ABSTRACT
Avian mycobacteriosis (AM) is a chronic and contagious disease of pet birds, captive exotic, wild and domestic fowl, and mammals. Mycobacterium avium subsp. avium is the most common cause of AM in poultry. For the first time, we report a chronic outbreak of AM in an Iranian breeder flock of 250 45-week-old turkeys (Meleagris gallopavo) with a morbidity and mortality rate of 91.6% and 80%, respectively. A well-defined clinical feature of the outbreak included a progressive weight loss, decreased egg production, listlessness, and lameness. Tuberculous nodules were seen on liver, spleen, ovary, and ribs. Granulomatous inflammation and acid-fast bacilli were confirmed by using Ziehl-Neelsen method on hepatic lesions. M. avium subsp. avium was identified by polymerase chain reaction techniques based on the presence of 16S ribosomal RNA gene and insertion elements IS1245 and IS901. In this report, we not only describe the epidemiological, pathological, and molecular characteristics of the outbreak in detail, but we also discuss multiple factors influencing the introduction and development of AM critically. In this case, wild feral pigeons might have been the source of infection, but further molecular-epidemiology studies are needed to understand the role of wild birds in the persistence and transmission of Mycobacterium.
RESEARCH HIGHLIGHTS
First report of avian mycobacteriosis in an Iranian commercial turkey flock is described in detail.
Risk factors intrinsic to the bird and mycobacteria, as well as extrinsic factors influencing the introduction and development of avian mycobacteriosis in birds, are critically discussed.
Introduction
Avian mycobacteriosis (AM) is a List B disease of the Office International des Epizootics (OIE) which is also considered to be of socio-economic and public health importance worldwide (OIE, Citation2018). Several mycobacterial species are responsible for AM, among which those of the Mycobacterium avium complex (MAC) including M. avium subsp. avium (MAA), M. avium subsp. paratuberculosis, M. avium subsp. silvaticum, and M. intracellulare, as well as M. genavense are the common agents involved in the aetiology. The pathogen most frequently involved in causing AM is MAA (Dhama et al., Citation2011; Riggs, Citation2011; Hodge et al., Citation2019).
AM is a significant disease of a wide variety of birds and also affects a range of mammalian species, including humans (Dhama et al., Citation2011; Fulton & Sanchez, Citation2013; OIE, Citation2018). Generally, avian susceptibility to disease varies across avian orders and species (Heatley et al., Citation2007; Witte et al., Citation2008; Dhama et al., Citation2011). In 1995, different species of domestic and free-living synanthropic birds were relatively classified according to the pathogenesis in experimental infections into highly susceptible, moderately susceptible, moderately resistant and highly resistant. However, to date, no reports exist of species totally resistant to mycobacteria (Hejlicek & Treml, Citation1995a; Riggs, Citation2011). However, much discrepancy still exists about how different bird species are sensitive to mycobacteriosis and which factors can favour the establishment of infection in birds. In the current literature, multiple risk factors are explored for mycobacterial infection; these can be listed as follows: (i) factors intrinsic to the bird such as species and breed (VanDerHeyden, Citation1997; Tell et al., Citation2001), gender (Hejlicek & Treml, Citation1995b), age at exposure (Kaboudi et al., Citation2017; OIE, Citation2018; Hodge et al., Citation2019), health and immune status (concurrent or preexisting infections, immunosuppression, etc.) (Hoenerhoff et al., Citation2004; Saggese et al., Citation2007; Witte et al., Citation2008), behaviour (feeding behaviour, and social integration/separation) (Gross et al., Citation1989; Cromie et al., Citation1991; Witte et al., Citation2008); (ii) factors related to bacteria such as strain pathogenicity and tissue predilection (Tell et al., Citation2001; Hoenerhoff et al., Citation2004; Witte et al., Citation2008); and (iii) extrinsic factors like demographic (Witte et al., Citation2010), temporal (Mutalib & Riddell, Citation1988; Witte et al., Citation2008), habitat (Tell et al., Citation2001; Riggs, Citation2011), climate (Cromie et al., Citation1991; Tell et al., Citation2001; Witte et al., Citation2008), and human interventions (raising birds in captivity, etc.) (Hoenerhoff et al., Citation2004; Gerhold & Fischer, Citation2005; OIE, Citation2018). Particularly in industrial poultry operations, the latter group appeared in the shape of faulty husbandry practices such as overcrowding (Witte et al., Citation2008; Dhama et al., Citation2011; Álvarez et al., Citation2017), poor biosecurity compliance (possible contact with wild birds, allowing birds to roam freely, mixture of avian and mammalian on the same farm, unknown provenance of birds, etc.) (Witte et al., Citation2008; Kelly et al., Citation2013; Álvarez et al., Citation2017; Kaboudi et al., Citation2017; OIE, Citation2018; Hodge et al., Citation2019), bad sanitation and hygienic conditions (Dhama et al., Citation2011; Álvarez et al., Citation2017), malnutrition (Mutalib & Riddell, Citation1988; Tell et al., Citation2001; Witte et al., Citation2008), poor environmental management (inadequate ventilation and excessive humidity) (Tell et al., Citation2001; Álvarez et al., Citation2017; Hodge et al., Citation2019), and movement and handling of birds (Witte et al., Citation2008; Hodge et al., Citation2019). AM has been reported in small poultry flocks of several U.S. states, Canada, Australia, the Czech Republic, Spain and, most recently, in China due to the above-mentioned factors (Gill et al., Citation1986; Mutalib & Riddell, Citation1988; González et al., Citation2002; Shitaye et al., Citation2008; Fulton & Sanchez, Citation2013; Zhu et al., Citation2016). At present, it is extremely rare due to the development of poultry husbandry practices in integrated commercial poultry farming (Dhama et al., Citation2011; Riggs, Citation2011; Fulton & Sanchez, Citation2013); however, there is still a need for further studies to eradicate AM in commercial poultry farms.
