ABSTRACT
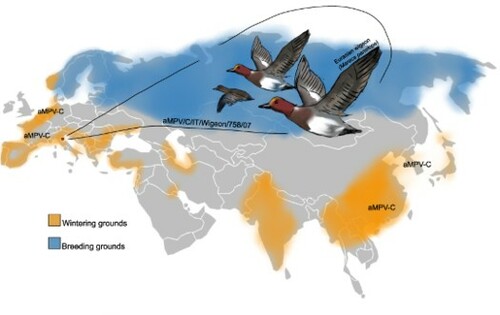
Avian metapneumovirus (aMPV) economically affects the global poultry industry causing respiratory and reproductive disorders. Considering the paucity of data on aMPV occurrence in European free-ranging avifauna, a molecular survey was conducted on wild birds of 23 species belonging to the orders Anseriformes, Charadriiformes or Passeriformes, captured alive and sampled in Northeast Italy as part of the national avian influenza virus (AIV) surveillance activities. A total of 492 oropharyngeal swabs, collected from 2007–2010, all AIV-negative, were screened from aMPV by subtype-specific qRT-PCR. An aMPV-C strain, named aMPV/C/IT/Wigeon/758/07, was found in a wintering young Eurasian wigeon (Mareca penelope) sampled in November 2007. The matrix, fusion, and attachment glycoprotein genes of the detected strain were subsequently amplified by specific independent RT-PCRs, then sequenced, and compared in a phylogenetic framework with known aMPV homologous sequences retrieved from GenBank. Close genetic relationships were found between the aMPV/C/IT/Wigeon/758/07 strain and subtype C Eurasian lineage strains isolated in the late 1990s in French domestic ducks, suggesting epidemiological links. Eurasian wigeons are medium/long-range migrant dabbling ducks that move along the Black Sea/Mediterranean flyway; our finding might, therefore, be related to migratory bridges between countries. To our knowledge, this is the first molecular evidence of the occurrence of aMPV subtype C in Italy and backdates the aMPV-C circulation to 2007. Moreover, the results suggest the susceptibility of Eurasian wigeons to aMPV. Broader investigations are needed to assess the role of wild ducks and the significance of the wildfowl/poultry interface in aMPV-C epidemiology.
Wild birds live-captured in Italy were tested for aMPV detection and characterization.
aMPV-C Eurasian lineage was found for the first time in a wintering Eurasian wigeon.
Migratory birds could be involved in the aMPV epidemiology.
RESEARCH HIGHLIGHTS
GRAPHICAL ABSTRACT
Introduction
The Metapneumovirus (MPV) genus belongs to the Pneumoviridae family, and includes non-segmented, single-stranded, negative-sense RNA viruses. To date, two MPV species are recognized: Avian metapneumovirus (aMPV) and Human metapneumovirus (hMPV) (Rima et al., Citation2017). Avian metapneumovirus is an emerging poultry pathogen mainly associated with respiratory and reproductive disorders in turkeys and chickens, resulting in severe economic losses to the domestic poultry industry.
To date, different aMPV subtypes have been recognized based on genetic and antigenic profiles (aMPV-A, -B, -C, -D) further showing diverse spatial distribution (Rautenschlein, Citation2020) and host range (Brown et al., Citation2019). aMPV-A and -B subtypes have been detected in Asia, Africa, Europe, and South America, whereas subtype D was only detected once in France (Bäyon-Auboyer et al., Citation2000). Subtype C, firstly reported in the US (Senne et al., Citation1997), genetically diverges from aMPV-A, -B and -D, and is closely related to hMPV, suggesting a possible common origin (Yunus et al., Citation2003). Two aMPV-C lineages have been recognized so far, according to the attachment glycoprotein (G) gene sequence: the North American lineage and the Eurasian lineage (Toquin et al., Citation2006). Moreover, two divergent aMPV strains have been recently detected in North America, one in a great black-backed gull (Larus marinus Linnaeus, 1758) (Canuti et al., Citation2019), and another in a monk parakeet chick (Myiopsitta monachus Boddaert 1783) (Retallack et al., Citation2019), tentatively increasing known aMPV subtypes to six.
