periportal non-suppurative hepatitis and occasional multifocal perivascular infiltrates of mononuclear cells in the brain (interpreted as a pre-existing lesion) surrounding congested vessels lined by plump endothelia, indicative of focal non-suppurative encephalitis.
Figure 7. Rock dove, pectoral muscle: Scattered myofibers show acute degeneration and necrosis (arrows), characterized by myofiber swelling, cytoplasmic vacuolation, eosinophilia and loss of striations, basophilic inclusions (interpreted as early mineralization), and pyknosis.
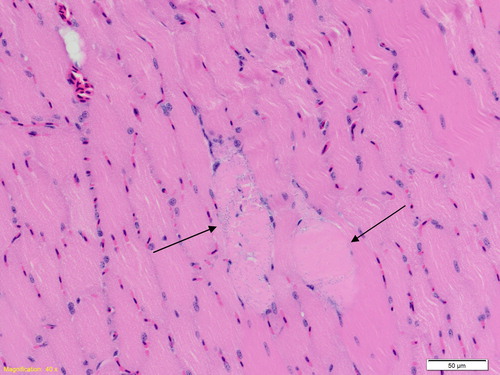
Table 5. Summary of blood results in rock doves. Values outside of the reference intervals are in bold.
Discussion
The changes in cloacal (body) temperature, heart rate and respiration rate in the present study were similar to those observed in anaesthetised experimental dog models for heatstroke (Bynum et al., Citation1977). The slight decrease in the cloacal temperature with time in control birds was not unexpected, as maintaining the body temperature of birds under general anaesthesia is difficult due to their large surface area to volume ratio (Boedeker et al., Citation2005; Phalen et al., Citation1996; Rembert et al., Citation2001). The increases in heart and respiration rates and subsequent decreases associated with heatstroke occurred at higher Tb in rock doves than in galahs. This was to be expected given the higher heat tolerance recently demonstrated in columbiform birds (McKechnie et al., Citation2016; Smith et al., Citation2015; Whitfield et al., Citation2015; Wolf et al., Citation2015), relative to psittaciform birds (McWhorter et al. Citation2018).
The mean Tb inflection point for heart rate increase in galahs was 43.63 ± 0.71°C and the mean Tb inflection point for heart rate decrease was 46.09 ± 0.16°C. The mean Tb inflection point for heart rate increase in rock doves was 44.59 ± 0.93°C and the mean Tb inflection point for heart rate decrease was 45.16 ± 0.91°C. Therefore, a rock dove experiencing heat stress under anaesthesia has a very narrow window between showing measurable signs of heat stress and experiencing irreversible heatstroke. Intervention by active cooling should therefore be instituted at Tb well below that of the lower inflection point.
The mean Tb inflection points for the respiration rate of both galahs and rock doves in the present study were similar, i.e. increasing just after Tb exceeded 41°C and decreasing just after Tb exceeded 44°C. McWhorter et al. (Citation2018) found that the ambient temperature (Ta) at the onset of panting for galahs was 42.7 ± 2.5°C, similar to the Tb of the galahs in this study when panting started. Similarly, the Ta at the onset of gular fluttering for four Southern hemisphere columbiform birds was found to range from 40.8–55.1°C, with the species closest in size to rock doves in that study, crested pigeons (Ocyphaps lophotes), at 48.0 ± 5.7°C (McKechnie, et al., Citation2016). The birds in these other studies were not under general anaesthesia, as the main purpose of these studies was to measure metabolic parameters to determine the heat tolerance limits and other physiological changes of birds when exposed to heat. The similarities in temperature of onset of panting in both conscious and anaesthetized birds indicate that general anaesthesia may not have significant effects on the ability of galahs and rock doves to utilize respiratory mechanisms of heat loss, even though the high inhalant oxygen concentration provided during inhalational anaesthesia may reduce the sensitivity of oxygen chemoreceptors in avian lungs, which could reduce respiratory drive and interfere with respiratory evaporative cooling. The humidity of the inspired gases was also not measured in our study, which may have been useful for determining the thermoregulatory efficiency of evaporative water loss, but those have already been studied in similar species (McKechnie, et al., Citation2016; McWhorter, et al., Citation2018).
