ABSTRACT
Mycoplasma gallisepticum is the primary causative agent of chronic respiratory disease in poultry, and vaccination is the measure most commonly used for its control. Pathological changes caused by M. gallisepticum are mainly observed in the trachea and air sacs, but assessment of air sac lesions is subjective. Standardized parameters for evaluation of pathological changes, and their reproducibility and discrimination in uninfected and infected groups, are critical when assessing the efficacy of M. gallisepticum vaccination. This study reviewed and critically appraised the published literature on evaluation of vaccine efficacy against pathological changes caused by M. gallisepticum in poultry in the trachea and air sacs. A search of four electronic databases, with subsequent manual filtering, identified 23 eligible papers published since 1962 describing the assessment of histopathological changes in the trachea using tracheal lesion scores and/or measurement of tracheal mucosal thicknesses and assessment of gross air sac lesions using lesion scores. Measurement of tracheal lesions proved a more reliable and robust method of assessing disease induced by M. gallisepticum when compared to assessment of air sac lesions, highlighting the importance of including assessment of tracheal lesions as the primary outcome variable in vaccine efficacy studies. In addition, this study also identified the necessity for use of a standardized model for evaluation and reporting on M. gallisepticum vaccines to minimize variations between vaccine efficacy studies and to allow direct comparisons between them.
RESEARCH HIGHLIGHTS
Tracheal and air sac lesions have been used to assess M. gallisepticum vaccine efficacy.
The specific parameters and statistical tests used to compare tracheal and air sac lesions vary greatly.
Measures of tracheal lesions are more discriminatory than measures of air sac lesions.
A standardized model is needed to evaluate vaccines against infection with M. gallisepticum.
Introduction
Mycoplasma gallisepticum is among the most important bacterial pathogens of poultry. It attaches to and replicates on the respiratory epithelial cells of the host, resulting in chronic inflammation in a range of respiratory tissues, including the trachea and air sacs (Nunoya et al., Citation1987; Wijesurendra et al., Citation2015). While M. gallisepticum can cause chronic respiratory disease in chickens as a sole pathogen (Whithear, Citation1993), in the field it is often a multifactorial disease. In this environment, M. gallisepticum is the primary aetiological agent but other pathogens, including Escherichia coli, Newcastle disease virus and infectious bronchitis virus exacerbate the course of disease (Ley, Citation2008).
Control of M. gallisepticum in commercial poultry production systems most commonly relies on routine vaccination using inactivated or live-attenuated vaccines (Kleven, Citation2008). The live-attenuated vaccines currently available in the global market, F strain, 6/85 and ts-11, are more effective than inactivated vaccines (Whithear, Citation1996), but each of these vaccines has limitations (Luginbuhl et al., Citation1967; Rodriguez & Kleven, Citation1980; Lin & Kleven, Citation1982; Abd-El-Motelib & Kleven, Citation1993; Noormohammadi, Browning et al., Citation2002; Noormohammadi, Jones et al., Citation2002). F strain has low to moderate virulence and transmissibility in broilers (Rodriguez & Kleven, Citation1980) and is virulent in turkeys (Lin & Kleven, Citation1982), 6/85 does not always induce a detectable serological response (Evans & Hafez, Citation1992; Abd-El-Motelib & Kleven, Citation1993) and has not been assessed in turkeys, and ts-11 is ineffective in turkeys and the serological response in chickens vaccinated with ts-11 can be highly variable (Noormohammadi, Browning et al., Citation2002; Noormohammadi, Jones et al., Citation2002).