In Italy, field studies revealed widespread circulation of aMPV in poultry mainly from densely populated poultry areas in the Northeastern part of the country. Even though the infection is mostly sustained by subtype B (Catelli et al., Citation2004; Listorti et al., Citation2014; Cecchinato et al., Citation2018; Tucciarone et al., Citation2018), aMPV-A has also been sporadically detected (Catelli et al, Citation2006; Lupini et al., Citation2011). Eventually, aMPV-C specific antibodies were demonstrated in intensively raised domestic mallards (Legnardi et al., Citation2021).
Since the first appearance of aMPV in South Africa in 1978 (Buys & du Preez, Citation1980) followed by an initial spread to Europe and Israel (Jones, Citation1996), migratory birds and respective flyways have been hypothetically linked to aMPV global transmission. Repeated detections of aMPV subtype C in free-living birds have been reported in North America (Shin et al., Citation2000; Bennett et al., Citation2002, Citation2004; Turpin et al., Citation2008; Cha et al., Citation2013; Jardine et al., Citation2018) and the periodic pattern of aMPV outbreaks in poultry has been linked to migratory movements (Shin et al., Citation2000). As regards to previous aMPV detections in European countries, aMPV has been detected in wild mallards (Anas platyrhynchos Linnaeus, 1758), greylag geese (Anser anser Linnaeus, 1758) and common gulls (Larus canus Linnaeus, 1758) in the Netherlands and characterized as subtype C according to partial polymerase gene (L) sequence analysis (van Boheemen et al., Citation2012). Serological (Catelli et al., Citation2001; Gethöffer et al., Citation2021) and molecular evidence (Curland et al., Citation2018) of aMPV in free-living pheasants (Phasianus colchicus Linnaeus, 1758) has been reported in Italy and Germany, without providing further indication regarding the subtype involved. Taken as a whole, all this evidence increasingly supports the possible role of wild birds as aMPV carriers or reservoir hosts.
To enlighten the status of migratory and resident free-living birds with respect to aMPV epidemiology, a molecular survey was performed in wild species sampled from 2007–2010 in northeast Italy for the avian influenza (AI) National Surveillance Plan.
Materials and methods
Ethical statement
All the samples were collected as part of Italy’s live wild bird avian influenza (AI) surveillance activities carried out by the Istituto Zooprofilattico delle Venezie, the Italian National Reference Centre and European Union Reference Laboratory for AI and Newcastle disease. Particularly, wild birds were captured alive during the authorized ringing activities conducted from 2007–2010 in accordance with the Italian Institute for Environmental Protection and Research (ISPRA – Higher Institute for Environmental Protection and Research). No supplementary permits or approvals were needed for sampling from wild birds captured alive for ringing activities; the sampling was conducted as part of the national avian influenza surveillance programme. All birds were handled in accordance with “Guidelines to the Use of Wild Birds in Research” (Fair et al., Citation2010).
Background and sample collection
Oropharyngeal swabs were obtained from 23 species of wild birds () sampled within the National Surveillance Plan for AI performed from spring to autumn 2007–2010. Swabs were individually collected and immersed in 1 ml of phosphate-buffered saline (PBS) with antibiotics and stored at −80°C until processing. Sample size was defined according to a previously published aMPV survey on wild birds (van Boheemen et al., Citation2012). Setting the average aMPV prevalence at 1%, a minimum of 299 samples was required to find at least one positive sample in an infinite population with a confidence of 95% (Cannon & Roe, Citation1982). A total number of 492 samples was eventually analyzed.
Table 1. Wild birds sampled from 2007 to 2010 during national avian influenza surveillance efforts in Northeastern Italian wetlands and tested for aMPV detection.
Of the birds studied, 53.9% (265/492) belonged to the order Anseriformes; 19.5% (96/492) to the order Charadriiformes, and 26.6% (131/492) to the order Passeriformes. According to age classes as defined by trained ornithologists, 27.8% (137/492) of the birds were adults, 40% (197/492) were juveniles (first calendar year), and 32.1% (158/492) were not categorized. The study area comprised wetlands of the Veneto and Emilia-Romagna regions located in the Po river delta, considered as strategic resting, wintering, and breeding sites for Anseriformes, Charadriiformes and Passeriformes species. All the samples screened for aMPV molecular detection tested negative for AI.
