ABSTRACT
Fowl adenoviruses (FAdV) are important infectious pathogens responsible for causing substantial economic losses to the poultry industry worldwide. One hundred and forty-six FAdV strains were continuously collected and analysed from 2013 to 2019 to understand the epidemiological change and nature of the virus in South Korea from two different standpoints, before and after the release of multiple commercial FAdV-4 vaccines. Phylogenetic analysis of the hexon loop-1 gene sequences showed that 92 strains belonged to FAdV-C (63%), 35 strains to FAdV-E (24%), 18 strains to FAdV-D (12.3%), and one strain to FAdV-A (0.7%), respectively. We provide evidence that the dominant FAdV serotype has recently changed from FAdV-4 to FAdV-8b, as reflected in the proportion of each serotype in field cases in 2019 (18.5% and 77.8%, respectively). The newly emerged FAdV-8b cluster was significantly noticeable compared to the old FAdV clusters, indicating that the development of a vaccine for FAdV-8b may be necessary. Overall, this new insight into FAdV prevalence provides a foundation for strategic control and the development of efficient vaccines against FAdV cases in chickens in South Korea.
RESEARCH HIGHLIGHTS
The dominant FAdV serotype in South Korea shifted from FAdV-4 to FAdV-8b in 2013–2019.
A new cluster of FAdV-8b has emerged in South Korea, indicating the development of new vaccines.
Introduction
Fowl adenoviruses (FAdV) are non-enveloped viruses containing a double-stranded DNA genome of 43–45 kb in size, and belonging to the genus Aviadenovirus, the family of Adenoviridae. The viruses are classified into five species (A to E) and 12 serotypes (1 to 8a and 8b to 11) (Hess, Citation2000; King et al., Citation2011). Among the structural proteins, the hexon is a major capsid protein exposed on the virus surface, having the loop-1 structure as subtype-specific antigenic determinants (Norrby, Citation1969). Therefore, the analysis of the hexon loop-1 gene allows for type inference, with wide application in routine diagnostics of FAdVs in the field (Raue & Hess, Citation1998). FAdV is widely prevalent in the poultry industry on a worldwide scale. This virus is capable of horizontal and vertical transmission (Yates & Fry, Citation1957; Hess, Citation2020) and infection by this virus is also associated with a wide range of diseases. Such diseases include hepatitis-hydropericardium syndrome (HHS) (Anjum et al., Citation1989), inclusion body hepatitis (IBH) (Helmboldt & Frazier, Citation1963), and gizzard erosion ulceration (GE) (Abe et al. Citation2001).
Comprehensive epidemiological studies based on genomic analysis have revealed that particular species or serotypes of FAdV are likely to be responsible for these pathological manifestations (Schachner et al., Citation2018). Various serotypes belonging to FAdV-D and FAdV-E are known to cause IBH, whereas some FAdV-4 strains from FAdV-C are responsible for inducing HHS, and FAdV-A strains are directly connected to GE (Hess, Citation2017). With the difference in their clinical manifestations, IBH, HHS, and GE case mortalities are likely to differ (Hess, Citation2020).
Subsequent to the first report on FAdV in South Korea (Kim et al., Citation2008), multiple types have been shown to be prevalent. The types reported as prevalent in South Korea were FAdV-1, FAdV-4, FAdV-8b, and FAdV-11 (Choi et al., Citation2012). Despite the development and application of multiple FAdV-4 vaccines, the prevalence of HHS and IBH did not show a significant decrease in the field during recent years. Moreover, the epidemiologic patterns regarding FAdV serotype distribution since the introduction of several commercial FAdV-4 vaccines in South Korea was so far unknown. In this study, we characterized FAdV strains isolated from clinical cases in South Korea between 2013 and 2019 in an attempt to investigate the current epidemiology of FAdV and to determine whether there was an epidemiologic shift in serotypes after introduction of the current FAdV-4 vaccines (Kim et al., Citation2010; Citation2014) were introduced.
