ABSTRACT
Since the emergence of low pathogenic avian influenza (LPAI) H9N2 viruses in Morocco in 2016, severe respiratory problems have been encountered in the field. Infectious bronchitis virus (IBV) is often detected together with H9N2, suggesting disease exacerbation in cases of co-infections. This hypothesis was therefore tested and confirmed in laboratory conditions using specific-pathogen-free chickens. Most common field vaccine programmes were then tested to compare their efficacies against these two co-infecting agents. IBV γCoV/chicken/Morocco/I38/2014 (Mor-IT02) and LPAI virus A/chicken/Morocco/SF1/2016 (Mor-H9N2) were thus inoculated to commercial chickens. We showed that vaccination with two heterologous IBV vaccines (H120 at day one and 4/91 at day 14 of age) reduced the severity of clinical signs as well as macroscopic lesions after simultaneous experimental challenge. In addition, LPAI H9N2 vaccination was more efficient at day 7 than at day 1 in limiting disease post simultaneous challenge.
RESEARCH HIGHLIGHTS
Simultaneous challenge with IBV and AIV H9N2 induced higher pathogenicity in SPF birds than inoculation with IBV or AIV H9N2 alone.
Recommended vaccination programme in commercial broilers to counter Mor-IT02 IBV and LPAIV H9N2 simultaneous infections: IB live vaccine H120 (d1), AIV H9N2 inactivated vaccine (d7), IB live vaccine 4-91 (d14).
Introduction
Avian respiratory viruses, including infectious bronchitis virus (IBV) and avian influenza virus (AIV), are among the primary causes of morbidity and mortality in the poultry industry worldwide and have been identified as the most economically important viral agents of acute and highly contagious diseases (Haghighat-Jahromi et al., Citation2008). IBV is a coronavirus, an enveloped positive strand RNA virus with a genome of 27 kb. It belongs to the family Coronaviridae, subfamily Coronavirinae, and to the genus Gammacoronavirus (Jackwood, Citation2012). AIVs are type A orthomyxoviruses and are classified as low pathogenic or highly pathogenic viruses based on their virulence in chickens or according to the presence of multiple basic amino acids at the cleavage site of the haemagglutinin (HA) precursor protein (Swayne et al., Citation2013). These pathogens are of major significance and have a large economic impact because they are able to induce disease independently or in association with other avian pathogens (Roussan et al., Citation2008).
AIV H9N2 is classified as a low pathogenic virus. However, high mortality has been reported in broiler farms in Morocco and in many other countries in Africa and Asia (Nili & Asasi, Citation2002, Citation2003; Al-Garib et al., Citation2016; El Houadfi et al., Citation2016; Ismail et al., Citation2018). In Morocco, the low pathogenic avian influenza virus (LPAIV) subtype H9N2 was first identified in poultry in January 2016 and spread very quickly throughout the country (El Houadfi et al., Citation2016). Phylogenetic analyses showed that Moroccan viruses belonged to the G1 H9N2 lineage and likely originated from the Middle East (El Houadfi et al., Citation2016).
Co-infections of poultry with multiple agents are common and have resulted in more severe clinical signs when compared to single agent infections (Bano et al., Citation2003; Stipkovits et al., Citation2012). Likewise, co-infections of LPAIV H9N2 with other respiratory pathogens, particularly with IBV, Mycoplasma gallisepticum, Staphylococcus aureus, Avibacterium paragallinarum, Escherichia coli, Ornithobacterium rhinotracheale and/or immunosuppressive agents can exacerbate LPAIV H9N2 infections and result in severe clinical disease with different rates of mortality (Nili & Asasi, Citation2002; Kishida et al., Citation2004; Perk et al., Citation2006). Previous studies have demonstrated longer H9N2 virus shedding, more severe clinical signs and higher mortality rates caused by a co-infection with LPAIV H9N2 and IBV, either as a vaccine or as a wild-type virus (Seifi et al., Citation2012; Hassan et al., Citation2017; Ismail et al., Citation2018).