Sample processing and RNA extraction
For elution, each sample was centrifuged at 1500 × g for 5 min at 4°C and processed in pools of five. Samples were pooled, whenever possible, according to the species. Total RNA was extracted from each pool using a protocol based on a guanidine-thiocyanate method (Chomczynski & Sacchi, Citation1987).
aMPV detection and subtyping by qRT-PCR
A total of 99 pools was screened by qRT-PCR, with minor modifications of the method described by Lemaitre et al. (Citation2018), for aMPV detection and subtyping. The reaction was performed with SuperScript™ III Platinum™ SYBR™ Green One-Step (Invitrogen, Waltham, MA, USA) on a Lightcycler® 96 instrument (Roche, Basel, Switzerland), using the primer pair PanMPV/N1fwdA and PanMPV/N1RevB (Lemaitre et al., Citation2018) targeting the nucleoprotein (N) gene. For each reaction, 2 µl of extracted RNA were added to a standard reaction mix composed of 1X SYBR® Green Reaction Mix, 0.2 µl of SuperScript III RT/Platinum Taq Mix, and 0.6 µM of each primer. Molecular biology grade water was added up to a final volume of 10 µl. The thermal protocol was set as follows: 50°C for 3 min for the reverse transcription phase, 95°C for 5 min for the initial activation step, 45 cycles at 95°C for 15 s for the denaturation phase and 60°C for 30 s for annealing and extension. Melting curve analysis was performed by progressively increasing the temperature from 40°C to 90°C and continuously monitoring the fluorescence data. Tentative strain subtyping was performed by melting temperature (Tm) evaluation and comparison with reference strains.
Pools positive for aMPV were traced back to the original samples, which underwent individual RNA extraction and subsequent qRT-PCR analysis.
aMPV subtype C molecular characterization
For molecular characterization of the aMPV subtype C positive samples, a RT-PCR targeting partial matrix (M) viral gene was performed as previously described (Seal, Citation1998). Furthermore, additional primers were designed on a previously published aMPV-C complete sequence (Brown et al., Citation2014) targeting the fusion (F) and attachment glycoprotein (G) viral genes (). The F and G genes were amplified using one reverse transcription (RT) and three overlapping independent PCRs for each gene. RT reactions were performed using a OneScript® Plus cDNA Synthesis Kit according to manufacturer instructions (Applied Biological Materials Inc., Richmond, BC, Canada). Random primers (1 µM) were used for total RNA transcription. The reactions were performed at 50°C for 15 min followed by RT inactivation at 85°C for 5 min.
Table 2. Sequences of primers used for PCR amplifications of F and G genes of aMPV subtype C.
The following reaction mix was used in each PCR: 0.25 μl of GoTaq® DNA Polymerase (Promega, Madison, WI, USA), 10 pmol of the appropriate primer pair, 1 μl of dNTPs (Promega), 10 μl of PCR GoTaq® 5x reaction buffer, 3.5 μl MgCl2 (3 mM), 31.25 μl of nuclease-free water (Promega) and 3 μl of cDNA. The PCR cycling parameters for both F and G gene PCR assays were as follows: a pre-cycle step at 95°C for 2 min, followed by 35 cycles of 95°C for 30 s, 50°C for 40 s and 72°C for 65 s, followed by a final extension at 72°C for 5 min.
PCR products were purified using Wizard® SV Gel and PCR Clean-Up System (Promega). Sequencing was performed by a commercial sequencing service (Macrogen Europe, Amsterdam, the Netherlands).
Nucleotide sequences were edited and assembled using BioEdit Sequence Alignment Editor version 7.2 (Hall & Carlsbad, Citation2011), then aligned against and compared with previously published aMPV sequences available on NCBI GenBank database (Tables S1, S2 and S3 in Supplemental Material), using MAFFT version 7.397 online service which applied automatic detection of the parameter set (Katoh et al., Citation2002). Best partition scheme, substitution model selection according to Bayesian information criterion (BIC), and maximum likelihood phylogenetic reconstruction for M, F and G genes were performed separately on the IQ-TREE web server (Trifinopoulos et al. Citation2016; Kalyaanamoorthy et al., Citation2017). The robustness of inferred clades was evaluated using 1000 ultrafast bootstrap replicates. Branches with bootstrap values ≥ 70 were considered reliable. Pairwise genetic p-distance and between-group mean distance were estimated using MEGA software version 11.0.10 (Kumar et al. Citation2018).