Effective, consistent and reproducible methods to assess pathological changes seen after challenge are critical for assessment and comparison of vaccine efficacy against any pathogen, including M. gallisepticum. The absence of standardized parameters for measuring the severity of disease, and the protection afforded by a vaccine, makes assessment of the efficacy of vaccine candidates problematic. The parameters that have been used in published papers to evaluate experimental infections with virulent M. gallisepticum in poultry include evaluation of the prevalence and load of M. gallisepticum in the respiratory tract (Moura et al., Citation2012; Sayed et al., Citation2018), the histopathological changes in the trachea (Nunoya et al., Citation1987), the gross pathological changes in the air sacs (Ferguson-Noel, Laibinis et al., Citation2012), clinical signs (Bíró et al., Citation2005), the extent of horizontal and vertical transmission (Barbour & Newman, Citation1990; Feberwee et al., Citation2006), the prevalence of serum antibodies against M. gallisepticum (Kanci et al., Citation2018), egg production and hatchability (Domermuth, Citation1962; Evans et al., Citation2007), and weight gain (Bíró et al., Citation2005). Some of these parameters are not suitable for all situations – for example, egg production and hatchability are age-dependent and applicable only in layers and broiler breeders (Glisson & Kleven, Citation1984) – and some strains of M. gallisepticum do not induce a strong serum antibody response in the host (Rodriguez & Kleven, Citation1980). Evaluation of histological changes in the trachea and gross changes in the air sacs are relatively convenient parameters to assess in most situations where efficacy against experimental infection is to be assessed (Nunoya et al., Citation1987). Although the consistency and the reproducibility of parameters for assessing vaccine efficacy have been reviewed previously for several other pathogens of poultry, including Eimeria species (Chapman et al., Citation2005; Soutter et al., Citation2020), avian influenza viruses (Swayne, Citation2009; Villanueva-Cabezas et al., Citation2017), infectious laryngotracheitis virus (Coppo et al., Citation2013) and Campylobacter jejuni (Pumtang-on et al., Citation2021), this has not been done for methods for assessing respiratory tract lesions caused by infection with M. gallisepticum.
This study systematically reviewed and critically appraised the designs of M. gallisepticum vaccination-challenge studies and the parameters used to evaluate the pathological changes in the trachea and air sacs. The aim was to describe and evaluate the vaccination and challenge strategies employed in terms of routes of vaccination and infection, age at vaccination, time between vaccination and challenge, and time between challenge and necropsy, and to evaluate the characteristics of the parameters used to assess the lesions in the trachea and air sacs, with a view to providing recommendations for future M. gallisepticum vaccination-challenge studies.
Materials and methods
Search strategy
A search strategy was initially developed in MEDLINE after identifying keywords and medical subject headings (MeSH). Peer-reviewed papers were retrieved by searching four electronic databases (Web of Science [all databases], CAB Direct, MEDLINE and Scopus). The search terms and Boolean operators included in the search strategy were (“Mycoplasma gallisepticum”) AND (“vaccine” OR “vaccination”) AND (“protection”) (). The resulting citations from all databases were exported to a reference manager (EndNote X8; Clarivate Analytics) and duplicate records were removed. The papers included were published between 1962 and August 2021.
Table 1. Comprehensive search strategy used for identifying papers.
All papers were screened based on their title and abstract. To be included, papers must have investigated the efficacy of at least one vaccine against M. gallisepticum by vaccination followed by infection with virulent M. gallisepticum. Review papers, opinions, editorials, newsletters, conference abstracts or proceedings, theses, dissertations, and books or book chapters were excluded. The papers for which the full text or an English translation were not available were also excluded. One paper was identified by a manual search of references of eligible papers. The selected papers were subjected to a full-text review to identify those describing an evaluation of pathological changes in both the trachea and the air sacs after infection with virulent M. gallisepticum in vaccinated and unvaccinated birds.
Data extraction and analyses
The following data were extracted and recorded in a Microsoft Excel spreadsheet: host, source of birds, group size, age of birds at vaccination, type of vaccine (live-attenuated, inactivated, or recombinant vaccine), route of vaccination, time between vaccination and experimental challenge, route of challenge, time between challenge and necropsy, inclusion of negative-control (unvaccinated-unchallenged) and/or positive-control (unvaccinated-challenged) groups, and parameters used to evaluate lesion/s in the trachea and air sacs. Any disparities were discussed among the authors before reaching consensus. Out of these papers, a subset of papers that fulfilled the following two criteria–(1) inclusion of two control groups (negative-control and positive- control) and (2) delivery of the challenge strain via a respiratory route – was generated. The data extracted from this subset of papers were: the parameters and statistical tests used to compare the lesions in the trachea and air sacs between groups; and whether a significant difference in those parameters was detected between the two control groups or not. A Fisher’s exact test was applied, and an odds ratio was calculated to compare differences between the proportion of trials that detected a significant difference in tracheal lesions and the proportion that detected a significant difference in air sac lesions between the two control groups using GraphPad Prism. A P-value of <0.05 was considered significant.