The aim of this study was to test the hypothesis that IBV exacerbates the pathogenic effect of LPAIV H9N2 in vivo and to determine the appropriate vaccination protocol allowing for good protection in the case of simultaneous challenge with IBV + H9N2. Firstly, we compared the pathogenicity of γCoV/chicken/Morocco/I38/2014 (Mor-IT02) and A/chicken/Morocco/SF1/2016(H9N2) (Mor-H9N2) alone and in combination in specific-pathogen-free (SPF) chickens. We then evaluated the protection conferred by different vaccination protocols against simultaneous co-infection with Mor-IT02 and Mor-H9N2.
Materials and methods
Viruses and vaccines
In order to stay as close as possible to field conditions, we selected two recently isolated Moroccan viruses for the co-infection experiments, representative of the viruses currently circulating in the country: γCoV/chicken/Morocco/I38/2014, referred to as Mor-It02 (accession number for its S1 gene sequence: KJ701020) (Fellahi et al., Citation2015) and A/chicken/Morocco/SF1/2016(H9N2), a LPAIV H9N2 referred to as Mor-H9N2 (accession number for its HA gene sequence: LT598497) (El Houadfi et al., Citation2016).
Challenge virus titrations were performed using 10-day-old SPF chicken embryos (CE) inoculated via the allantoic cavity. For IBV, the CEs were examined daily to check the viability of the embryos up to 6 days post inoculation. After placing the eggs at 4°C for 24 h, the embryos were examined for characteristic IBV lesions (curling and dwarfing). For AIV, after 72 h of incubation, surviving CEs were chilled and allantoic fluid collected and tested for positive haemagglutination with chicken erythrocytes.
The viral titres of the two viruses (IBV and AIV) were calculated following the method described by Reed and Muench (Citation1938) and expressed as the 50% egg infective dose 50 per ml (EID50/ml).
IB commercial live attenuated vaccines used in this study were Mass H120 (Bioral H120, batch number: L418386, 3.7–5.0 log10 EID50) and 4/91 (Nobilis IB 4-91, batch number: A188CJ01, 3.6 log10 EID50). Vaccines were delivered in 0.2 ml/dose via the ocular and nasal routes as recommended by the vaccines' manufacturers. Commercial inactivated vaccine against avian influenza H9N2 (G1 Middle Eastern strain) was used with 0.3 ml/dose of vaccine administered by subcutaneous injection as recommended by the vaccine manufacturer.
Experimental design
In all experiments, management procedures for all groups were identical. Experiments were conducted in accordance with European and French legislations on Laboratory Animal Care and Use (French Decree 2001-464 and European Directive CEE86/609) and animal protocols were approved by the Ethics Committee “Sciences et santé animale”, committee number 115.
Experiment 1: pathogenicity study in SPF chicks
Twenty-eight SPF chicks were divided into four groups, individually identified and housed in negative pressure High Efficiency Particulate Air isolators (Allentown, NJ, USA and Noroit, Bouaye, France) at the Joined Research Unit Host–Pathogen Interactions (1225) at the National Veterinary School of Toulouse (France). The birds were housed in animal biosafety level 2 facilities and had free access to food and water. They were infected at 21 days of age with a total volume of 0.2 ml by the oculo-nasal route. Doses of 103 EID50 of IBV and 106 EID50 of AIV were administered. In this pilot experiment, the birds were not vaccinated. The four groups were as follows: GIB, infected with Mor-IT 02 virus (n = 5 birds); GAI, infected with Mor-H9N2 virus (n = 9); GIB/AI, infected simultaneously with Mor-IT02 and Mor-H9N2 viruses (n = 9); GNI, not infected (n = 5).