Results
Among all the tested samples, a young male Eurasian wigeon (Mareca penelope Linnaeus, 1758) captured alive in November 2007 in the Po river delta area (Rovigo province, Veneto) was positive by qRT-PCR for aMPV subtype C (qRT-PCR cycle threshold value of the positive pooled sample: 35; qRT-PCR cycle threshold value of the positive individual sample: 33.5). The strain, named aMPV/C/IT/Wigeon/758/07 following the nomenclature reported by Mescolini et al. (Citation2021), was molecularly characterized. Partial M and F gene sequences of 400 and 1620 bases, respectively, and the complete sequence of the G gene (2160 bases) were obtained by RT-PCR and sequencing. Sequence data were submitted to the NCBI GenBank database under accession numbers OM021855, OM021856, and OM021857.
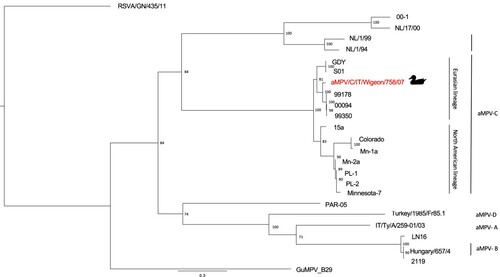
The phylogenetic tree constructed on the G gene sequence () demonstrated the clustering of the aMPV/C/IT/Wigeon/758/07 strain within the Eurasian lineage of aMPV-C, which includes French and Chinese subtype C field strains isolated from domestic duck flocks (Toquin et al., Citation1999, Citation2006; Sun et al., Citation2014). Specifically, the aMPV/C/IT/Wigeon/758/07 strain was closely related to a clade including strains isolated from Muscovy ducks (99178 and 99350 strains) and White Pekin ducks (00094 strain) in France (Toquin et al., Citation2006). Between-group mean distance, measured among the G gene sequence obtained in the present study and the French clade, was 0.042.
Figure 1. Phylogenetic tree based on G gene nucleotide sequences of aMPV/C/IT/Wigeon/758/07 strain detected in the study (marked by duck symbol), aMPV and hMPV reference strains obtained from NCBI GenBank database. Only bootstrap values ≥ 70 are shown. The G sequence of the human respiratory syncytial virus strain RSVA/GN/435/11 (accession number JX627336.1) was included and used as an outgroup. Sequence data are reported in Table S1 (Supplemental Material).
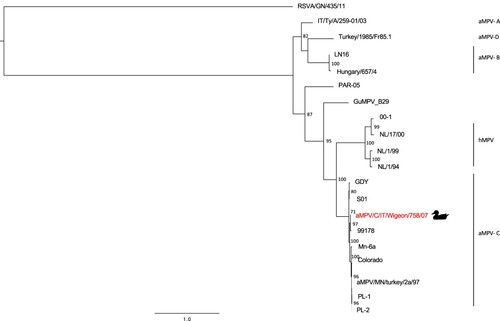
M gene and F gene phylogenetic trees ( and , respectively), confirming the results obtained with the G gene, showed a close relationship between the aMPV/C/IT/Wigeon/758/07 strain and the 99178 strain (Brown et al., Citation2014). Pairwise p-distance values obtained comparing the above-mentioned strains were 0.020 in M gene and 0.014 in F gene, respectively.
Figure 2. Phylogenetic tree based on partial M gene nucleotide sequences (from nucleotide 2514 to 2913 in the genome) of aMPV/C/IT/Wigeon/758/07 strain detected in the study (marked by duck symbol) and aMPV-C strains obtained from NCBI GenBank database. Only bootstrap values ≥ 70 are shown. The partial M sequence of the human respiratory syncytial virus strain RSVA/GN/435/11 (accession number JX627336.1) was included and used as an outgroup. Sequence data are reported in Table S2 (Supplemental Material).