The wild turkey (Meleagris gallopavo) is native to the North American continent, domesticated in Europe, and distributed worldwide. Between the end of the nineteenth century and the 1930s, wild turkey populations in the United States experienced a dip to the lowest number, followed by a dramatic restoration and growth in domestic turkey production (Brant, Citation1998). In 1931, the first case of turkey tuberculosis was reported in a wild turkey with cutaneous and subcutaneous lesions (Scrivner & Elder, Citation1931). After a few years, tuberculosis of turkeys was further described according to the incidences in the United States (Hinshaw et al., Citation1932; Hinshaw, Citation1937). A laboratory survey from 1985 to 2001, in the Midwestern United States, reported no cases of AM in commercial turkeys among a total of 15,097 bird submissions received. Furthermore, based on the only visual inspection data recorded by the National Agricultural Statistics Service (NASS) of the U.S. Department of Agriculture (USDA) from 1995 to 2011, AM in turkey was detected only in 2003 with an incidence rate of 0.04 per 10 million mature turkeys slaughtered (Fulton & Sanchez, Citation2013). More recently, in 2005 and 2006, two papers were published in the American Midwest; one described a susceptibility to a natural infection with MAA in a wild turkey (Gerhold & Fischer, Citation2005) while the other concluded a high resistance to experimental infection with M. bovis in wild turkeys (Clarke et al., Citation2006).
Here, we report the first case of severe natural infection with MAA in a commercial turkey breeder flock diagnosed based on necropsy, microscopic, and molecular findings. This paper also seeks to discuss a variety of factors implicated in the introduction and development of severe AM in this outbreak. Commercial and backyard turkey flocks have been increased considerably during recent years in Iran with low standard conditions (Rassouli et al., Citation2016). To the author’s knowledge, this is the first report of AM in Iranian commercial poultry flocks.
Materials and methods
Flock history
An outbreak of AM occurred in a 45-week-old breeder flock of 250 (226 female and 24 male) Bourbon Red turkeys, in Saveh, Markazi Province, Iran. The first-affected cases were found accidentally by the owner during necropsy while being confused with tumour and mycosis. A month after the onset of the outbreak, the owner moved the entire flock to a second farm in Takestan, Qazvin Province, Iran. However, the disease still developed and killed 200 turkeys over 6 months, from December 2018 to May 2019. All interventions were unsuccessful, including intermittent antibiotic therapies; using acidified copper sulfate (either as a sanitizer or as anti-fungal and mould inhibitor feed supplement); and also treating the flock with hepatoprotector supplements.
Ethical considerations
Tissue samples were collected during routine necropsy on farm. No other in vivo experiments, clinical or epidemiological trials are linked to this report. The Research Ethics Committee at the Ferdowsi University of Mashhad was informed of publishing the diagnostic findings, and a formal waiver of ethics approval was granted. After a positive diagnosis of AM and, according to the Terrestrial Animal Health Code (Terrestrial Code) and guidance of the national veterinary authorities (Hinshaw, Citation1937; Fulton & Sanchez, Citation2013; OIE, Citation2018, Citation2019), the owner slaughtered and burnt the remaining flock, and followed a cleanout and disinfection programme of all contaminated premises and facilities to ensure freedom from AM in other commercial farms, wildlife, and susceptible human population.
Microscopic examination
Two liver samples of one-year-old hens were submitted to confirm the presumptive diagnosis by histopathological examination. After recording of gross characteristics, impression smears were stained with the Ziehl-Neelsen (Z-N) method for acid-fast bacilli. Then, samples were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with haematoxylin and eosin (H&E) and Z-N staining methods.
Molecular identification
To identify the causative agent, DNA extraction and PCR protocol were carried out on two liver samples. Additionally, this report led us to present a streamlined pre-extraction technique on the formalin-fixed paraffin-embedded (FFPE) liver tissue which has been optimized by slight modifications at the Tuberculosis Reference Laboratory, Razi Vaccine and Serum Research Institute, Tehran, Iran as follows.
Preparation of tissue sections
After trimming the extra wax, the paraffin block of the liver was thin-sectioned with a sterile scalpel under a stereomicroscope to ensure the presence of nodular tissue in chosen sections (the sections were less than 0.5 mm thick). A single section was placed in a sterile 1.5 ml microtube.
Deparaffinizing sections
One ml of absolute xylene was added to the microtube, and the tissue section was immersed for 15 min. Then, a gentle vortex agitation for 2 min and centrifugation for 5 min at 18,000 g were performed. The supernatant solvent was decanted with a single-use, fine-tipped glass pipette. This washing step was repeated twice. To remove xylene residue, the tissue pellet was washed twice with 1 ml of absolute ethanol, and then centrifuged at 18,000 g for 15 min. Subsequently, a gradual ethanol dehydration using 1 ml of 80%, 60%, and 40% ethanol washes was performed (10 min at room temperature for each step followed by the same centrifugation). Finally, the tissue pellet was allowed to dry at 37°C for 15 min.