Materials and methods
Sample collection
Among the field cases submitted to the Avian Disease Laboratory, College of Veterinary Medicine, Chungbuk National University between 2013 and 2019, cases involving breeders, broilers, layers, and native chickens that were suspected of FAdV, characterized by gross lesions such as haemorrhagic hepatitis, pericardial effusion, gizzard erosion, and pancreatitis were selected. The liver, pancreas, and gizzard tissues from necropsy samples were homogenized in phosphate-buffered saline (PBS) and centrifuged at 8000 × g for 10 min. The supernatants of tissue homogenates were collected, and polymerase chain reaction (PCR) was directly carried out from these homogenates for sequencing. Tissue samples were also collected for histological evaluation. The history of the selected cases was provided either directly by producers or through case application forms.
DNA extraction, PCR and sequencing
The viral DNA was extracted using the Patho Gene-spin DNA/RNA Extraction Kit (iNtRON Biotechnology Inc., Seongnam, South Korea) following the manufacturer's instructions. The 897 base pairs of the hexon gene containing the hexon loop-1 region were amplified using the Ex Taq DNA polymerase (Takara, Tokyo, Japan) and HxA 5′-CAARTTCAGRCAGACGGT-3′ and HxB 5′-TAGTGATGMCGSGACATCAT-3′ primers following PCR conditions described elsewhere (Meulemans et al., Citation2001). After electrophoresis at 135 V for 25 min, the PCR product was purified from 1.5% agarose gel using the Expin gel SV kit (GeneAll Biotechnology, Seoul, South Korea). The extracted DNA was sequenced by Cosmogentech (Seoul, South Korea).
Phylogenetic analysis
The partial hexon sequences of the FAdV isolates were aligned with homologous sequences using the CLC sequence 7 software (Qiagen, Germantown, MD, USA). A phylogenetic tree of the hexon gene loop-1 region was constructed using the maximum likelihood (ML) method by Mega version 10 (http://www.megasoftware.net). The FAdV strains were typed based on the phylogenetic analysis of the hexon gene by using reference strains from previous studies and GenBank (https://www.ncbi.nlm.nih.gov/genbank/) (Supplementary data, Table 1).
Statistical analysis
All diagnostic data were analysed in Excel and GraphPad Prism software (v.8.1; GraphPad Software, La Jolla, CA, USA).
Results
Post mortem examinations and epidemiological observations
FAdV cases were widespread at a national scale in South Korea, regardless of provinces (a). A total of 146 field cases submitted from 2013 to 2019, representing nine broiler breeder, 76 broiler, 43 layer, and 18 native chicken flocks, were identified as FAdV-positive by PCR and histopathology (b). It was shown that FAdV-4 could infect most chicken types, with the primary hosts centred on layers and broilers, while FAdV-8b and FAdV-11 mostly affected broilers. Cases of FAdV-8b and FAdV-11 in native chickens were not confirmed. For epidemiological observations, FAdV strains were isolated from cases from all eight provinces, with Gyeonggi province (36 cases) having the highest prevalence followed by Chonnam (28 cases) and Chungnam (27 cases). FAdV-4 was the most frequently isolated serotype (63%), followed by serotype 8b (24%) and serotype 11 (12.3%), respectively (c). Only a single serotype was shown to be primarily responsible for each case, which was either FAdV-4, FAdV-8b, FAdV-11, or FAdV-1. The mortality