Results
Literature search and eligible papers
In total, 333 papers were identified by the search of the four databases. After removal of duplicates (n = 179 papers), 154 papers were screened based on their title and abstract, and 87 papers were excluded based on the inclusion and exclusion criteria, leaving 66 papers (Figure S1, online supplemental data). The full-text screening of these papers and another identified by manual search (n = 67 papers) excluded a further 44 papers. Of these, 18/44 did not evaluate lesions in the trachea or the air sacs, 5/44 papers evaluated lesions only in the trachea and 21/44 papers evaluated lesions only in the air sacs. Hence, data were extracted from 23 eligible papers that described evaluation of lesions in both the trachea and air sacs. These papers were published between 1992 and August 2021.
General characteristics of studies included in the review
The majority of the studies were undertaken in Australia (10/23 studies), followed by the USA (7/23 studies), Thailand (4/23 studies), Japan (1/23 studies) and Hungary (1/23 studies). Of these studies, 11/23 included only specific pathogen-free (SPF) chickens, 10/23 included only commercial chickens (8/10 on layers, 1/10 on broiler breeders, and 1/10 on both layers and broilers), 1/23 included only commercial turkeys (Kanci et al., Citation2018) and 1/23 included both commercial chickens and turkeys (Ferguson et al., Citation2004). There were multiple trials in most of the papers, yielding 48 trials investigating the efficacy of vaccines against M. gallisepticum. Multiple trials included in each paper differed in the host species (chicken or turkey), the type of chickens (layers, breeders or broilers), the commercial line/breed, the age at vaccination, the time between vaccination and challenge, or the time between challenge and necropsy. These 48 vaccination-and-challenge trials were analysed in detail (Dataset S1).
Measures of pathological changes in the trachea
Infection with M. gallisepticum causes inflammation in the trachea and air sacs in poultry. Histological lesions are more apparent than the gross lesions in the trachea and include increased tracheal mucosal thickness due to leukocyte infiltration, loss of cilia, epithelial cell degeneration, mucosal oedema, squamous cell metaplasia and luminal exudation (Nunoya et al., Citation1987). The methods used in each paper to measure the histological lesions in the trachea are outlined in .
Table 2. Methods and parameters reported for the evaluation of histological lesions in the trachea and gross lesions in the air sacs.
Tracheal mucosal thicknesses
Tracheal mucosal thicknesses were measured in 32/48 trials and all these trials reported the mean tracheal mucosal thickness values of each group of birds (). Measurement of the same parameter in the majority of the trials facilitates comparisons of the different vaccination trials. However, there were variations in the anatomical level of trachea used to measure the tracheal mucosal thicknesses. A number of trials reporting tracheal mucosal thicknesses (14/32) measured tracheal mucosal thicknesses at three levels of the trachea (upper, middle and lower) and compared the thicknesses at each level separately (Shil et al., Citation2011; Tseng et al., Citation2017; Kanci et al., Citation2018; Noormohammadi & Whithear, Citation2019; Kanci Condello, Kulappu Arachchige, et al., Citation2020; Kanci Condello, Underwood, et al., Citation2020; Kulappu Arachchige et al., Citation2021) or compared only the average of the three measurements at the three different levels (Noormohammadi et al., 2002; Gates et al., Citation2008; Leigh et al., Citation2012) between the groups. A smaller proportion (10/32 trials) calculated the average of the mucosal thicknesses of the upper and lower trachea (Gaunson et al., Citation2006a, Citationb), 7/32 trials measured only the thickness of the upper trachea (Ferguson et al., Citation2004; Raviv et al., Citation2008; Ferguson-Noel, Cookson, et al., Citation2012; Ferguson-Noel, Laibinis, et al., Citation2012; Ferguson-Noel & Williams, Citation2015), and 1/32 trials did not explicitly indicate which level of the trachea was measured (Bíró et al., Citation2005). There were also variations in the statistical tests applied to compare the mean tracheal mucosal thicknesses between the trials. Of the 32 trials that measured the mean tracheal mucosal thicknesses, 31 applied parametric statistical tests, including Student’s t-test (Noormohammadi et al., 2002; Gaunson et al., Citation2006a, Citationb; Noormohammadi & Whithear, Citation2019), a one-way analysis of variance (ANOVA) (Shil et al., Citation2011; Leigh et al., Citation2012; Tseng et al., Citation2017; Kanci et al., Citation2018; Kanci Condello, Kulappu Arachchige, et al., Citation2020; Kanci Condello, Underwood, et al., Citation2020; Kulappu Arachchige et al., Citation2021), Tukey’s pair-wise comparison test (Bíró et al., Citation2005) or the Tukey–Kramer highly significant difference (HSD) test (Ferguson et al., Citation2004; Raviv et al., Citation2008; Ferguson-Noel, Cookson et al., Citation2012; Ferguson-Noel, Laibinis et al., Citation2012; Ferguson-Noel & Williams, Citation2015). Only in one trial was a nonparametric statistical test, the Kruskal–Wallis test, used (Gates et al., Citation2008).