DNA extraction
This step was performed according to the procedure developed by van Soolingen et al. (Citation1991) with some modifications. Briefly, the pellet was solubilized in 400 μl of 1X TE buffer (10 mM Tris-HCl [Basel, Switzerland] and 1 mM EDTA [Sigma-Aldrich, Saint-Louis, MO, US], pH 8.0) and incubated in an 80°C water bath for 20 min. Then 50 μl of 10 mg/ml lysozyme (Merck, Darmstadt, Germany) was added and shortly vortexed to dissolve, followed by overnight incubation at 37°C. The mixture, after addition of 75 μl SDS/proteinase K solution (5 μl of 10 mg/ml proteinase K [Sigma, Germany] diluted in 70 μl of 10% sodium dodecyl sulphate [Merck, Germany]) was briefly vortexed and incubated for 10 min at 65°C. Next, 100 μl of 5 M NaCl and 100 μl of CTAB/NaCl solution (4.1 g NaCl dissolved in 80 ml of distilled water with 10 g N-Cetyl-N,N,N Trimethyl Ammonium Bromide [Sigma, Germany] and the volume adjusted to 100 ml with distilled water and prewarmed to 65°C) were added to the mixture. After 10 min incubation at 65°C, the mixture was extracted with 750 μl of chloroform/isoamyl alcohol (24:1 v/v) (Merck, Germany), vortexed for 10 s and centrifuged at room temperature, 4000 g for 15 min. The supernatant (approximately 180 μl) was transferred to a sterile microtube, and 450 μl ice-cold isopropanol was added. The tube was placed at −20°C for 30 min and then the precipitate was collected by centrifugation at 4000 g for 15 min. The pellet was washed once with 70% ethanol and dried thoroughly. The final yield of DNA was redissolved in 20 μl of 1X TE buffer, and the concentration was determined by Epoch™ microplate spectrophotometer (BioTek instruments, Winooski, VT, USA).
PCR assay
Extracted DNA was used as a template for PCR amplification targeting a 543-bp fragment of the 16S ribosomal RNA (16S rRNA) gene with the forward primer 5′-ACGGTGGGTACTAGGTGTGGGTTTC-3′ and reverse primer 5′-TCTGCGATTACTAGCGACTCCGACTTCA-3′ for the genus Mycobacterium (Huard et al., Citation2003). To determine the contribution of MAC, a 427-bp fragment of IS1245 was amplified using forward primer 5′-AGGTGGCGTCGAGGAAGAC-3′ and reverse primer 5′- GCCGCCGAAACGATCTAC-3′ (Guerrero et al., Citation1995). Finally, the forward primer 5′-GCAACGGTTGTTGCTTGAAA-3′ and reverse primer 5′-TGATACGGCCGGAATCGCGT-3′ were used to amplify an 1108 bp fragment of IS901 to identify MAA (Kunze et al., Citation1991). Each PCR contained 3 μl of the DNA, 0.4 μl of each forward and reverse primer (50 μg/μl) (Eurofins, Germany), 2.5 μl 10×Tth polymerase PCR buffer (Roche, Germany), 0.5 μl deoxynucleoside triphosphate (2.5 mM of each dNTP) (Roche, Germany), 1 μl MgCl2 (Roche, Germany), 0.125 μl Taq polymerase (5 U/μl) (Roche, Germany) and 17 μl water. PCR amplification for IS901 was run with the following thermal cycling profile: an initial denaturation at 94°C for 3 min, 30 cycles (30 s denaturation at 94°C, 30 s annealing at 65°C, 150 s extension at 72°C), an annealing cycle at 65°C for 2 min and a final elongation step at 72°C for 5 min. PCR conditions for amplifying both IS1245 and 16S rRNA included an initial denaturation at 94°C for 5 min followed by 30 cycles of 94°C for 1 min, annealing at 60°C and 65°C (for 16S rRNA and IS1245, respectively) for 1 min, 72°C for 2 min, and a final extension step for 10 min at 72°C. Positive control (MAA D4 strains, ATCC number 35713), negative controls (double-distilled water and M. bovis AN5 strain, ATCC number 35726) were included in tests. The PCR amplicons were detected using ethidium bromide-stained 2% agarose gels in a submerged electrophoresis system.
Results
On-farm and clinical findings
The house and facilities of the first farm were repurposed after 10 years with poor biosecurity conditions. Near to this farm, there was no livestock, no poultry farm, no processing plant, and no village road, except a derelict building as a loft for roosting and reproducing of more than 1000 feral pigeons. The loft was located at an aerial distance of 400 m from the breeder premises. Pigeons were hunted as a hobby and also eaten by guard dogs. The owner used to implement minimum sanitation procedures, an acceptable feeding plan, and routine vaccination programmes against Newcastle disease (Razi Vaccine and Serum Research Institute, Iran), H9N2 avian influenza (Razi Vaccine and Serum Research Institute, Iran), and fowl pox (Razi Vaccine and Serum Research Institute, Iran). The previous flock had a history of pox and Newcastle disease. No microbial contamination of the water supply had been found during recent years.