Experiment 2: study comparing vaccination regimens in commercial broilers
Eighty commercial day-old broiler chicks from a Moroccan commercial hatchery were used to perform two experiments, each with 40 chicks, and each divided into four groups (n = 10 birds per group) with two groups (GH120/4-91/H9 and GNEG-CON groups) repeated in both experiments due to laboratory space constraints (six groups could not have been separated in a single experiment). The chicks were housed at the Avian Pathology Unit of the Agricultural and Veterinary Institute (Rabat, Morocco). The experimental protocol of the two combined experiments (experiments 2a and 2b) is presented in .
Table 1. Experimental design.
In both experiments 2a and 2b, the chickens were individually identified and then co-infected simultaneously at 28 days of age by the oculonasal route with 103 EID50/0.2 ml of IBV Mor-IT02 and 106EID50/0.2 ml of AIV Mor-H9N2.
Clinical signs
In all experiments, daily clinical observations were performed by the same investigator for consistency reasons. The clinical signs included respiratory signs such as dyspnoea, rales, nasal discharge and respiratory distress. Strict measures were applied to avoid cross contamination between different groups. The clinical signs were recorded until 10 days post challenge (dpc).
Macroscopic lesions
In SPF chicks (experiment 1), at 5 dpc, three birds from each group were euthanized and macroscopic lesions observed in all organs were recorded. The remaining birds were euthanized at day 10pc. All gross lesions were recorded. In commercial broilers (experiment 2), post mortem examination at 5 dpc was performed on five birds from each group. The remaining birds were humanely sacrificed at 9 and 10 dpc in experiments 2a and 2b, respectively.
Microscopic lesions
Trachea and lung samples from SPF birds (experiment 1) were taken from each sacrificed bird at 5 dpc. Samples were fixed in 10% formalin for the determination of microscopic lesions. After fixation, tissues were routinely processed in paraffin blocks, sectioned at 4 µm and stained with haematoxylin and eosin for microscopic examination.
Serology
Blood was collected when the chickens were 1, 14, 28, and 37–38 days of age: at arrival, before vaccine boost, pre-challenge, and 10 dpc. IBV and AIV antibody titres of commercial broilers were measured using Synbiotics ProFlock ELISA kits (Zoetis, Kansas City, MO) following the manufacturer instructions.
Virus shedding measured by real-time RT–PCR
Oropharyngeal swabs were collected at 3, 5 and 7 dpc in experiment 1 (SPF birds) and at 3, 5, 7 and 9 dpc in experiment 2 (commercial broilers) to assess virus shedding. The swabs were stored in 1 ml of PBS containing 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen) and kept at −80°C until further use.
IBV and AIV viral RNA were extracted using the Macherey-Nagel viral RNA extraction kit (Düren, Germany) following the instructions of the manufacturer. The extracted RNA was subjected to real-time RT–PCR in two steps. First, a reverse transcription was carried out using the Revertaid kit (Thermo Fisher Scientific) on an Applied Biosystems thermocycler (Foster City, USA). Primers used for IBV detection targeted the 5′ untranslated region of IBV and were designed by Callison et al. (Citation2006): IBV5′GU391, 5′ GCTTTTGAGCCTAGCGTT 3′; IBV5′GL533, 5′ GCCATGTTGTCACTGTCTATTG 3′; and IBV5′G Taqman®, 5′ FAM-CACCACCAGAACCTGTCACCTC-BHQ1 3′. For AIV detection, the primers and probe targeted the matrix gene and were described by Spackman et al. (Citation2002): AIV-M-P1, 5′ AGATGAGTCTTCTAACCGAGGTCG 3′; AIV-M-P2, 5′ TGCAAAAACATCTTCAAGTCTCTG 3′; and AIV-M-Probe, 5′ FAM-TCAGGCCCCCTCAAAGCCGA-TAMRA 3′. PCRs were carried out using the iTaq Universal Probes Supermix kit (BioRad, Foster City, USA). The reaction mix consisted of 5 µl of 2x itaq mix, 1.75 µl nuclease free water, 0.5 µl of each primer (10 µM), 0.25 µl of probe (10 µM), and 2 µl cDNA with the following programme: initial denaturation: 94°C for 2 min, followed by 40 cycles of denaturation at 94°C for 10s and hybridization/elongation for 30sec at 60°C. A LightCycler 480 (Roche, Penzberg, Germany) was used.