The lack of obvious and consistent EMG indications of cramps suggest that heat cramps are not a common phenomenon in galahs and rock doves, unlike in humans, where heat cramps are common in early stages of heat illnesses (Leon & Kenefick, Citation2012). It is possible that birds may experience heat cramps with more chronic heat exposure, but that was not tested in our study.
Biochemical changes suggestive of skeletal muscle and hepatocellular injury were often observed in heat-exposed galahs and rock doves after heatstroke was induced; however, other biochemical changes were more variable. In both galahs and rock doves, the hyperkalaemia, hyperphosphataemia (galahs only) and elevated AST and CK were suggestive of skeletal muscle degeneration and/or necrosis at the onset of heatstroke; however, transcellular shifts in electrolytes associated with acid–base disturbances and effects of haemoconcentration must also be considered. Galah 7 (control group) had elevated AST, GLDH and CK at the final blood collection before euthanasia. These values from this control galah were left out for generation of reference intervals for the blood results. We suspect that this control galah had underlying disease that predisposed it to developing these hepatic changes just through the stress of handling and anaesthesia.
In humans with heatstroke, hypokalaemia usually occurs first (Bouchama & Knochel, Citation2002), either due to elevated catecholamine levels, respiratory alkalosis secondary to panting or loss in sweat and through the kidneys secondary to hyperaldosteronism (Grogan & Hopkins, Citation2002). Hypokalaemia, as a result of loss in sweat and via kidneys, is more commonly observed with exertional heat illnesses in humans (Grogan & Hopkins, Citation2002). However, prolonged hyperthermia, hypoxia and hypoperfusion of several hours will lead to failure of the Mg2+-dependent Na+/K+-ATPase pump, causing K+ to leak extracellularly, resulting in hyperkalaemia (Grogan & Hopkins, Citation2002). Hyperkalaemia was the most common electrolyte abnormality in both bird species studied, which was possibly a sequela to hypoxia and hypoperfusion following the onset of heatstroke, but these were not specifically measured in our study.
Hypocalcaemia was observed in the majority of heat-exposed galahs, but not in the rock doves. Hypercalcaemia and hyperproteinaemia commonly result from dehydration and haemoconcentration associated with heatstroke (Bouchama & Knochel, Citation2002). However, hypocalcaemia associated with rhabdomyolysis and acute renal failure is more commonly observed in exertional heatstroke (Shieh et al., Citation1992). Profound hypercalcaemia (8.96 and 4.40 mmol/l compared with the control reference interval of 0.626–2.152 mmol/l) occurred in two heat-exposed rock doves which could not be explained by physiological or pathological reasons. Therefore, analytical error was considered the most likely cause and they were excluded from analyses.
Hypophosphataemia has also been commonly observed in humans suffering from heatstroke (Bouchama & Knochel, Citation2002) and has been suggested to result from the increased glucose phosphorylation seen in acute alkalotic conditions (Grogan & Hopkins, Citation2002). Hyperuricaemia was observed in one heat-exposed rock dove. Hyperuricaemia develops due to the release of purines from injured muscle (Grogan & Hopkins, Citation2002). The elevations in GLDH, AST and CK in rock doves indicate they may be likely to develop hepatocellular injury and rhabdomyolysis, respectively, from heatstroke. Exertional heatstroke tends to result in higher levels of CK elevation associated with rhabdomyolysis (Hsu et al., Citation1997), but even heatstroke patients with no history of exercise exhibit CK elevation to a certain extent (Dematte et al., Citation1998). However, severe liver damage is more common in exertional heatstroke due to direct cell damage and hypoxia (Giercksky et al., Citation1999; Saissy, Citation1996).