During winter 2018, the onset of a slight but steady rise in the number of turkeys manifesting progressive and debilitating disease was noted at 45 weeks of age. Early affected birds were easily identified by listlessness and a firm swollen abdomen. Sick hens gradually ceased laying and became severely emaciated over a month while they kept a good appetite. Incidences and severity of the disease increased as the flock aged. In extreme cases, hens lost more than 3 kg in weight during a month, manifesting a sharp-edged keel and a smaller face. By the end stages of the disease, turkeys showed dropped wings, unilateral lameness, or hyperextension of both legs while few sitting on hocks. The dark and ruffled appearance of feathers was reported. Appetite diminished and crop emptying was occasionally seen when carrying a dying bird. No signs referable to the respiratory or nervous system were evident. Over 6 months, the morbidity and mortality rate were measured at 91.6% (226 hens and three toms) and 80% (199 hens and one tom), respectively.
Post mortem findings
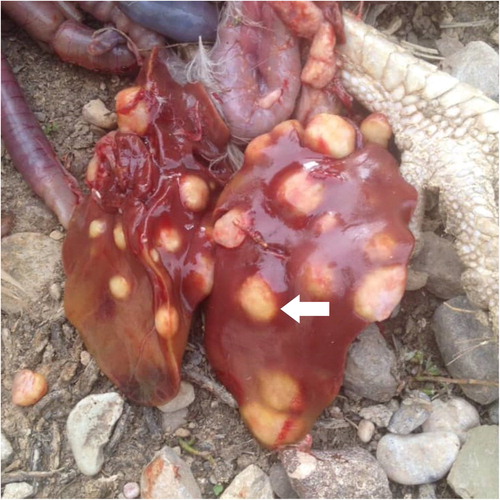
Typical AM nodules were distributed throughout liver, spleen, ovaries, and ribs. All of these organs were enlarged and manifested irregular nodular contours. Nodules were yellowish in colour and varied from 0.1 mm to 30 mm in diameter. In the early stages, numerous necrotic foci were observed on the liver capsule and parenchyma. Nevertheless, in advanced cases, the liver was enlarged twice or more featuring multiple discrete nodular lesions, either bulging from the capsular surface or deep in the parenchyma (). Swelling of the hock joint was reported in some affected birds.
Microscopic findings
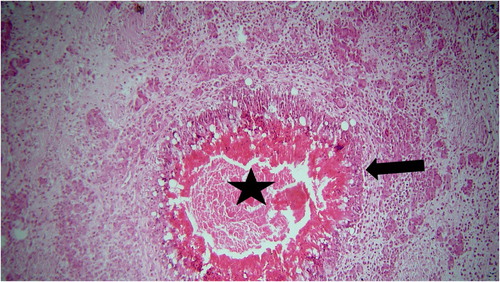
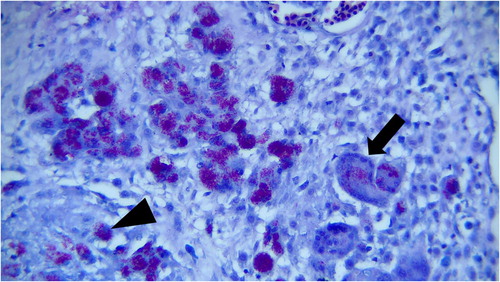
Histopathological examination revealed numerous focal granulomatous inflammations in affected livers. Most of these foci had central caseous necrosis that was surrounded by high numbers of epithelioid macrophages and multinucleated giant cells. Other inflammatory cells, such as lymphocytes and plasma cells, and severe fibrosis were seen in the periphery of the caseating hepatic granulomas and between them (). A large number of acid-fast bacilli of Mycobacterium species were observed within the cytoplasm of epithelioid macrophages and multinucleated giant cells by the Z-N staining method ().
Molecular findings
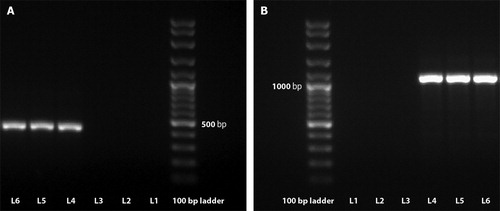
The molecular typing revealed specific bands of PCR amplicons in gel electrophoresis consistent with 16S rRNA, IS1245, and IS901 in both FFPE liver samples ().
Figure 4. PCR amplification of DNA from paraffin-embedded liver sections of the turkeys infected with Mycobacterium avium subsp. avium. The 427-bp specific fragment from IS1245 (A) and the 1108-bp specific fragment from IS901 (B) are shown in relation to molecular size marker with 100 bp rungs. L1 and L2, negative controls (no template added); L3, Mycobacterium bovis AN5 strain (ATCC 35726) (amplification product was not obtained); L4, Mycobacterium avium subsp. avium D4 strain (ATCC 35713) (amplification product was obtained); L5 and L6, double-checked samples tested for Mycobacterium avium subsp. avium (amplification product was obtained).
Discussion
AM is an undoubtedly rare occurrence in commercial turkeys (Fulton & Sanchez, Citation2013) and has not been reported till now. We described an outbreak of severe AM caused by MAA for the first time in a turkey breeder farm where 80% of the flock died. The diagnosis was made using farm history, necropsy, microscopic examinations, and molecular assays, but late after the infection had been creeping into the entire flock. Quite contrary to the expectations of little susceptibility (or even moderate resistance) of domestic turkeys to M. avium (Tell et al., Citation2001; Dhama et al., Citation2011; Fulton & Sanchez, Citation2013), this report critically highlights how a variety of factors could be implicated in the severity of an AM outbreak.