Tracheal lesion scores
Tracheal lesions were scored in 29/48 trials and all of them used a scale of 0-3, based on the prevalence and severity of the lesions. Some of these trials (13/29 trials) also measured tracheal mucosal thicknesses (Noormohammadi et al., 2002; Gaunson et al., Citation2006b; Gates et al., Citation2008; Shil et al., Citation2011; Leigh et al., Citation2012; Tseng et al., Citation2017; Kanci et al., Citation2018), and some (5/29 trials) also reported the proportion of birds with tracheal lesions (Yagihashi et al., Citation1992; Leigh et al., Citation2012) (). The use of the same scale to score the lesions across all the trials enables comparisons between studies, but, of the 29 trials scoring tracheal lesions, 18/29 trials reported mean scores, while the rest (11/29) reported median scores, making direct comparisons more problematic. There was also variation in the anatomical levels of the tracheas that were used for lesion scoring. A number of the trials (12/29) scored lesions at four levels (upper, two middle levels and lower) of the trachea (Pakpinyo et al., Citation2013; Pakpinyo et al., Citation2015; Limsatanun et al., Citation2016, Citation2018), while 7/29 trials scored lesions at three levels (upper, middle and lower) (Noormohammadi et al., 2002; Gates et al., Citation2008; Shil et al., Citation2011; Leigh et al., Citation2012; Tseng et al., Citation2017; Kanci et al., Citation2018), 6/29 trials scored lesions at two levels (upper and lower) (Gaunson et al., Citation2006b), and 4/29 trials (Yagihashi et al., Citation1992) did not specify the level of the trachea that was scored. Of these 29 trials, three performed comparisons of the lesions at the different levels of the trachea separately (Shil et al., Citation2011; Tseng et al., Citation2017; Kanci et al., Citation2018), while the rest of the trials calculated the average of the scores at the different levels for individual birds. One of the trials comparing tracheal lesion scores used a parametric test (ANOVA) to assess differences between the scores for the groups (Leigh et al., Citation2012). While some of the remaining trials reported mean lesion scores in the results, they used nonparametric statistical tests, including Mann–Whitney and/or Kruskal–Wallis tests, to compare the lesion scores.
Measures of pathological changes in the air sacs
Unlike tracheal lesions, air sac lesions are apparent grossly as well as histologically, but scoring of gross lesions has been preferred over assessment of histological lesions in the air sacs after infection with M. gallisepticum (Nunoya et al., Citation1987). The gross lesions seen in the air sacs range from a cloudy appearance due to thickening of the air sac membrane, to air sacs with a few to multiple yellowish foci, to diffuse yellowish thickening with caseous exudates and a partially to completely meaty consistency (Kleven et al., Citation1972; Nunoya et al., Citation1987; Kulappu Arachchige et al., Citation2021). Air sac lesion scores were reported in 46/48 trials as either a mean or a median score, and some of these trials also reported the proportion of birds with air sac lesions (). A small proportion of the trials (2/48) reported only the proportion of birds with air sac lesions (Bíró et al., Citation2005; Raviv et al., Citation2008). Two scoring scales have been used to score the gross lesions in the air sacs, one with a range from 0–3 and the other with a range from 0–4, with 35/48 trials either explicitly stating that lesions were scored on a scale of 0-4 or referring to a previous paper describing the 0-4 scoring system. The remaining 13/48 trials (Yagihashi et al., Citation1992; Noormohammadi et al., 2002; Gates et al., Citation2008; Shil et al., Citation2011; Leigh et al., Citation2012; Tseng et al., Citation2017; Kanci et al., Citation2018; Noormohammadi & Whithear, Citation2019) either explicitly stated that the lesions were scored on a scale of 0-3 or referred to a previous paper describing the 0-3 scoring system. The specific air sacs that were scored also varied between the trials. Lesions in the thoracic and abdominal air sacs were scored in 18/48 trials, only