Dogs with heatstroke are considered less useful as a model for human heatstroke studies as they display different changes (Damanhouri & Tayeb, Citation1992), but heatstroke in dogs is relatively better understood compared to other species in veterinary medicine due to their popularity as pets in all parts of the world, including those that experience high environmental temperatures. The most common biochemical changes observed in dogs with heatstroke are elevated CK, ALT, AST, ALP and creatinine (Bruchim et al., Citation2006). Some of these changes were found in the birds in our study. Hypoglycaemia is also frequently observed, and the possible association with heatstroke includes increased glucose utilization due to high body temperature, sepsis and liver failure (Bruchim, et al., Citation2006). Disseminated intravascular coagulation (DIC) is also a common sequelae of heatstroke (Bruchim, et al., Citation2006). The birds in our study were not kept alive long enough post-heatstroke to determine if DIC is a feature of avian heatstroke, and future studies could be performed to examine this.
Microscopic examination did not reveal any significant cardiac changes, but acute congestion in the lungs was consistent with those in a case report of a sun conure that was suspected to have suffered heatstroke under general anaesthesia (Hofmeister, Citation2005). In this sun conure, there was also mild multifocal acute degeneration and contraction band necrosis of the biceps femoris muscle, as well as diffuse moderate acute congestion of the lungs (Hofmeister, Citation2005). Some of our heat-exposed rock doves also had indications of necrosis in the pectoral muscle (), similar to that of the sun conure, which were consistent with the elevated blood CK levels observed. Significant hepatic changes were also observed in some heat-exposed galahs, but not in any of the rock doves. For example, in galah 5, the histopathological findings of congestion, mild endothelial hypertrophy, and scattered single cell necrosis and random foci of hepatocellular degeneration in the liver corresponded with its biochemical abnormalities. There may therefore be species differences in the organs most affected by heatstroke. The nonsuppurative encephalitis observed in rock dove 8 (control group) were chronic and likely due underlying conditions that were unrelated to the study.
Histopathological reports of birds dying from heatstroke are uncommon because the quick onset of autolysis under high ambient and body temperatures precludes any useful post mortem examination unless the carcasses were collected and examined immediately or stored appropriately until examination could be carried out. Overstreet and Rehak (Citation1982) reported that chicks of the least tern (S. albifrons) suffering from heatstroke after being exposed to high environmental temperatures at a narrow beach nesting area in Mississippi, USA had microscopic evidence of early ischaemic focal necrosis in the brain, characterized by neuronal degeneration, leukocytic infiltration and degeneration of the neuropil surrounding some cortical peripheral blood vessels (Overstreet & Rehak, Citation1982). Also the splenic sinuses were packed with red blood cells, an indicator of circulatory collapse (Overstreet & Rehak, Citation1982). There were small areas of degeneration in the liver, kidneys, and intestine (Overstreet & Rehak, Citation1982); however, the lungs, heart, and other tissues were not examined. None of these changes were identified in our study, which is likely to be because the birds were euthanized promptly after the onset of heatstroke. It is possible that the birds in our study may have developed similar histopathological changes if they were kept alive after the onset of heatstroke; however, due to ethical and welfare considerations, they were not kept alive.
In a baboon heatstroke model, severely affected animals had multifocal injury to the liver with intrasinusoidal and central vein accumulation of erythrocytes and neutrophils (Bouchama, et al., Citation2005). There was also injury to the jejunal villi with tissue loss, desquamation and exposure of the lamina propria (Bouchama, et al., Citation2005). Central nervous system changes included cytoplasmic eosinophilia and nuclear pyknosis in the scattered neurons of the hippocampus, pallidum and cerebellar Purkinje cells (Bouchama, et al., Citation2005). All these changes were much more pronounced in baboons with severe heatstroke (heat-exposed until hypotension occurred) and minimal in those with moderate heatstroke (heat-exposed until Tb was 42.5°C). If similar changes were to be expected in birds, then the lack of histopathological changes in our study would indicate that these birds only suffered moderate heatstroke, and a longer heat exposure or longer delay between the onset of heatstroke and euthanasia may be required to illicit similar histopathological changes.