The disease was characterized by a latency phase in which infected birds remained asymptomatic. As indicated earlier, this feature points to the long incubation period of mycobacterial infection (González et al., Citation2002; Coles et al., Citation2007; OIE, Citation2018). No clinical signs were reported in the flock before the birds were 1 year of age. Although the incubation is governed by the host condition and the load of exposure (Hoefer, Citation1997), the disease is most frequently expected in flocks older than a year (Tell et al., Citation2001; Casaubon-Huguenin & Brugère-Picoux Citation2015; Kaboudi et al., Citation2017). Therefore, it seems that the chronic, insidious nature of mycobacteriosis could be responsible for prolonged bacterial shedding and dissemination inside the flock. However, the distribution of heavy mycobacterial load at the onset of this outbreak can only be partially blamed for the high morbidity and mortality.
The latency phase was followed by a six-month protracted course of clinical manifestations, which was characterized by an early coelomic distention, wasting, marked emaciation, egg drop, and lameness, almost similar to the classic scenario of signs described in other avian species (Tell et al., Citation2001). As previously argued in the literature, all clinical signs were typical of non-pathognomonic findings (Dhama et al., Citation2011), so they were not useful as an ante mortem change to allow early detection of underlying mycobacteriosis, and to alleviate the severity of the outbreak.
The severity of granulomatous lesions of visceral organs was gradually increased during the outbreak. Diffuse miliary foci of visceral organs in first cases later changed to large granular tuberculous nodules in advanced cases. Miliary tuberculosis of liver and spleen was observed by Hejlícek and Treml (Citation1995b) in turkeys under experimental infection. Formation of large tuberculous nodules could cautiously be attributed to the chronic spread of the organism throughout the flock followed by different induced granulomatous responses of birds. However, there is a paucity of information on the immune response against AM in bird species (Hodge et al., Citation2019). Considering the chronic nature of the outbreak and low stocking density, no ruptures were seen in livers and spleens. Thus, no sudden death was reported (OIE, Citation2018). However, these two reticuloendothelial organs were the main seats for nodular lesions. This finding matches those observed by Francis (Citation1958) for predominantly affected organs of turkeys. Generally, a greater number of organs tend to be infected in turkeys than in chickens (Hinshaw, Citation1937). What we are unable to account for is the fact that, even in advanced cases, no findings were seen in the intestinal tract, while it is known as the most essential site for entry and primary localization of AM (Haridy et al., Citation2014; OIE, Citation2018; Hodge et al., Citation2019). OIE noted that nearly always primary lesions are expected in the intestinal tract of birds infected (OIE, Citation2018). In the first studies of turkey tuberculosis in 1932, 45.65% of lesions were found in the intestinal tract (Hinshaw et al., Citation1932). Furthermore, we did not observe any gross finding in the respiratory tract, but involvement of lungs, though uncommon (Beytut et al., Citation2001; Tell et al., Citation2001), is expected in severe cases (Fulton & Sanchez, Citation2013; OIE, Citation2018; Hodge et al., Citation2019). We think different breeds of turkeys may be differently susceptible to the respiratory tract infection, which requires further investigations of pathogenesis. Curiously, ovaries, ribs, and sternum were other prominent organs which manifested severe lesions in all hens resulting in egg drop and locomotion problems. Moreover, the susceptibility of female breeder turkeys was higher than males in the current outbreak, which is previously noted by Hejlicek and Treml (Citation1995b). Therefore, there might be a correlation between the latter two findings and the physiological and/or anatomical characteristics of females. In contrast to the first case of AM in a turkey with cutaneous and subcutaneous lesions (Scrivner & Elder, Citation1931), no evidence of an external sign was observed. Inevitably, post mortem findings could not be all-inclusive due to the on-farm necropsies. Previously, reports varied from generalized forms to lesions limited to one or few organs. In localized forms, some infected organs may be underreported due to visual inspection or the paradigm of alimentary nature of infection in birds (Mayahi et al., Citation2013; Kaboudi et al., Citation2017; Hodge et al., Citation2019). Taken together, the variety and number of organs involved might be explained by factors linked to species, breed, sex, and immune status as well as tissue predilection, pathogenicity, and entry route of the mycobacterial strain. Although necropsy is the most expedient AM diagnosis tool (Fulton & Sanchez, Citation2013), misdiagnosis with tumours and mycosis is plausible (Hinshaw, Citation1937). In the current outbreak, lack of knowledge, negligence and misdiagnosis are mostly blamed for the unexpected development of the outbreak. However, treatment of AM in any situation is not suggested (Fulton & Sanchez, Citation2013).
The diagnosis was confirmed by acid-fast staining of smears and histopathology of the liver (Fulton & Sanchez, Citation2013; OIE, Citation2018). Moreover, the large number of bacilli reveals a typical finding of M. avium infections while differs from rare organisms found within tubercles for other species like M. tuberculosis or M. bovis (Tell et al., Citation2001). It may mirror the severity of infection due to the pathogenicity of the involved strain or different host immune response (Hodge et al., Citation2019). Identification of the causative agent at subspecies level targeted the IS901 element, which is an identifier for MAA and also a high pathogenicity determinant of serotype involved (Dhama et al., Citation2011; OIE, Citation2018). Different strains of MAA, with infectivity higher than that expected, are responsible for frequent outbreaks in Iranian flocks of domestic pigeons (Bolfion et al., Citation2010; Mayahi et al., Citation2013; Parvandar-Asadollahi et al., Citation2015). Wild birds are one of the main sources of infection and were present nearby the current farm. Therefore, pigeons could be the major (if not the only) shedders and reservoirs (Fulton & Sanchez, Citation2013; Casaubon-Huguenin & Brugère-Picoux, Citation2015; Álvarez et al., Citation2017). This also raises questions about feed, water, and litter as important and probable sources of environmental contamination (Casaubon-Huguenin & Brugère-Picoux Citation2015). We should sound a note of caution concerning such a finding for interspecies transmission. Given the inevitable uncertainties in our understanding of the other before-mentioned extrinsic factors, poor biosecurity compliance could well be responsible for the establishment of the disease in this flock.