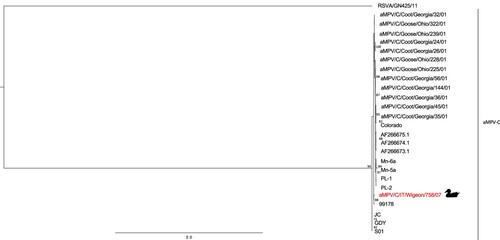
Figure 3. Phylogenetic tree based on partial F gene nucleotide sequences (from nucleotide 3319 to 4650 in the genome) of aMPV/C/IT/Wigeon/758/07 strain detected in the study (marked by duck symbol), aMPV and hMPV reference strains obtained from NCBI GenBank database. Only bootstrap values ≥ 70 are shown. The partial F sequence of the human respiratory syncytial virus strain RSVA/GN/435/11 (accession number JX627336.1) was included and used as an outgroup. Sequence data are reported in Table S3 (Supplemental Material).
Discussion
We herein report the detection of an aMPV subtype C in a juvenile Eurasian wigeon (M. penelope) wintering in northeast Italy. To our knowledge, this is the first molecular evidence of the occurrence of aMPV subtype C in Italy and it backdates the aMPV-C circulation to 2007. Moreover, our finding suggests, for the first time, the susceptibility to aMPV infection of Eurasian wigeons.
Close genetic relationships were found between the detected strain and strains of the subtype C Eurasian genetic lineage, particularly those isolated in the late 1990s in French domestic ducks. This might be related to migratory bridges between countries, along the Black Sea/Mediterranean flyway. Eurasian wigeons are considered to be medium- to long-range migrant dabbling ducks. Whilst major concentrations of wintering individuals are found in Northwestern Europe and Eastern Asia, remarkable numbers are also reported in France and other Mediterranean countries, including Italy (Atkinson et al., Citation2006). Furthermore, recovery of ringed birds showed a direct connection between Italian and French wetlands (Atkinson et al., Citation2007; Spina & Volponi, Citation2008). Diverse factors such as migratory movements, dense congregations of birds, and presence of immunologically naïve juveniles are considered relevant drivers in disease dynamics as already known for a major avian viral disease such as AI (Gaidet et al., Citation2010; van Dijk et al., Citation2014). Our finding suggests that these factors might also be relevant for the aMPV-C epidemiology. Considering that the geographical separation of wild hosts undertaking seasonal movements along different migration routes was linked to the initial diversification of AI viruses into Eurasian and American clades (Krauss et al., Citation2007), the presence of two distinct genetic lineages of aMPV-C could also be associated with migratory movements of wild hosts.
Recent serological findings of aMPV-C infection in Italian asymptomatic intensively raised domestic mallards (Legnardi et al., Citation2021) further underline the potential epidemiological role of ducks for aMPV-C circulation. Considering that the Eurasian lineage of aMPV-C is well adapted to domestic ducks, as demonstrated by in vivo experimental trials (Brown et al., Citation2019), and that domestic duck breeds were domesticated from wild mallards (Qu et al., Citation2009), it is plausible that wild duck species might act as carrier and reservoir hosts for this viral lineage. Our detection of aMPV-C in an additional wild anatid species further supports the latter hypothesis. However, given the paucity of aMPV-C Eurasian-origin strains found, the inferred relationship might be affected by a sampling bias and only partially represents the actual scenario. Thus, broader investigations are needed to assess the role of wild ducks and the significance of the wildfowl/poultry interface in aMPV-C epidemiology.
Eventually, evaluation of the biological features of aMPV strains circulating in wild species through in vitro and in vivo studies and facilitation of whole genome sequencing and viral isolation, not performed herein, would be essential.