In dogs that presented to university teaching veterinary hospitals with heatstroke and subsequently died, post mortem examinations revealed that the most consistent histopathological changes were hyperaemia and diffuse oedema in the skin, lungs, brain and bone marrow (Bruchim, et al., Citation2009). There was also congestion of the splenic pulp and hepatic sinusoids, as well as necrosis in the small intestinal mucosa, large intestinal mucosa, renal tubular epithelium, hepatic parenchyma and brain neural tissue (Bruchim, et al., Citation2009). However, in dogs where heatstroke was induced as an experimental model, congestion of the liver, kidney and lung, as well as karyorrhexis of lymphocytes in the spleen and mesenteric lymph nodes, were the only consistent findings (Bynum, et al., Citation1977). The difference in histopathological findings between these two studies may be due to differences in Tb reached, extent and duration at the exposed temperature and how soon after heating they were examined. Further studies in birds will need to be performed by exposing them to different durations and degrees of heat exposure to determine if this is the case. The birds in our study may have developed additional histopathological changes if maintained alive, under general anaesthesia, for a period of time post heat exposure.
The physiological, biochemical and histopathological changes associated with heatstroke have not been previously documented in galahs and rock doves. Our study has provided a baseline for further studies, as well as indications for diagnosing heatstroke in clinical cases presenting with a history of heat exposure. The study also provides Tb data to indicate when intervention is necessary under general anaesthesia to prevent overheating. Rock doves especially require intervention early on as they are overcome by irreversible heatstroke very quickly once Tb exceeds the critical point. There were also significant species differences in the physiological, biochemistry and histopathological changes, which indicate that bird species should be studied separately for clinical syndromes such as heatstroke. Further studies should concentrate on bird species more vulnerable to heatstroke, e.g. Psittaciform birds with lower tolerance for heat (McWhorter, et al., Citation2018), as well as investigate if chronic exposure to heat may result in different clinical and histopathological changes.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Shangzhe Xie http://orcid.org/0000-0001-7880-5261
Todd J. McWhorter http://orcid.org/0000-0002-4746-4975
References
- Abdelatif, A. & Modawi, S.M. (1994). Effect of hyperthermia on blood constituents in the domestic rabbit (lepus cuniculus). Journal of Thermal Biology, 19, 357–363. doi: 10.1016/0306-4565(94)90034-5
- Boedeker, N.C., Carpenter, J.W. & Mason, D.E. (2005). Comparison of body temperatures of pigeons (Columba livia) anesthetized by three different anesthetic delivery systems. Journal of Avian Medicine and Surgery, 19, 1–6. doi: 10.1647/2002-026
- Bouchama, A. & Knochel, J.P. (2002). Heat stroke. New England Journal of Medicine, 346, 1978–1988. doi: 10.1056/NEJMra011089
- Bouchama, A., Roberts, G., Al Mohanna, F., El-Sayed, R., Lach, B., Chollet-Martin, S., Ollivier, V., Al Baradei, R., Loualich, A., Nakeeb, S. & Eldali, A. (2005). Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. Journal of Applied Physiology, 98, 697–705. doi: 10.1152/japplphysiol.00461.2004
- Bricknell, M. (1995). Heat illness-a review of military experience (part 1). Journal of the Royal Army Medical Corps, 141, 157–166. doi: 10.1136/jramc-141-03-06
- Bruchim, Y., Klement, E., Saragusty, J., Finkeilstein, E., Kass, P. & Aroch, I. (2006). Heat stroke in dogs: a retrospective study of 54 cases (1999–2004) and analysis of risk factors for death. Journal of Veterinary Internal Medicine, 20, 38–46.