One unanticipated issue which emerged from the molecular investigation was DNA extraction from formaldehyde-fixed and the FFPE liver specimens. Most previously described methods report a roughly 10-fold reduction in sensitivity and an amplification success rate between 60% and 80% because of DNA degradation (Bréchot, Citation1993; Coura et al., Citation2005). Likewise, the method introduced by van Soolingen et al. (Citation1991) failed to reveal amplification directly on either formaldehyde-fixed or FFPE specimens while the pre-extraction technique presented in this report showed DNA can indeed be recovered. Recovery of nucleic acid from archived tissues was accomplished as early as 1985, employing proteinase K and SDS (Goelz et al., Citation1985; Dubeau et al., Citation1986). A similar pretreatment technique was described by other authors (Miller et al., Citation1999; Shi et al., Citation2002; Coura et al., Citation2005), but the presented technique has been optimized and used in our laboratory in recent years.
In conclusion, turkeys can be either preferentially resistant (Clarke et al., Citation2006) or quite susceptible (OIE, Citation2018), depending on many factors implicated in the introduction and development of mycobacteriosis. Rather than those intrinsic to the bird, this paper has drawn attention to the extrinsic factors which facilitated the introduction of infection into the flock (from the most probable source of wild pigeons), followed by deterioration into a severe AM outbreak. Factors related to mycobacteria such as longevity in the environment, high shedding through a long incubation period, problematic and unreliable ante mortem diagnosis, and refractive and impractical treatment are significant obstacles in the management of AM. Under these perspectives, addressing the health and economic impact of mycobacteriosis needs further molecular-epidemiology studies to identify reservoirs, environmental sources, risk pathways for interspecies transmission, and the interdependence of human and avian species in poultry farms, wild birds, and zoological collections.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Iman Salamatian http://orcid.org/0000-0001-9855-3583
Abolfazl Ghaniei http://orcid.org/0000-0003-0341-6987
Nader Mosavari http://orcid.org/0000-0002-3480-3376
Hossein Nourani http://orcid.org/0000-0002-4035-1118
Rouholah Keshavarz http://orcid.org/0000-0003-4976-1508
Mohammad Eslampanah http://orcid.org/0000-0002-2585-9001
References
- Álvarez, P.P., Moroni, M. & Verdugo, C. (2017). Avian tuberculosis in a Lady Amherst’s pheasant Chrysolophus amherstiae. Austral Journal of Veterinary Sciences, 49, 213–215. doi: 10.4067/S0719-81322017000300213
- Beytut, E., Atabay, H. & Akça, A. (2001). Tuberculosis and sarcosporidiosis in the periorbital locations in a hen. Kafkas Universitesi Veteriner Fakültesi Dergisi, 71, 213–217.
- Bolfion, M., Salehi, M., Helan, J.A., Soleimani, K., Keshavarz, R., Pajoohi, R.A., Taheri, M.M., Tadayon, K. & Mosavari, N. (2010). Outbreak of avian mycobacteriosis in flocks of domestic pigeons: an epidemiological approach. Iranian Journal of Microbiology, 2, 189–193.
- Brant, A. (1998). A brief history of the turkey. World’s Poultry Science Journal, 54, 365–373. doi: 10.1079/WPS19980027
- Bréchot, C. (1993). Polymerase chain reaction for the diagnosis of hepatitis B and C viral hepatitis. Journal of Hepatology, 17, S35–S41. doi: 10.1016/S0168-8278(05)80421-0
- Casaubon-Huguenin, M.T. & Brugère-Picoux, J. (2015). Avian tuberculosis. In J. Brugère-Picoux, J.P. Vaillancourt, H.L. Shivaprasad, D. Venne & M. Bouzouaia (Eds.), Manual of poultry Diseases (1st ed., pp. 361–364). Paris: AFAS.
- Clarke, K., Fitzgerald, S., Hattey, J., Bolin, C., Berry, D., Church, S. & Reed, W. (2006). Experimental inoculation of wild turkeys (Meleagris gallopavo) with Mycobacterium bovis. Avian Diseases, 50, 131–134. doi: 10.1637/7456-101405R.1
- Coles, P.C., Cicuta, M., Zumarraga, M., Etchetchoury, M., Lertora, J. & Ramirez, G. (2007). Avian mycobacteriosis in chickens from a rural area of Argentina. Revista Veterinaria, 18, 72–77.
- Coura, R., Prolla, J., Meurer, L. & Ashton-Prolla, P. (2005). An alternative protocol for DNA extraction from formalin fixed and paraffin wax embedded tissue. Journal of Clinical Pathology, 58, 894–895. doi: 10.1136/jcp.2004.021352
- Cromie, R., Brown, M., Price, D. & Stanford, J. (1991). Susceptibility of captive wildfowl to avian tuberculosis: the importance of genetic and environmental factors. Tubercle, 72, 105–109. doi: 10.1016/0041-3879(91)90036-R
- Dhama, K., Mahendran, M., Tiwari, R., Dayal Singh, S., Kumar, D., Singh, S. & Sawant, P.M. (2011). Tuberculosis in birds: insights into the Mycobacterium avium infections. Veterinary Medicine International, 2011, 1–14. doi: 10.4061/2011/712369
- Dubeau, L., Chandler, L.A., Gralow, J.R., Nichols, P.W. & Jones, P.A. (1986). Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Research, 46, 2964–2969.