Supplemental Material
Download MS Word (34.9 KB)Acknowledgements
The authors thank Professor Marco Martini (University of Padua, Department of Animal Medicine, Production and Health) for the statistical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Atkinson, P.W., Clark, J.A., Delany, S., Diagana, C.H., du Feu, C., Fiedler, W., Fransson, T., Gaulthier-Clerc, M., Grantham, M.J., Gschweng, M., Hagemeijer, W., Helmink, T., Johnson, A., Khomenko, S., Martakis, G., Overdijk, O., Robinson, R.A., Solokha, A., Spina, F., Sylla, S.I., Veen, J. & Visser, D. (2006). Urgent preliminary assessment of ornithological data relevant to the spread of Avian Influenza in Europe. Retrieved from https://ec.europa.eu/environment/nature/conservation/wildbirds/birdflue/docs/rep_spread_avian_influenza_report.pdf
- Atkinson, P.W., Robinson, R.A., Clark, J.A., Miyar, T., Downie, I.S., du Feu, C.R., Fiedler, W., Fransson, T., Grantham, M.J., Gschweng, M., Spina, F. & Crick, H.Q.P. (2007). Migratory movements of waterfowl: a web-based mapping tool. EURING report to the EU Commission. Retrieved from http://blx1.bto.org/ai-eu/
- Bäyon-Auboyer, M.H., Arnauld, C., Toquin, D. & Eterradossi, N. (2000). Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subtype. Journal of General Virology, 81, 2723–2733.
- Bennett, R.S., McComb, B., Shin, H.J., Njenga, M.K., Nagaraja, K.V. & Halvorson, D.A. (2002). Detection of avian pneumovirus in wild Canada (Branta canadensis) and blue-winged teal (Anas discors) geese. Avian Diseases, 46, 1025–1029.
- Bennett, R.S., Nezworski, J., Velayudhan, B.T., Nagaraja, K.V., Zeman, D.H., Dyer, N., Graham, T., Lauer, D.C., Njenga, M.K. & Halvorson, D.A. (2004). Evidence of avian pneumovirus spread beyond Minnesota among wild and domestic birds in central North America. Avian Diseases, 48, 902–908.
- Brown, P.A., Allée, C., Courtillon, C., Szerman, N., Lemaitre, E., Toquin, D., Mangart, J.M., Amelot, M. & Eterradossi, N. (2019). Host specificity of avian metapneumoviruses. Avian Pathology, 48, 311–318.
- Brown, P.A., Lemaitre, E., Briand, F.X., Courtillon, C., Guionie, O., Allée, C., Toquin, D., Bayon-Auboyer, M.-H., Jestin, V. & Eterradossi, N. (2014). Molecular comparisons of full length metapneumovirus (MPV) genomes, including newly determined French AMPV-C and -D isolates, further supports possible subclassification within the MPV genus. PLoS One, 9, e102740.
- Buys, S.B. & du Preez, J.H. (1980). A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempts to attenuate the virus. Turkeys, 36, 56.
- Cannon, R.M. & Roe, R.T. (1982). Livestock disease surveys. A field manual for veterinarians. Canberra: Australian Government Publishing Service.
- Canuti, M., Kroyer, A., Ojkic, D., Whitney, H.G., Robertson, G.J. & Lang, A.S. (2019). Discovery and characterization of novel RNA viruses in aquatic North American wild birds. Viruses, 11, 768.
- Catelli, E., Cecchinato, M., Delogu, M., De Matteo, P., Ortali, G., Franciosi, C., De Marco, M.A. & Naylor, C.J. (2004). Avian pneumovirus infection in turkey and broiler farms in Italy: a virological, molecular, and serological field survey. Italian Journal of Animal Science, 3, 287–292.
- Catelli, E., Cecchinato, M., Savage, C.E., Jones, R.C. & Naylor, C.J. (2006). Demonstration of loss of attenuation and extended field persistence of a live avian metapneumovirus vaccine. Vaccine, 24, 6476–6482.
- Catelli, E., De Marco, M.A., Delogu, M., Terregino, C. & Guberti, V. (2001). Serological evidence of avian pneumovirus infection in reared and free-living pheasants. Veterinary Record, 149, 56–57.
- Cecchinato, M., Lupini, C., Silveira, F., Listorti, V., Mescolini, G., Morandini, E., Franzo, G. & Catelli, E. (2018). Molecular characterization of avian metapneumovirus from guinea fowls (Numida meleagridis). Pakistan Veterinary Journal, 38, 419–423.
- Cha, R.M., Qingzhong, Y. & Zsak, L. (2013). The pathogenicity of avian metapneumovirus subtype C wild bird isolates in domestic turkeys. Virology Journal, 38, 1–8.
- Chomczynski, P. & Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry, 162, 156–159.