ranged from 0.5% to 4% at the time of case submission, usually 3–5 days after the outbreak when the mortality rate peaks, making it difficult to accurately pinpoint the time when the actual infection has occurred. Additionally, only a single case of GE caused by FAdV-1 was confirmed within the seven-year survey period. d shows the proportion of cases of co-infection of FAdV with other pathogens, with bacterial co-infections (E.coli: 27 cases, Staphylococcus spp: 4 cases, Salmonella spp: 4 cases, Mycoplasma synoviae: 1 case) making up 25% of all FAdV cases, followed by other viral diseases (4%) and coccidiosis (3%). Among the viruses associated with FAdV cases, only two cases of infectious bursal disease virus (IBDV) were detected. The co-infection of FAdVs with coccidiosis was only observed in native chicken farms. Regarding cases from 2013 to 2016, FAdV-4 was the dominant serotype with an average proportion of over 90%, which peaked at 95.2% in 2015 (). After introducing multiple commercial inactivated FAdV-4 vaccines, the proportion of FAdV-4 cases rapidly declined to as low as 18.5% in 2019. However, the prevalence of FAdV-8b and FAdV-11 showed a significant increase over the same timeframe, with FAdV-8b being the most frequently isolated serotype in 2019, making up 77.8% of total cases. The number of FAdV related clinical cases in 2016 and 2017 was lower than in other years, which was probably due to the massive depopulations of poultry that were implemented to prevent the spread of highly pathogenic avian influenza outbreaks during that period. Chickens infected with FAdV were grouped based on the age reported when the case was confirmed (). FAdV-4 cases occurred as early as one day old in broilers to late as 588 days old in layers, while most of the FAdV-8b and FAdV-11 cases occurred in young chickens, with broilers being infected at an average age of 17.4 and 16.9 days old, respectively. In cases of native chickens, the average age when FAdV-4 cases mainly occurred was 53.5 days old (from 35 to 89 days old), which was disparate with FAdV-4 cases in broilers (15.2 days old). Post mortem examinations demonstrated that the features of gross lesions and histological lesions were distinctive between serotypes, as described in previous reports. However, most of the IBH cases caused by FAdV-8b and FAdV-11 in 2018 and 2019 were associated with severe necrotic pancreatitis and intranuclear inclusion bodies (INIB) in young broilers less than 24 days old ().
Figure 1. Summary of clinical cases (n = 146) of fowl adenovirus (FAdV) in Korea between 2013 and 2019. (a) Geographical distribution of clinical cases of FAdV. (b) Clinical cases of FAdV associated with different types of chickens. (c) Number and proportion of clinical cases per FAdV serotype. (d) Number and proportion of co-infection cases of FAdVs associated with other pathogens. Number of bacterial co-infection cases: E.coli: 27, Staphylococcus spp: 4, Salmonella spp: 4, Mycoplasma synoviae: 1.
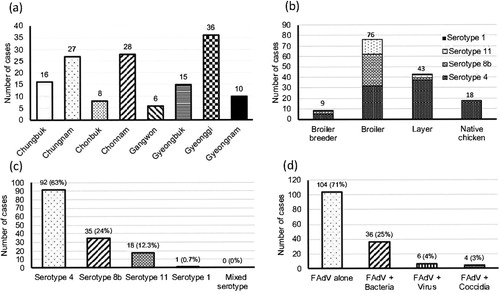
Figure 2. The annual proportion of clinical cases for each FAdV serotype from 2013 to 2019. Absolute numbers of cases are given in parentheses.
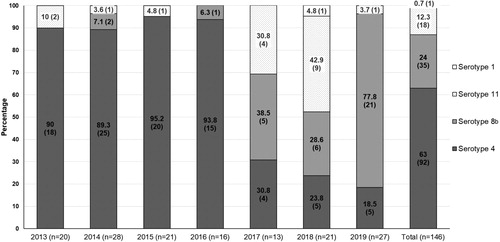
Figure 3. The age of chickens at the time when FAdV infection was confirmed. (a) FAdV-4, (b) FAdV-8b, (c) FAdV-11, (d) FAdV-1. Horizontal lines present the average age of infection.