- Francis, J. (1958). Tuberculosis in animals and man. A study in comparative pathology. London: Cassell.
- Fulton, R.M. & Sanchez, S. (2013). Tuberculosis. In D.E. Swayne, J.R. Glisson, L.R. McDougald, L.K. Nolan, D.L. Suarez & V. Nair (Eds.), Diseases of poultry (13th ed., pp. 1008–1017). Ames, IA: Wiley-Blackwell.
- Gerhold, R.W. & Fischer, J.R. (2005). Avian tuberculosis in a wild turkey. Avian Diseases, 49, 164–166. doi: 10.1637/7245-072204R
- Gill, I.J., Blandy, M.L., Hanson, W.D., Witkowski, J.F., Barber, D.T., Currier, D.R., Hynes, K.F., Li, W.G., Baker, T.C. & Chamberlain, W.F. (1986). Control of avian tuberculosis in a commercial poultry flock. Australian Veterinary Journal, 63, 422–423. doi: 10.1111/j.1751-0813.1986.tb15923.x
- Goelz, S.E., Hamilton, S.R. & Vogelstein, B. (1985). Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochemical and Biophysical Research Communications, 130, 118–126. doi: 10.1016/0006-291X(85)90390-0
- González, M., Rodriguez-Bertos, A., Gimeno, I., Flores, J.M. & Pizarro, M. (2002). Outbreak of avian tuberculosis in 48-week-old commercial layer hen flock. Avian Diseases, 46, 1055–1061. doi: 10.1637/0005-2086(2002)046[1055:OOATIW]2.0.CO;2
- Gross, W., Falkinham III, J. & Payeur, J. (1989). Effect of environmental-genetic interactions on Mycobacterium avium challenge infection. Avian Diseases, 33, 411–415. doi: 10.2307/1591097
- Guerrero, C., Bernasconi, C., Burki, D., Bodmer, T. & Telenti, A. (1995). A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. Journal of Clinical Microbiology, 33, 304–307. doi: 10.1128/JCM.33.2.304-307.1995
- Haridy, M., Fukuta, M., Mori, Y., Ito, H., Kubo, M., Sakai, H. & Yanai, T. (2014). An outbreak of Mycobacterium genavense infection in a flock of captive diamond doves (Geopelia cuneata). Avian Diseases, 58, 383–390. doi: 10.1637/10775-011714-Reg.1
- Heatley, J.J., Mitchell, M.M., Roy, A., Cho, D.Y., Williams, D.L. & Tully, T.N. (2007). Disseminated mycobacteriosis in a bald eagle (Haliaeetus leucocephalus). Journal of Avian Medicine and Surgery, 21, 201–209. doi: 10.1647/1082-6742(2007)21[201:DMIABE]2.0.CO;2
- Hejlicek, K. & Treml, F. (1995a). Comparison of the pathogenesis and epizootiologic importance of avian mycobacteriosis in various types of domestic and free-living syntropic birds. Veterinarni Medicina, 40, 187–194.
- Hejlicek, K. & Treml, F. (1995b). Pathogenesis of avian mycobacteriosis in the domestic turkey (Meleagris gallopavo f. domestica) and Guinea fowl (Numida meleagris f. domestica). Veterinarni Medicina, 40, 123–127.
- Hinshaw, W., Niemann, K. & Busic, W. (1932). Studies of tuberculosis of turkeys. Journal of the American Veterinary Medical Association, 80, 765–777.
- Hinshaw, W.R. (1937). Diseases of turkeys. California, CA: University of California College of Agriculture, Agricultural Experiment Station.
- Hodge, P., Sandy, J. & Noormohammadi, A. (2019). Avian mycobacteriosis in captive brolgas (Antigone rubicunda). Australian Veterinary Journal, 97, 81–86. doi: 10.1111/avj.12784
- Hoefer, H.L. (1997). Practical avian medicine. Trenton, NJ: Veterinary Learning Systems.
- Hoenerhoff, M., Kiupel, M., Sikarskie, J., Bolin, C., Simmons, H. & Fitzgerald, S. (2004). Mycobacteriosis in an American bald eagle (Haliaeetus leucocephalus). Avian Diseases, 48, 437–441. doi: 10.1637/7133
- Huard, R.C., de Oliveira Lazzarini, L.C., Butler, W.R., van Soolingen, D. & Ho, J.L. (2003). PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. Journal of Clinical Microbiology, 41, 1637–1650. doi: 10.1128/JCM.41.4.1637-1650.2003
- Kaboudi, K., Amara, A. & Bouzouaia, M. (2017). Avian tuberculosis in a backyard poultry flock in Tunisia: case report. International Journal of Veterinary Sciences and Animal Husbandry, 2, 34–37.