- Curland, N., Gethöffer, F., van Neer, A., Ziegler, L., Heffels-Redmann, U., Lierz, M., Baumgärtner, W., Wohlsein, P., Völker, I., Lapp, S., Bello, A., Pfankuche, V.M., Braune, S., Runge, M., Moss, A., Rautenschlein, S., Jung, A., Teske, L., Strube, C., Schulz, J., Bodewes, R., Osterhaus, A.D.M.E. & Siebert, U. (2018). Investigation into diseases in free-ranging ring-necked pheasants (Phasianus colchicus) in northwestern Germany during population decline with special reference to infectious pathogens. European Journal of Wildlife Resources, 64, 1–12.
- Fair, J., Paul, E. & Jones, J. (2010). Guidelines to the use of wild birds in research. Washington, DC: Ornithological Council. Retrieved from https://birdnet.org/info-for-ornithologists/guidelines-english-3rd-edition-2010/
- Gaidet, N., Cappelle, J., Takekawa, J.Y., Prosser, J., Iverson, S.A., Douglas, D.C., Perry, W.M., Mundkur, T. & Newman, S.H. (2010). Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large-scale satellite telemetry. Journal of Applied Ecology, 47, 1147–1157.
- Gethöffer, F., Curland, N., Voigt, U., Woelfing, B., Ludwig, T., Heffels-Redmann, U., Hafez, H.M., Lierz, M. & Siebert, U. (2021). Seroprevalences of specific antibodies against avian pathogens in free-ranging ring-necked pheasants (Phasianus colchicus) in Northwestern Germany. PLoS One, 16, e0255434.
- Hall, T. & Carlsbad, C. (2011). Bioedit: an important software for molecular biology. GERF Bulletin of Biosciences, 2, 60–61.
- Jardine, C.M., Parmley, E.J., Buchanan, T., Nituch, L. & Ojkic, D. (2018). Avian metapneumovirus subtype C in wild waterfowl in Ontario, Canada. Transboundary and Emerging Diseases, 65, 1098–1102.
- Jones, R.C. (1996). Avian pneumovirus infection: questions still unanswered. Avian Pathology, 25, 639–648.
- Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., von Haeseler, A. & Jermiin, L.S. (2017). Modelfinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589.
- Katoh, K., Misawa, K., Kuma, K. & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066.
- Krauss, S., Obert, C.A., Franks, J., Walker, D., Jones, K., Seiler, P., Niles, L., Pryor, S.P., Obenauer, J.C., Naeve, C.W., Widjaja, L., Webby, R.J. & Webster, R.G. (2007). Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathogens, 3, e167.
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018). MEGA x: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
- Legnardi, M., Allée, C., Franzo, G., Cecchinato, M. & Brown, P. (2021). Detection of avian metapneumovirus subtype C specific antibodies in a mallard flock in Italy. Poultry Science, 100, 101186.
- Lemaitre, E., Allée, C., Vabret, A., Eterradossi, N. & Brown, P.A. (2018). Single reaction, real time RT-PCR detection of all known avian and human metapneumoviruses. Journal of Virological Methods, 251, 61–68.
- Listorti, V., Lupini, C., Cecchinato, M., Pesente, P., Rossi, G., Giovanardi, D., Naylor, C.J. & Catelli, E. (2014). Rapid detection of subtype B avian metapneumoviruses using RT-PCR restriction endonuclease digestion indicates field circulation of vaccine-derived viruses in older turkeys. Avian Pathology, 43, 51–56.
- Lupini, C., Cecchinato, M., Ricchizzi, E., Naylor, C.J. & Catelli, E. (2011). A turkey rhinotracheitis outbreak caused by the environmental spread of a vaccine-derived avian metapneumovirus. Avian Pathology, 40, 525–530.
- Mescolini, G., Lupini, C., Franzo, G., Quaglia, G., Legnardi, M., Cecchinato, M., Tucciarone, C.M., Blanco, A., Turblin, V., Biarnés, M., Tatone, F., Falchieri, M. & Catelli, E. (2021). What is new on melecular characteristics of avian metapneumovirus strains circulating in Europe? Transboundary and Emerging Diseases, 68, 1314–1322.