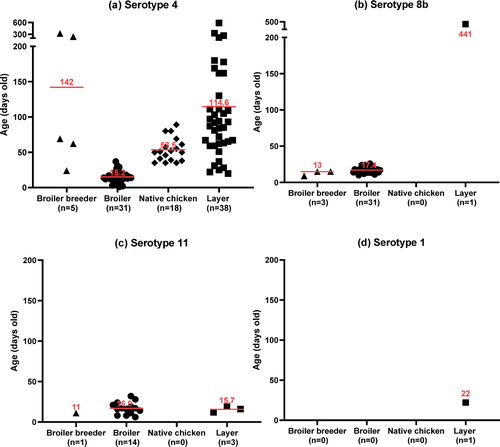
Figure 4. Post mortem and histological examinations of FAdV cases in South Korea. (a), (b), FAdV-8b: (a) Multifocal necrosis in the pancreas; (b) Large necrotic area associated with intranuclear inclusion bodies (INIB, arrow). (c), (d) FAdV-1: (c) Focal erosion and haemorrhage in the ventriculus; (d) Lymphocyte infiltration and INIB in the mucosal area (arrow). (e), (f) IBH: (e) Enlarged liver with multiple petechial haemorrhages; (f) Focal necrosis of hepatocytes associated with basophilic INIB (arrow).
Phylogenetic analysis
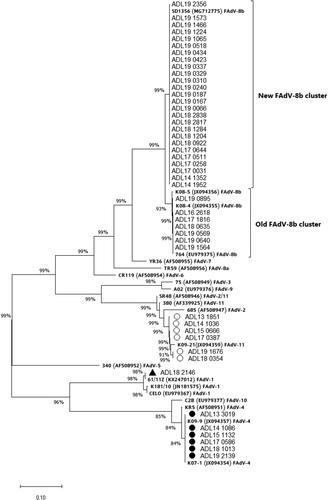
The phylogenetic analysis results based on the hexon loop-1 gene nucleotide sequence from FAdV isolates are presented in . The FAdV-4 and FAdV-11 strains isolated in this study shared similarities with the previously prevalent groups in South Korea. Compared with reference strains, the FAdV-4 group showed a 98.8% similarity with the KR5 strain, while the FAdV-11 group shared 97.2–97.4% similarity with the FAdV-11 380 strain. The isolated FAdV-8b strains were grouped into two separate clusters, the old cluster and a supposedly newly emerging cluster, which shared 99.8–100% and 97.3% homology with the 764 reference strain. The new FAdV-8b cluster consisted of strains that were being commonly isolated from more recent IBH outbreaks compared to the strains in the old cluster. One FAdV-1 strain shared a 98.9% similarity with the CELO strain, but it eventually became most similar to the 61/11z strain, originating from Poland. This strain is different from the South Korean strain K181/10 (98.7% homology).
Figure 5. Phylogenetic analysis of FAdV isolates based on the nucleotide sequences of the hexon loop-1 gene. The tree was constructed by the maximum likelihood (ML) method with Mega version 10. The strains sequenced for this study are designated by names starting with ADL. Reference strains are in bold text with accession numbers in parentheses. For the FAdV-4 and 11 groups, a strain isolated from each year (2013–2019) was selected and listed in the tree as a representative. Solid circles indicate representative strains for the isolated FAdV-4 serotypes. Representative strains for the isolated FAdV-11 serotypes are indicated by open circles. The only FAdV-1 strain (ADL18 2146) from this study is indicated by a solid triangle. The old and the newly formed clusters of FAdV-8b are shown on the right. Sequence data of the isolated FAdV strains were submitted to GenBank under the accession numbers MN737046-MN737093. SR48 (AF508949) is officially designated as FAdV-2; however, recent publications suggest a reclassification of this strain into FAdV-11 (Steer et al., Citation2011; Marek et al., Citation2016; Feichtner et al., Citation2018; Schachner et al., Citation2019).