- Kelly, P., Jahns, H., Power, E., Bainbridge, J., Kenny, K., Corpa, J.M., Cassidy, J.P. & Callanan, J.J. (2013). Mycobacteriosis in ostriches (Struthio camelus) due to infection with Mycobacterium bovis and Mycobacterium avium complex. Avian Diseases, 57, 808–811. doi: 10.1637/10581-052313-Case.1
- Kunze, Z., Wall, S., Appelberg, R., Silva, M., Portaels, F. & McFadden, J. (1991). IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Molecular Microbiology, 5, 2265–2272. doi: 10.1111/j.1365-2958.1991.tb02157.x
- Mayahi, M., Mosavari, N., Esmaeilzadeh, S. & Parvandar-Asadollahi, K. (2013). Avian tuberculosis in naturally infected lofts of domestic pigeons, isolation, molecular identification and study of necropsy findings. The International Journal of Applied Research in Veterinary Medicine, 11, 194–201.
- Miller, J.M., Jenny, A.L. & Ellingson, J.L. (1999). Polymerase chain reaction identification of Mycobacterium avium in formalin-fixed, paraffin-embedded animal tissues. Journal of Veterinary Diagnostic Investigation, 11, 436–440. doi: 10.1177/104063879901100508
- Mutalib, A.A. & Riddell, C. (1988). Epizootiology and pathology of avian tuberculosis in chickens in Saskatchewan. The Canadian Veterinary Journal, 29, 840–842.
- OIE. (2018). Chapter 3.3.6. Avian tuberculosis. In Manual of diagnostic tests and vaccines for terrestrial animals 2019. Retrieved 13 March 2020, from https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.03.06_AVIAN_TB.pdf
- OIE. (2019). Chapter 7.6. Killing of animals for disease control purposes. In Terrestrial Animal Health Code 2019. Retrieved 13 March 2020, from https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_aw_killing.pdf
- Parvandar-Asadollahi, K., Mosavari, N. & Mayahi, M. (2015). Genotyping of Mycobacterium avium subsp. avium isolates from naturally infected lofts of domestic pigeons in Ahvaz by IS901 RFLP. Iranian Journal of Microbiology, 7, 260–264.
- Rassouli, M., Darvishi, M.M. & Lima, S.R.R. (2016). Ectoparasite (louse, mite and tick) infestations on female turkeys (Galliformes, Phasianidae. Meleagris gallopavo) in Iran. Journal of Parasitic Diseases, 40, 1226–1229. doi: 10.1007/s12639-015-0657-1
- Riggs, G. (2011). Avian mycobacterial disease. In R.E. Miller & M.E. Fowler (Eds.), Fowler’s zoo and wild animal medicine (pp. 266–274). St. Louis, MO: Elsevier Saunders.
- Saggese, M.D., Riggs, G., Tizard, I., Bratton, G., Taylor, R. & Phalen, D.N. (2007). Gross and microscopic findings and investigation of the aetiopathogenesis of mycobacteriosis in a captive population of white-winged ducks (Cairina scutulata). Avian Pathology, 36, 415–422. doi: 10.1080/03079450701595909
- Scrivner, L. & Elder, C. (1931). Cutaneous and subcutaneous tuberculosis in turkeys. Journal of the American Veterinary Medical Association, 79, 244–247.
- Shi, S.R., Cote, R.J., Wu, L., Liu, C., Datar, R., Shi, Y., Liu, D., Lim, H. & Taylor, C.R. (2002). DNA extraction from archival formalin-fixed, paraffin-embedded tissue sections based on the antigen retrieval principle: heating under the influence of pH. Journal of Histochemistry & Cytochemistry, 50, 1005–1011. doi: 10.1177/002215540205000802
- Shitaye, J., Matlova, L., Horvathova, A., Moravkova, M., Dvorska-Bartosova, L., Treml, F., Lamka, J. & Pavlik, I. (2008). Mycobacterium avium subsp. avium distribution studied in a naturally infected hen flock and in the environment by culture, serotyping and IS901 RFLP methods. Veterinary Microbiology, 127, 155–164. doi: 10.1016/j.vetmic.2007.07.026
- Tell, L.A., Woods, L. & Cromie, R. (2001). Mycobacteriosis in birds. Revue Scientifique et Technique-Office International des Epizooties, 20, 180–203. doi: 10.20506/rst.20.1.1273
- van Soolingen, D., Hermans, P., De Haas, P., Soll, D. & Van Embden, J. (1991). Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. Journal of Clinical Microbiology, 29, 2578–2586. doi: 10.1128/JCM.29.11.2578-2586.1991
- VanDerHeyden, N. (1997). Clinical manifestations of mycobacteriosis in pet birds. In M. Hernández (Ed.), Seminars in avian and exotic pet medicine (pp. 18–24). Philadelphia, PA: WB Saunders.
- Witte, C.L., Hungerford, L.L., Papendick, R., Stalis, I.H. & Rideout, B.A. (2008). Investigation of characteristics and factors associated with avian mycobacteriosis in zoo birds. Journal of Veterinary Diagnostic Investigation, 20, 186–196. doi: 10.1177/104063870802000207
- Witte, C.L., Hungerford, L.L., Papendick, R., Stalis, I.H. & Rideout, B.A. (2010). Investigation of factors predicting disease among zoo birds exposed to avian mycobacteriosis. Journal of the American Veterinary Medical Association, 236, 211–218. doi: 10.2460/javma.236.2.211
- Zhu, D.K., Song, X.H., Wang, J.B., Zhou, W.S., Ou, X.M., Chen, H.X., Liu, M.F., Wang, M.S., Jia, R.Y., Chen, S. & Chen, S. (2016). Outbreak of avian tuberculosis in commercial domestic Pekin ducks (Anas platyrhynchos domestica). Avian Diseases, 60, 677–680. doi: 10.1637/11396-021916-ResNote.1