those in the thoracic air sacs were scored in 12/48 trials (Pakpinyo et al., Citation2013, Citation2015; Limsatanun et al., Citation2016, Citation2018), and lesions in the thoracic air sacs and the peritoneum were scored in 1/48 trials (Bíró et al., Citation2005), while in 17/48 trials the air sacs that were scored were not indicated (Ferguson et al., Citation2004; Gaunson et al., Citation2006a, Citationb; Raviv et al., Citation2008; Ferguson-Noel, Cookson et al., Citation2012; Ferguson-Noel, Laibinis et al., Citation2012; Ferguson-Noel & Williams, Citation2015). Most of the trials (45/48 trials) applied nonparametric statistical tests, including the Mann–Whitney and/or the Kruskal–Wallis tests, to compare the lesion scores between the groups. One trial used a parametric statistical test (Leigh et al., Citation2012), one used a multivariate statistical method (Bíró et al., Citation2005) and another compared proportions of birds with air sac lesions using Fisher’s exact test (Tseng et al., Citation2017).
Level of discrimination in measures of lesions in the trachea and air sacs between the positive and negative control groups
Of the 23 eligible papers included in this review, only 17/23 included negative-control and positive-control groups (Dataset S2), while the rest of the papers (6/23 studies) did not have a negative-control group, but did have a positive-control group (Yagihashi et al., Citation1992; Noormohammadi et al., 2002; Gaunson et al., Citation2006a, Citationb; Ferguson-Noel, Laibinis et al., Citation2012; Kanci Condello, Underwood, et al., Citation2020). These 17 papers included a total of 30 different vaccination-challenge trials. Of these 30, five trials compared both tracheal mucosal thicknesses and tracheal lesion scores between the groups and a significant difference was detected between the negative-control group and the positive-control group in all of them (Gates et al., Citation2008; Shil et al., Citation2011; Tseng et al., Citation2017; Kanci et al., Citation2018) except one (Leigh et al., Citation2012). All the trials (13/30) that compared only the tracheal mucosal thicknesses (Ferguson et al., Citation2004; Bíró et al., Citation2005; Raviv et al., Citation2008; Ferguson-Noel, Cookson et al., Citation2012; Ferguson-Noel & Williams, Citation2015; Noormohammadi & Whithear, Citation2019; Kanci Condello, Kulappu Arachchige, et al., Citation2020; Kulappu Arachchige et al., Citation2021) and all the trials (12/30) that compared only the tracheal lesion scores (Pakpinyo et al., Citation2013, Citation2015; Limsatanun et al., Citation2016, Citation2018) detected a significant difference between the negative-control group and the positive-control group (). Similarly, there was a significant difference in the air sac lesion scores between the negative-control group and the positive-control group in 17/30 trials (). However, there was no significant difference detected in air sac lesion scores for the negative-control group and the positive-control group in 12/30 trials (Pakpinyo et al., Citation2013, Citation2015; Limsatanun et al., Citation2016, Citation2018; Tseng et al., Citation2017; Kulappu Arachchige et al., Citation2021) (). A single trial did not report the results of statistical comparisons of the air sac lesion scores for the control groups (Bíró et al., Citation2005).
Table 3. Proportion of trials that detected significant differences between the negative-control group and positive-control group in measurements of tracheal lesions and air sac lesions.
There was a highly significant difference (P = 0.0004) between the proportion of trials (29/30 trials) that detected a significant difference in measures of tracheal lesions compared to the proportion of trials (17/29 trials) that detected a significant difference in air sac lesion scores between the negative-control group and the positive-control group. The odds of detection of a significant difference in tracheal lesions between the negative-control group and the positive-control group was 20.47 times the odds of detection of a significant difference in air sac lesions between the negative-control group and the positive-control group.