Results
Experiment 1: Increased pathogenicity with IBV-H9N2 virus co-infection as compared to single infections in SPF chickens
No mortality was reported in any group. No clinical signs, lesions or virus were detected in the negative control group. As early as 24 h post challenge (hpc), birds infected with IBV and AIV separately (GIB and GAI) or co-infected simultaneously with both viruses (GIB/AI) started to show clinical signs. The peak was observed at 5 dpc. The co-infected group (GIB/AI) showed significantly more respiratory signs than its counterparts. The birds showed intense depression and nasal discharge with some birds having very severe respiratory signs (). Similarly, birds in the GIB/AI group showed the most severe macroscopic lesions at necropsy 5 dpc, ranging from tracheal congestion and air sac thickening to fibrinous sinusitis in one bird out of three. Histopathology investigation revealed a constant subacute tracheitis in all infected groups. However, lesions were again more intense in the GIB/AI group (). Interestingly, virus shedding was similar in the mono- and co-infected groups, with 7 days of shedding for both viruses, and comparable Ct values by real-time PCR ().
Table 2. Pathogenicity comparison after mono- and co-infections with Mor-IT02 and Mor-H9N2 in SPF chickens.
In summary, the pilot experiment confirmed that IBV and AIV co-infection exacerbates the clinical disease. Indeed, co-infection with Mor-IT02 and Mor-H9N2 viruses led to more severe disease and lesions than mono-infections without higher or longer replication of either of the two viruses in SPF birds.
Experiment 2: Vaccination programmes and IBV-AIV simultaneous challenge: moderate to severe clinical signs except for the GH120/4-91/H9D7 birds
Respiratory signs were detected in infected commercial broilers as early as 48 hpc with varying intensity between groups. The clinical signs peaked at 5 dpc. For all groups, respiratory signs ranged from mild respiratory distress to severe rales, most commonly reported in the GPOS-CON group (the unvaccinated co-infected positive control group) and GH120/4-91/H9D1 group (vaccinated with two heterologous IB vaccines and AI vaccination at one day of age). In the GH120/4-91/H9D1 group, at 5 dpc intense clinical signs were recorded and one chick out of 10 died at 5 dpc (the only mortality in this study). At 9 dpc, 100% of the chicks still showed clinical signs and one chick out of five developed respiratory gasps ().
Table 3. Comparison of protection conferred by different vaccination programmes against Mor-IT02 and Mor-H9N2 co-infection in commercial broilers.
In the GH120/4-91/H9D7 group, moderate clinical signs were noted with a maximum severity at 5 dpc with four chicks out of 20 with respiratory rales. In addition, at 6 dpc, the chicks started to recover. At 9 dpc, only three out of 10 chickens showed slight respiratory signs ().
As for the GH120/H120/H9D7 group, 100% of the chicks showed respiratory signs as early as 48 hpc and the maximum intensity of clinical signs was noted at 4 and 5 dpc. In this group, chickens showed more severe respiratory signs than in the GH120/4-91/H9D7 group, such as the presence of severe rales, respiratory dyspnoea and intense depression. Chickens started to recover at 6 dpc and no mortality was observed. Similarly, moderate respiratory signs were noted as early as 48 hpc in the GH120/H9 group. At 9 dpc, 100% of the chicks still showed clinical signs ().
In the GPOS-CON group, the clinical signs observed were severe rales, intense respiratory distress, lacrimation and presence of whitish diarrhoea in five chicks at 3 dpc. In this unvaccinated and co-infected group, the chicks started to recover at 6 dpc as in the other groups, but three out of five chickens still showed marked respiratory distress at 9 dpc. In contrast and as expected, no clinical signs were recorded in the negative control group GNEG-CON ().