Discussion
Epidemiological observations revealed that FAdVs are widespread in South Korean poultry farms, regardless of provinces, affecting various ages of susceptible poultry. Although FAdV-4 was the most common serotype, making up 63% of all confirmed cases, the proportion of FAdV-4 related cases decreased rapidly from 93.8% in 2016 down to 18.5% in 2019 after the introduction of multiple commercial inactivated FAdV-4 vaccines in late 2015 (Kim et al., Citation2010, Citation2014). The current analysis indicates that, after over a year, the vaccines have shown high efficacy in controlling the prevalence of FAdV-4 in the field by preventing transmission (). The temporal epidemiological pattern of FAdVs during recent years in South Korea revealed by this study was very interesting. While FAdV-4 was overwhelmingly prevalent during the first four years (2013–2016), after the rapid decline in FAdV-4 case numbers in 2017 due to vaccination programmes, FAdV-8b and FAdV-11 started to take over for the next three years (2017–2019). Interestingly, cases of FAdV-11 that were steadily increasing following the decline of FAdV-4 cases showed a sharp decrease in 2019, with FAdV-8b making up to nearly 80% of confirmed cases. It was obvious that, although the vaccines have worked effectively as intended against FAdV-4, they did not exhibit any cross-protectivity against FAdV-8b and 11, which was not surprising as cross-protection between different serotypes is unlikely to happen unless they are in the species of FAdV (Erny et al., Citation1995; Steer et al., Citation2011; Venne, Citation2013). Although a previous experimental study showed that inactivated FAdV-4 vaccines could cross-protect against both FAdV-8b and FAdV-11 (Kim et al., Citation2014), based on our study results this was not true in the field as outbreaks caused by these serotypes rapidly increased after implementing FAdV-4 vaccination programmes. Moreover, most IBH cases in 2019 were caused by FAdV-8b, two years after the FAdV-4 vaccines were introduced (). The drastic increase of FAdV-8b and FAdV-11 followed by the sharp drop of FAdV-4 cases further proves the lack of cross-protectivity of the current FAdV-4 vaccines. A study by Steer and colleagues confirmed FAdV-11 field infections in birds that were vaccinated with live FAdV-8b vaccine (Steer et al., Citation2011), and poor cross-protectivity between FAdV-8b and 11 was also reported in Canada (Venne, Citation2013), supporting the contrasting dynamics and lack of cross-protectivity between these two types in our study. A possible explanation behind the discrepancy between the rapidly declining FAdV-11 and soaring FAdV-8b case numbers in 2019 is that birds would likely have acquired immunity against FAdV-11 due to frequent natural exposure. It seemed apparent that the rapid reduction of FAdV-11 cases after a temporal delay of two years (2017–2018) was a manifestation of the protective effect conferred by naturally acquired immunity. The extent of natural spreading in flocks resulting in protective immunity may have varied between FAdV-8b and 11. Regarding naturally acquired immunity, parental birds that were infected would have needed to seroconvert and transmit protective maternal antibodies to progeny, which requires a tight timeframe of activity (Schachner et al., Citation2018). The existence of neutralizing antibodies in breeder flocks has been suggested to be accountable for preventing vertical transmission in some field cases (Philippe et al., Citation2007; Grafl et al., Citation2012). However, it seems evident that, even for birds carrying neutralizing antibodies for FAdV-11, it would not have helped reduce transmission of FAdV-8b. In fact, it is not uncommon to isolate multiple heterologous strains from a single bird harbouring high levels of neutralizing antibodies against a single serotype due to lack of cross-protectivity (Hess, Citation2020). In that aspect, regular monitoring of antibodies against specific serotypes in parental birds before the onset of egg production would likely help formulate protection strategies (Schachner et al., Citation2020).