- Bruchim, Y., Loeb, E., Saragusty, J. & Aroch, I. (2009). Pathological findings in dogs with fatal heatstroke. Journal of Comparative Pathology, 140, 97–104. doi: 10.1016/j.jcpa.2008.07.011
- Bynum, G., Patton, J., Bowers, W., Leav, I., Wolfe, D., Hamlet, M. & Marsili, M. (1977). An anesthetized dog heatstroke model. Journal of Applied Physiology, 43, 292–296. doi: 10.1152/jappl.1977.43.2.292
- Chen, C.-M., Hou, C.-C., Cheng, K.-C., Tian, R.-L., Chang, C.-P. & Lin, M.-T. (2006). Activated protein C therapy in a rat heat stroke model. Critical Care Medicine, 34, 1960–1966. doi: 10.1097/01.CCM.0000224231.01533.B1
- Damanhouri, Z.A. & Tayeb, O.S. (1992). Animal models for heat stroke studies. Journal of Pharmacological and Toxicological Methods, 28, 119–127. doi: 10.1016/1056-8719(92)90073-A
- Dechesne, C.J., Kim, H.N., Nowak, T.S. & Wenthold, R.J. (1992). Expression of heat shock protein, HSP72, in the Guinea pig and rat cochlea after hyperthermia: immunochemical and in situ hybridization analysis. Hearing Research, 59, 195–204. doi: 10.1016/0378-5955(92)90116-5
- Dematte, J.E., O'Mara, K., Buescher, J., Whitney, C.G., Forsythe, S., McNamee, T., Adiga, R.B. & Ndukwu, I.M. (1998). Near-fatal heat stroke during the 1995 heat wave in Chicago. Annals of Internal Medicine, 129, 173–181. doi: 10.7326/0003-4819-129-3-199808010-00001
- Friedrichs, K.R., Harr, K.E., Freeman, K.P., Szladovits, B., Walton, R.M., Barnhart, K.F. & Blanco-Chavez, J. (2012). ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Veterinary Clinical Pathology, 41, 441–453. doi: 10.1111/vcp.12006
- Geffré, A., Concordet, D., Braun, J.P. & Trumel, C. (2011). Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with microsoft excel. Veterinary Clinical Pathology, 40, 107–112. doi: 10.1111/j.1939-165X.2011.00287.x
- Giercksky, T., Boberg, K.M., Farstad, I., Halvorsen, S. & Schrumpf, E. (1999). Severe liver failure in exertional heat stroke. Scandinavian Journal of Gastroenterology, 34, 824–827. doi: 10.1080/003655299750025778
- Grogan, H. & Hopkins, P.M. (2002). Heat stroke: implications for critical care and anaesthesia. British Journal of Anaesthesia, 88, 700–707. doi: 10.1093/bja/88.5.700
- Hofmeister, E.H. (2005). Anesthesia case of the month. Journal of the American Veterinary Medical Association, 227, 718–720. doi: 10.2460/javma.2005.227.718
- Hsu, Y.-D., Lee, W.-H., Chang, M.-K., Shieh, S.-D. & Tsao, W.-L. (1997). Blood lactate threshold and type II fibre predominance in patients with exertional heatstroke. Journal of Neurology, Neurosurgery & Psychiatry, 62, 182–187. doi: 10.1136/jnnp.62.2.182
- Johnson, S.I., McMichael, M. & White, G. (2006). Heatstroke in small animal medicine: a clinical practice review. Journal of Veterinary Emergency and Critical Care, 16, 112–119. doi: 10.1111/j.1476-4431.2006.00191.x
- Leon, L.R. & Kenefick, R. (2012). Pathophysiology of heat-related illnesses. In P.S. Auerbach (Ed.), Wilderness medicine (6th ed). Missouri: Mosby.