Vaccination programmes and IBV-AIV simultaneous challenge: macroscopic lesions 5 days post challenge except for the GH120/4-91/H9D7 birds
The autopsy performed on day 5 after experimental simultaneous challenge revealed that the lesions were limited to the respiratory tract with variable intensities in the different infected groups (). The lesions observed were tracheal congestion, airsacculitis, thickening of air sacs, and lung congestion. Fibrin deposit was formed in the trachea and bronchial bifurcation, in abdominal airsacs and sinuses. Different organs such as the liver, kidney, spleen and thymus were congested.
The most severe lesions were observed in the GPOS-CON group with 80% of the chicks showing tracheal congestion and thickening of the air sacs. In addition, a fibrin deposit was observed at the bronchial bifurcation in 40% of examined GPOS-CON chicks. Similar lesions were observed in the GH120/4-91/H9D1 group with a case of mortality reported at 5 dpc. The necropsy carried out on the dead chicken revealed the presence of fibrin deposits at the bronchial bifurcation, throughout the trachea and on the air sacs.
In the GH120/H120/H9D7 group, 80% of the birds had tracheitis, 80% airsacculitis, and 40% fibrinous sinusitis at 5 dpc. Similar lesions with similar percentages of birds affected were found in the GH120/H9D7 group. As far as the GH120/4-91/H9D7 group was concerned, moderate lesions were observed as compared to the four other infected groups.
As expected, no lesions were observed in the negative control group ().
Vaccination programmes and IBV-AIV shedding: faster virus clearance for the GH120/4-91/H9D7 birds
IBV (Mor-IT02) and AIV (Mor-H9N2) shedding was assessed by real-time PCR on oropharyngeal swabs collected from chickens after infection. No significant difference was observed in IBV titres between the infected groups, but viral shedding was of shorter duration in the GH120/4-91/H9D7 group compared to the other groups (clearance by day 7 for GH120/4-91/H9D7 versus after day 9 for all other groups). The percentage of chickens shedding IBV differed among groups with 75% of the GH120/4-91/H9D7 birds shedding virus at 3 dpc (before a decrease in the number of positive chicks on the later days), as compared to 100% of the chicks in the GH120//H120/H9D7, GH120/HD79, GH120/4-91/H9D1 and GPOS-CON groups (A).
Figure 1. Virus shedding in commercial broilers after different vaccine regimens. A. IBV shedding. B. AIV shedding. Groups of birds are indicated on the x axis. The four panels for each virus correspond to the four days for which swabs were tested: 3 dpc (closed black circle shaped symbols), 5 dpc (closed black square shaped symbols), 7 dpc (closed black triangle shaped symbols), and 9 dpc (closed black diamond shaped symbols). Ct: cycle threshold.
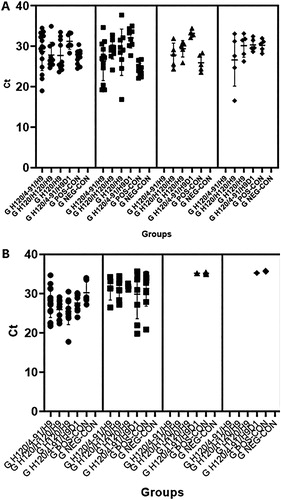
As for the Mor-H9N2 virus (AIV), the maximum percentage of positive birds was noted at 3 dpc for all infected groups. Viral clearance was recorded at 7 dpc in all vaccinated groups irrespective of the IBV vaccine programme (GH120/H120/H9, GH120/H120/H9 and GH120/H9), except for 20% of the GH120/4-91/H9D1 birds that still shed virus 9 dpc. Similarly, 40% of the GPOS-CON chicks tested positive for AIV at 9 dpc (B).
No chicken tested positive in the negative control group (GNEG-CON) for either virus tested.
Seroconversion after vaccination and after challenge in commercial chickens
All birds had high levels of maternally-derived antibodies against both IBV and AIV at 1 day of age, at 14 days of age antibodies were below the threshold of detection. IBV antibodies were just above the detection threshold at 28 days of age (just before challenge) but most birds were seronegative for AIV at 28 days. All infected birds had seroconverted against both IBV and AIV at 37–38 days (10 dpc, data not shown).