An intriguing shift in prevalence patterns after introducing FAdV-4 vaccines at a national scale was previously seen in a region geographically close to South Korea. Since 2015, similar to South Korea, clinical cases associated with FAdV-4 have been increasing in China, affecting large-scale broiler farms causing substantial economic losses to the industry (Niu et al., Citation2016; Zhang et al., Citation2016). A similar epidemiological survey took place in China by collecting clinical cases of FAdV and typing strains to investigate the dominant serotypes from 2007 to 2017 (Wang et al., Citation2018). The study results showed that the cases of FAdV-11 decreased alongside FAdV-4 after clinical application of inactivated FAdV-4 vaccines, with FAdV-8b emerging as the dominant serotype two years later. These findings from the Chinese study suggest that FAdV-4 vaccines may have controlled not only FAdV-4 but also FAdV-11. This was not the case in our study as FAdV-11 was not reduced alongside FAdV-4 after broad administration of FAdV-4 vaccines, but rather increased after a year from 30.8% to 42.9% (). However, the rapid emergence of FAdV-8b as the dominant serotype after the reduction of FAdV-4 and FAdV-11 cases was something that our study and the Chinese study had in common. The phylogenetic tree from our study suggested the emergence of a new cluster consisting of FAdV-8b strains similar to the SD1356 strain from China (Huang et al., Citation2019) which was reported in 2014. Since then, the strains from the new cluster have been more frequently associated with IBH clinical cases than the strains from the old FAdV-8b cluster. The pathogenic properties of the strains belonging to this new cluster were shown to be similar to the SD1356 strain, which displayed broad tissue tropism in chickens without causing significant mortality (Huang et al., Citation2019). As it seemed clear that current vaccines could not protect against FAdV-8b (), developing specific vaccines should be seriously considered.
In conclusion, FAdV-4, FAdV-8b, and FAdV-11 were the most prevalent serotypes in South Korea associated with HHS and IBH from 2013 to 2019. The use of commercial inactivated FAdV-4 vaccines resulted in a shift from dominating FAdV-4 to FAdV-8b, with the new cluster emerging and spreading nationwide. This study will hopefully aid in developing future strategies to counter the newly emerging FAdV strains and to determine the next FAdV strain candidate for vaccine development in South Korea.
Supplemental Material
Download MS Word (26 KB)Supplemental Material
Download MS Word (25.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abe, T., Nakamura, K., Tojo, T. & Yuasa, N. (2001). Gizzard erosion in broiler chicks by group I avian adenovirus. Avian Diseases, 45, 234–239.
- Anjum, A., Sabri, M. & Iqbal, Z. (1989). Hydropericarditis syndrome in broiler chickens in Pakistan. Veterinary Record, 124, 247.
- Choi, K., Kye, S., Kim, J., Jeon, W., Lee, E., Park, K. & Sung, H. (2012). Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poultry Science, 91, 2502–2506.
- Erny, K., Pallister, J. & Sheppard, M. (1995). Immunological and molecular comparison of fowl adenovirus serotypes 4 and 10. Archives of Virology, 140, 491–501.
- Feichtner, F., Schachner, A., Berger, E. & Hess, M. (2018). Fiber-based fluorescent microsphere immunoassay (FMIA) as a novel multiplex serodiagnostic tool for simultaneous detection and differentiation of all clinically relevant fowl adenovirus (FAdV) serotypes. Journal of Immunological Methods, 458, 33–43.
- Grafl, B., Aigner, F., Liebhart, D., Marek, A., Prokofieva, I., Bachmeier, J. & Hess, M. (2012). Vertical transmission and clinical signs in broiler breeders and broilers experiencing adenoviral gizzard erosion. Avian Pathology, 41, 599–604.
- Helmboldt, C. & Frazier, M. (1963). Avian hepatic inclusion bodies of unknown significance. Avian Diseases, 7, 446–450.
- Hess, M. (2000). Detection and differentiation of avian adenoviruses: a review. Avian Pathology, 29, 195–206.
- Hess, M. (2017). Commensal or pathogen a challenge to fulfil Koch’s postulates. British Poultry Science, 58, 1–12.
- Hess, M., (2020). Aviadenovirus infections. In Diseases of poultry (14th ed., pp. 322–331). John Wiley & Sons, Inc.
- Huang, Q., Ma, X., Huang, X., Huang, Y., Yang, S., Zhang, L., Cui, N. & Xu, C. (2019). Pathogenicity and complete genome sequence of a fowl adenovirus serotype 8b isolate from China. Poultry Science, 98, 573–580.