- Lin, M. & Lin, S. (1992). Cerebral ischemia is the main cause for the onset of heat stroke syndrome in rabbits. Experientia, 48, 225–227. doi: 10.1007/BF01930459
- McKechnie, A.E., Hockey, P.A. & Wolf, B.O. (2012). Feeling the heat: Australian landbirds and climate change. Emu - Austral Ornithology, 112, i–vii. doi: 10.1071/MUv112n2_ED
- McKechnie, A.E., Whitfield, M.C., Smit, B., Gerson, A.R., Smith, E.K., Talbot, W.A., McWhorter, T.J. & Wolf, B.O. (2016). Avian thermoregulation in the heat: efficient evaporative cooling allows for extreme heat tolerance in four southern Hemisphere columbids. Journal of Experimental Biology, 219, 2145–2155. doi: 10.1242/jeb.138776
- McKechnie, A.E. & Wolf, B.O. (2010). Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biology Letters, 6, 253–256. doi: 10.1098/rsbl.2009.0702
- McWhorter, T.J., Gerson, A.R., Talbot, W.A., Smith, E.K., McKechnie, A.E. & Wolf, B.O. (2018). Avian thermoregulation in the heat: evaporative cooling capacity and thermal tolerance in two Australian parrots. Journal of Experimental Biology.
- Muggeo, V.M. (2008). Segmented: an R package to fit regression models with broken-line relationships. R News, 8, 20–25.
- Oglesbee, M., Diehl, K., Crawford, E., Kearns, R. & Krakowka, S. (1999). Whole body hyperthermia: effects upon canine immune and hemostatic functions. Veterinary Immunology and Immunopathology, 69, 185–199. doi: 10.1016/S0165-2427(99)00053-7
- Overstreet, R.M. & Rehak, E. (1982). Heat-stroke in nesting least tern chicks from gulfport, Mississippi, during June 1980. Avian Diseases, 26, 918–923. doi: 10.2307/1589880
- Phalen, D.N., Mitchell, M.E. & Cavazos-Martinez, M.L. (1996). Evaluation of three heat sources for their ability to maintain core body temperature in the anesthetized avian patient. Journal of Avian Medicine and Surgery, 10, 174–178.
- Rembert, M.S., Smith, J.A., Hosgood, G., Marks, S.L. & Tully Jr, T.N. (2001). Comparison of traditional thermal support devices with the forced-air warmer system in anesthetized hispaniolan Amazon parrots (amazona ventralis). Journal of Avian Medicine and Surgery, 15, 187–193. doi: 10.1647/1082-6742(2001)015[0187:COTTSD]2.0.CO;2
- Roberts, G.T., Ghebeh, H., Chishti, M.A., Al-Mohanna, F., El-Sayed, R., Al-Mohanna, F. & Bouchama, A. (2008). Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1130–1136. doi: 10.1161/ATVBAHA.107.158709
- Saissy, J. (1996). Liver transplantation in a case of fulminant liver failure after exertion. Intensive Care Medicine, 22, 831–831. doi: 10.1007/BF01709530
- Saunders, D.A., Mawson, P. & Dawson, R. (2011). The impact of two extreme weather events and other causes of death on carnaby’s black cockatoo: a promise of things to come for a threatened species? Pacific Conservation Biology, 17, 141–148. doi: 10.1071/PC110141
- Shieh, S.D., Lin, Y.F., Lu, K.C., Li, B.L., Chu, P., Shyh, T.P. & Diang, L.K. (1992). Role of creatine phosphokinase in predicting acute renal failure in hypocalcemic exertional heat stroke. American Journal of Nephrology, 12, 252–258. doi: 10.1159/000168454
- Smith, E.K., O'Neill, J., Gerson, A.R. & Wolf, B.O. (2015). Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert doves and quail. Journal of Experimental Biology, 218, 3636–3646. doi: 10.1242/jeb.128645
- Whitfield, M.C., Smit, B., McKechnie, A.E. & Wolf, B.O. (2015). Avian thermoregulation in the heat: scaling of heat tolerance and evaporative cooling capacity in three southern African arid-zone passerines. The Journal of Experimental Biology, 218, 1705–1714. doi: 10.1242/jeb.121749
- Wolf, B., McKechnie, A., Gerson, A., Whitfield, M., Smit, B., Talbot, W., O’Neill, J. & McWhorter, T. (2015). Avian thermoregulation in the heat: Tolerance to heat stress varies greatly among species. The Physiologist, 58, 56.