Discussion
Despite its “low pathogenic” qualification, AIV H9N2 causes frequent outbreaks of disease with high mortality and significant economic losses in different poultry production types around the world (Nili & Asasi, Citation2002, Citation2003; Swayne et al., Citation2013; Parvin et al., Citation2014, Citation2015; Al-Garib et al., Citation2016; El Houadfi et al., Citation2016). In the field, co-infections are frequently reported and might be responsible for the severe clinical signs observed (Nili & Asasi, Citation2003; Kishida et al., Citation2004; Perk et al., Citation2006).
Aouini et al. (Citation2018) evaluated viral interactions in vitro between an IBV live vaccine strain (H120) and LPAIV H9N2 that were simultaneously or sequentially inoculated, and showed an antagonism and interference between them which led to decreased viral growth. Likewise, in the case of super-infection (when AIV and IBV were inoculated with a 1 h interval), the second virus, either AIV or IBV, induced a decrease in the growth of the first inoculated virus (Aouini et al., Citation2018).
In contrast to these findings, co-infection with respiratory pathogens, such as IBV circulating in the field or live IBV vaccine, was shown to be one of the causes of increased virus pathogenicity and prolonged H9N2 viral shedding (Hassan et al., Citation2017; Ismail et al., Citation2018). Live IBV vaccine was indeed shown to provide proteases that increased the pathogenicity of AIV H9N2, due to a trypsin-like serine domain encoded by IBV (Liu et al., Citation1995; Ng & Liu, Citation2000). The present study confirmed the hypothesis that IBV exacerbates the pathogenic effects of AIV H9N2 on SPF chickens. Our first experiment indeed suggested a synergistic effect of the two pathogens as was reported in other studies (Karimi-Madab et al., Citation2010; Seifi et al., Citation2012; Hassan et al., Citation2017). Haghighat-Jahromi et al. (Citation2008) and Tavakkoli et al. (Citation2011) reported that H9N2 viral shedding reached higher titres and lasted longer, when birds were vaccinated with a live IBV vaccine (Haghighat-Jahromi et al., Citation2008; Tavakkoli et al., Citation2011). The differences observed when using live IBV vaccine versus wild-type virus, and when looking in vitro versus in vivo, are still poorly understood and require further studies. However, most studies (including ours), and more importantly in vivo experiments and field observations, tend to suggest a synergetic effect of the two pathogens. The putative role of vaccination with live vaccines in pathogenesis in cases of co-infections is of great animal health importance and should be investigated.
We also performed this study to understand the role of maternally derived antibodies in the synergy of IBV and AIV. The commercial birds used in the present study (experiment 2) had high levels of maternally-derived antibodies that waned within the first 2 weeks of the bird's life. The combination of H120 and 4/91 IBV vaccines showed not only the best protection against Mor-IT02 infection, as already reported (Belkasmi et al., Citation2017), but also against Mor-IT02 and Mor-H9N2 simultaneous challenge. The AIV H9N2 vaccination at day 7 rather than day 1 provided much better protection, likely due to lower levels of maternally-derived antibodies at day 7 than at day 1 and/or to a more mature immune system at day 7 than at day 1.
Whether the two viruses, AIV H9N2 and IBV, are synergistic or interfering, should be assessed with different times of infection, with not only simultaneous but also sequential inoculations, likely closer to field situations. In addition, vaccines (both live and inactivated) against each disease should be looked at separately and the age of vaccination should be assessed taking into account the level of maternally-derived antibodies. Clinical signs may indeed be reduced in the field if one makes sure that maternally-derived antibodies do not neutralize the vaccines used.
In conclusion, the results of these studies showed that vaccination with two heterologous IBV vaccines (H120 and 4/91) combined with AIV H9N2 vaccination at day 7 reduced the severity of clinical signs and macroscopic lesions following experimental simultaneous challenge with Mor-IT02 and Mor-H9N2. This vaccination programme could be recommended to poultry farms with a similar epidemiological status as the one described here.