- Kim, J., Kim, J. & Mo, I. (2010). Safety and efficacy of fowl adenovirus serotype-4 inactivated oil emulsion vaccine. Korean Journal of Poultry Science, 37, 255–263.
- Kim, J.N., Byun, S.H., Kim, M.J., Kim, J.J., Sung, H.W. & Mo, I.P. (2008). Outbreaks of hydropericardium syndrome and molecular characterization of Korean fowl adenoviral isolates. Avian Diseases, 52, 526–530.
- Kim, M.-S., Lim, T.H., Lee, D.H., Youn, H.N., Yuk, S.S., Kim, B.Y., Choi, S.W., Jung, C.H., Han, J.H. & Song, C.S. (2014). An inactivated oil-emulsion fowl adenovirus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl adenovirus. Vaccine, 32, 3564–3568.
- King, A.M., Lefkowitz, E., Adams, M.J. & Carstens, E.B. (2011). Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Elsevier.
- Marek, A., Kaján, G.L., Kosiol, C., Benkő, M., Schachner, A. & Hess, M. (2016). Genetic diversity of species fowl aviadenovirus D and fowl aviadenovirus E. Journal of General Virology, 97, 2323–2332.
- Meulemans, G., Boschmans, M., Van den Berg, T. & Decaesstecker, M. (2001). Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathology, 30, 655–660.
- Niu, Y.J., Sun, W., Zhang, G.H., Qu, Y.J., Wang, P.F., Sun, H.L., Xiao, Y.H. & Liu, S.D. (2016). Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. Journal of General Virology, 97, 2684–2690.
- Norrby, E. (1969). The structural and functional diversity of adenovirus capsid components. Journal of General Virology, 5, 221–236.
- Philippe, C., Grgiæ, H., Ojkiæ, D. & Nagy, É. (2007). Serologic monitoring of a broiler breeder flock previously affected by inclusion body hepatitis and testing of the progeny for vertical transmission of fowl adenoviruses. Canadian Journal of Veterinary Research, 71, 98.
- Raue, R. & Hess, M. (1998). Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differentiation of fowl adenoviruses and egg drop syndrome virus. Journal of Virological Methods, 73, 211–217.
- Schachner, A., Matos, M., Grafl, B. & Hess, M. (2018). Fowl adenovirus-induced diseases and strategies for their control – a review on the current global situation. Avian Pathology, 47, 111–126.
- Schachner, A., Gonzalez, G., Endler, L., Ito, K. & Hess, M. (2019). Fowl adenovirus (FAdV) recombination with intertypic crossovers in genomes of FAdV-D and FAdV-E, displaying hybrid serological phenotypes. Viruses, 11, 1094.
- Schachner, A., Grafl, B. & Hess, M. (2020). Spotlight on avian pathology: fowl adenovirus (FAdV) in chickens and beyond – an unresolved host-pathogen interplay. Avian Pathology, 50, 1–4.
- Steer, P., O'Rourke, D., Ghorashi, S. & Noormohammadi, A. (2011). Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Australian Veterinary Journal, 89, 184–192.
- Venne, D. (2013). Field data of the changing clinical picture over time of inclusion body hepatitis in Canada with an emphasis on diagnosis, prevention and trials on supportive treatments. In 24th Annual Australian Poultry Science Symposium (p. 222).
- Wang, J., Wang, S., Zou, K., Zhang, Y., Xu, S. & Yin, Y. (2018). Variant serotypes of fowl adenovirus isolated from commercial poultry between 2007 and 2017 in some regions of China. Avian Diseases, 62, 171–176.
- Yates, V.J. & Fry, D.E. (1957). Observations on a chicken embryo lethal orphan (CELO) virus. American Journal of Veterinary Research, 18, 657–660.
- Zhang, T., Jin, Q., Ding, P., Wang, Y., Chai, Y., Li, Y., Liu, X., Luo, J. & Zhang, G. (2016). Molecular epidemiology of hydropericardium syndrome outbreak-associated serotype 4 fowl adenovirus isolates in central China. Virology Journal, 13, 188.