- Qu, L., Liu, W., Yang, F., Hou, Z., Zheng, J., Xu, G. & Yang, N. (2009). Origin and domestication history of Peking ducks determined through microsatellite and mitochondrial marker analysis. Science in China. Series C, Life Sciences, 52, 1030–1035.
- Rautenschlein, S. (2020). Avian metapneumovirus. In D.E. Swayne, M. Boulianne, C.M. Logue, L.R. McDougald, V. Nair, D.L. Suarez, S. de Wit, T. Grimes, D. Johnson, M. Kromm, T.Y. Prajitno, I. Rubinoff & G. Zavala (Eds.), Diseases of poultry (pp. 135–143). Hoboken, NJ: Wiley Blackwell.
- Retallack, H., Clubb, S. & DeRisi, J.L. (2019). Genome sequence of a divergent avian metapneumovirus from a monk parakeet (Myiopsitta monachus). Microbiology Resource Announcements, 8, e00284–19.
- Rima, B., Collins, P., Easton, A., Fouchier, R., Kurath, G., Lamb, R.A., Lee, B., Maisner, A., Rota, P., Wang, L. & ICTV Report Consortium (2017). ICTV virus taxonomy profile: Pneumoviridae. Journal of General Virology, 98, 2912–2913.
- Seal, B.S. (1998). Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains. Virus Research, 58, 45–52.
- Senne, D., Edson, J.C. & Pederson, B.P. (1997). Avian pneumovirus update. In Proceedings of the 134th annual convention of the American Veterinary Medical Association (p. 190). Reno, Nevada.
- Shin, H.J., Njenga, M.K., McComb, B., Halvorson, D.A. & Nagaraja, K.V. (2000). Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys. Journal of Clinical Microbiology, 38, 4282–4284.
- Spina, F. & Volponi, S. (2008). Atlante della migrazione degli uccelli in italia: Non-passeriformi. Roma: Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA).
- Sun, S., Chen, F., Cao, S., Liu, J., Lei, W., Li, G., Song, Y., Lu, J., Liu, C., Qin, J. & Li, H. (2014). Isolation and characterization of a subtype C avian metapneumovirus circulating in Muscovy ducks in China. Veterinary Research, 45, 1–13.
- Toquin, D., Bäyon-Auboyer, M.H., Eterradossi, N., Jestin, V. & Morin, H. (1999). Isolation of a pneumovirus from a Muscovy duck. The Veterinary Record, 145, 680.
- Toquin, D., Guionie, O., Jestin, V., Zwingelstein, F., Allee, C. & Eterradossi, N. (2006). European and American subtype C isolates of avian metapneumovirus belong to different genetic lineages. Virus Genes, 32, 97–103.
- Trifinopoulos, J., Nguyen, L.T., von Haeseler, A. & Minh, B.Q. (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research, 44.
- Tucciarone, C.M., Franzo, G., Lupini, C., Torres Alejo, C., Listorti, V., Mescolini, G., Branão, P.E., Martini, M., Catelli, E. & Cecchinato, M. (2018). Avian metapneumovirus circulation in Italian broiler farms. Poultry Science, 97, 503–509.
- Turpin, E.A., Stallknecht, D.E., Slemons, R.D., Zsak, L. & Swayne, D.E. (2008). Evidence of avian metapneumovirus subtype C infection of wild birds in Georgia, South Carolina, Arkansas and Ohio, USA. Avian Pathology, 37, 343–351.
- van Boheemen, S., Bestebroer, T.M., Verhagen, J.H., Osterhaus, A.D.M.E., Pas, S.D., Herfst, S. & Fouchier, R.A. (2012). A family-wide RT-PCR assay for detection of paramyxoviruses and application to a large-scale surveillance study. PLoS One, 7, 1–9.
- van Dijk, J.G., Hoye, B.J., Verhagen, J.H., Nolet, B.A., Fouchier, R.A. & Klaassen, M. (2014). Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. Journal of Animal Ecology, 83, 266–275.
- Yunus, A.S., Govindarajan, D., Huang, Z. & Samal, S.K. (2003). Deduced amino acid sequence of the small hydrophobic protein of US avian pneumovirus has greater identity with that of human metapneumovirus than those of non-US avian pneumoviruses. Virus Research, 93, 91–97.