Acknowledgements
The authors would like to thank the Hubert Curien Partnership (PHC) (grant Toubkal/16/25–Campus France 34654NL entitled “Coronavirus aviaries émergents et réémergents au Maroc”) for financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Mariette F. Ducatez http://orcid.org/0000-0001-9632-5499
Additional information
Funding
References
- Al-Garib, S., Agha, A. & Al-Mesilaty, L. (2016). Low pathogenic avian influenza H9N2: world-wide distribution. World’s Poultry Science Journal, 72, 125–136. doi: 10.1017/S0043933915002603
- Aouini, R., Laamiri, N. & Ghram, A. (2018). Viral interference between low pathogenic avian influenza H9N2 and avian infectious bronchitis viruses in vitro and in ovo. Journal of Virological Methods, 259, 92–99. doi: 10.1016/j.jviromet.2018.06.011
- Bano, S., Naeem, K. & Malik, S.A. (2003). Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens. Avian Diseases, 47, 817–822. doi: 10.1637/0005-2086-47.s3.817
- Belkasmi, S.F.Z., Fellahi, S., Umar, S., Delpont, M., Delverdier, M., Lucas, M.-N., Bleuart, C., Kichou, F., Nassik, S., Guerin, J.-L., Fihri, O.F., Ducatez, M.F. & El Houadfi, M. (2017). Efficacy of Massachusetts and 793B vaccines against infectious bronchitis Moroccan-Italy 02 virus in specific-pathogen-free chickens and commercial broilers. Avian Diseases, 61, 466–471. doi: 10.1637/11686-060817-Reg.1
- Callison, S.A., Hilt, D.A., Boynton, T.O., Sample, B.F., Robison, R., Swayne, D.E. & Jackwood, M.W. (2006). Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods, 138, 60–65. doi: 10.1016/j.jviromet.2006.07.018
- El Houadfi, M., Fellahi, S., Nassik, S., Guérin, J.-L. & Ducatez, M.F. (2016). First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. Virology Journal, 13, 140. doi: 10.1186/s12985-016-0596-1
- Fellahi, S., El Harrak, M., Ducatez, M., Loutfi, C., Koraichi, S.I., Kuhn, J.H., Khayi, S., El Houadfi, M. & Ennaji, M.M. (2015). Phylogenetic analysis of avian infectious bronchitis virus S1 glycoprotein regions reveals emergence of a new genotype in Moroccan broiler chicken flocks. Virology Journal, 12, 116. doi: 10.1186/s12985-015-0347-8
- Haghighat-Jahromi, M., Asasi, K., Nili, H., Dadras, H. & Shooshtari, A.H. (2008). Coinfection of avian influenza virus (H9N2 subtype) with infectious bronchitis live vaccine. Archives of Virology, 153, 651–655. doi: 10.1007/s00705-008-0033-x
- Hassan, K.E., Ali, A., Shany, S.A.S. & El-Kady, M.F. (2017). Experimental co-infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Research in Veterinary Science, 115, 356–362. doi: 10.1016/j.rvsc.2017.06.024
- Ismail, Z.M., EL-Deeb, A.H., EL-Safty, M.M. & Hussein, H.A. (2018). Enhanced pathogenicity of low-pathogenic H9N2 avian influenza virus after vaccination with infectious bronchitis live attenuated vaccine. Veterinary World, 11, 977–985. doi: 10.14202/vetworld.2018.977-985
- Jackwood, M.W. (2012). Review of infectious bronchitis virus around the world. Avian Diseases, 56, 634–641. doi: 10.1637/10227-043012-Review.1
- Karimi-Madab, M., Ansari-Lari, M., Asasi, K. & Nili, H. (2010). Risk factors for detection of bronchial casts, most frequently seen in endemic H9N2 avian influenza infection, in poultry flocks in Iran. Preventive Veterinary Medicine, 95, 275–280. doi: 10.1016/j.prevetmed.2010.03.010
- Kishida, N., Sakoda, Y., Eto, M., Sunaga, Y. & Kida, H. (2004). Co-infection of Staphylococcus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza A virus infection in chickens. Archives of Virology, 149, 2095–2104. doi: 10.1007/s00705-004-0372-1
- Liu, C., Eichelberger, M.C., Compans, R.W. & Air, G.M. (1995). Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. Journal of Virology, 69, 1099–1106.
- Ng, L.F. & Liu, D.X. (2000). Further characterization of the coronavirus infectious bronchitis virus 3C-like proteinase and determination of a new cleavage site. Virology, 272, 27–39. doi: 10.1006/viro.2000.0330
- Nili, H. & Asasi, K. (2002). Natural cases and an experimental study of H9N2 avian influenza in commercial broiler chickens of Iran. Avian Pathology, 31, 247–252. doi: 10.1080/03079450220136567
- Nili, H. & Asasi, K. (2003). Avian influenza (H9N2) outbreak in Iran. Avian Diseases, 47, 828–831. doi: 10.1637/0005-2086-47.s3.828
- Parvin, R., Heenemann, K., Halami, M.Y., Chowdhury, E.H., Islam, M.R. & Vahlenkamp, T.W. (2014). Full-genome analysis of avian influenza virus H9N2 from Bangladesh reveals internal gene reassortments with two distinct highly pathogenic avian influenza viruses. Archives of Virology, 159, 1651–1661. doi: 10.1007/s00705-014-1976-8
- Parvin, R., Shehata, A.A., Heenemann, K., Gac, M., Rueckner, A., Halami, M.Y. & Vahlenkamp, T.W. (2015). Differential replication properties among H9N2 avian influenza viruses of Eurasian origin. Veterinary Research, 46, 75. doi: 10.1186/s13567-015-0198-8
- Perk, S., Panshin, A., Shihmanter, E., Gissin, I., Pokamunski, S., Pirak, M. & Lipkind, M. (2006). Ecology and molecular epidemiology of H9N2 avian influenza viruses isolated in Israel during 2000-2004 epizootic. Developments in Biologicals, 124, 201–209.
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty percent endpoints. American Journal of Epidemiology, 27, 493–497. doi: 10.1093/oxfordjournals.aje.a118408
- Roussan, D.A., Haddad, R. & Khawaldeh, G. (2008). Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poultry Science, 87, 444–448. doi: 10.3382/ps.2007-00415
- Seifi, S., Asasi, K. & Mohammadi, A. (2012). An experimental study on broiler chicken co-infected with the specimens containing avian influenza (H9 subtype) and infectious bronchitis (4/91 strain) viruses. Iranian Journal of Veterinary Research, 13, 138–142.
- Spackman, E., Senne, D.A., Myers, T.J., Bulaga, L.L., Garber, L.P., Perdue, M.L., Lohman, K., Daum, L.T. & Suarez, D.L. (2002). Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology, 40, 3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002
- Stipkovits, L., Glavits, R., Palfi, V., Beres, A., Egyed, L., Denes, B., Somogyi, M. & Szathmary, S. (2012). Pathologic lesions caused by coinfection of Mycoplasma gallisepticum and H3N8 low pathogenic avian influenza virus in chickens. Veterinary Pathology, 49, 273–283. doi: 10.1177/0300985811415702
- Swayne, D.E., Suarez, L.S. & Sims, L.D. (2013). Influenza. In D.E. Swayne (Ed.). Diseases of Poultry 13th edn (pp.181–218). Ames: Iowa State Press.
- Tavakkoli, H., Asasi, K. & Mohammadi, A. (2011). Effectiveness of two H9N2 low pathogenic avian influenza conventional inactivated oil emulsion vaccines on H9N2 viral replication and shedding in broiler chickens. Iranian Journal of Veterinary Research, 12, 214–221.