Variations among vaccination-challenge experiments
Types of vaccines
The vaccines assessed in the vaccination-and-challenge trials included different live-attenuated vaccines, inactivated M. gallisepticum bacterins and a recombinant fowl poxvirus-M. gallisepticum (rFPV-MG) vaccine (). The majority of the trials (33/48 trials) used live-attenuated vaccines, including the three commercial M. gallisepticum vaccine strains (F-strain, 6/85 and ts-11), and novel vaccine candidates. These trials either assessed the efficacy of a single strain (22/33 trials), compared the efficacy of a novel vaccine candidate to that of a commercial strain (5/33 trials) (Ferguson et al., Citation2004; Shil et al., Citation2011; Ferguson-Noel & Williams, Citation2015; Kanci Condello, Underwood, et al., Citation2020), compared the efficacies of two commercial vaccine strains (2/33 trials) (Noormohammadi & Whithear, Citation2019), compared efficacies of the three commercial vaccine strains (2/33 trials) (Pakpinyo et al., Citation2013), or compared the efficacies of novel vaccine candidates to each other (Ferguson-Noel, Laibinis et al., Citation2012). The efficacies of inactivated M. gallisepticum bacterins were investigated in 11/48 trials, while 3/48 trials compared the efficacies of a live-attenuated vaccine and a rFPV-MG vaccine (Pakpinyo et al., Citation2015) and one trial compared the efficacies of a bacterin, a live-attenuated vaccine and a rFPV-MG vaccine (Ferguson-Noel, Cookson et al., Citation2012).
Figure 1. Numbers of trials and the routes used to deliver each different Mycoplasma gallisepticum vaccine. Eleven different live-attenuated strains, 1 recombinant fowl pox virus – M. gallisepticum vaccine (rFPV-MG) and various inactivated M. gallisepticum bacterins were assessed, with the different vaccines delivered by eye-drop, aerosol, intratracheal instillation, or intravenous or intramuscular injection.
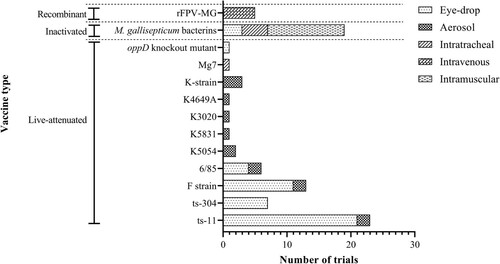
Age at vaccination
There was considerable variation between the trials in the age at vaccination. The youngest age at vaccination was 1 week (Gaunson et al., Citation2006a), while the oldest age at vaccination was 12 weeks (Ferguson-Noel & Williams, Citation2015). In between these two extremes, birds were vaccinated at 2, 3, 4, 5, 6, 7, 8, 10 or 11 weeks of age. In some trials (11/48 trials), birds were vaccinated with two doses of the same vaccine or two different vaccines, 2 days apart (Gates et al., Citation2008), 2 weeks apart (Yagihashi et al., Citation1992), 3 weeks apart (Pakpinyo et al., Citation2015) or 4 weeks apart (Limsatanun et al., Citation2018).
Route of vaccination
The method of delivery of the vaccines varied depending on the strain, type of vaccine or the commercial manufacturer. In several trials, different routes were used for the same vaccine in different groups of chickens with the aim of investigating the effect of the route of vaccination on the efficacy of the vaccine (Yagihashi et al., Citation1992; Limsatanun et al., Citation2018; Noormohammadi & Whithear, Citation2019). However, there were a few vaccination-challenge trials in separate papers that used different routes for the same commercial vaccine (Ferguson-Noel, Cookson et al., Citation2012; Leigh et al., Citation2012; Ferguson-Noel & Williams, Citation2015). The most common route of vaccination was the intraocular route (eye-drop) (), as the recommended route of delivery for the commercial M. gallisepticum ts-11 live-attenuated vaccine is the intraocular route and this was the vaccine evaluated in 20/48 trials, either as the only vaccine evaluated (13/20 trials) or as one of several vaccines evaluated (8/20 trials) (Ferguson et al., Citation2004; Shil et al., Citation2011; Pakpinyo et al., Citation2013; Ferguson-Noel & Williams, Citation2015; Noormohammadi & Whithear, Citation2019; Kanci Condello, Underwood, et al., Citation2020). The routes of administration used for other vaccines included aerosol exposure, intratracheal inoculation, or intravenous or intramuscular injection ().
Route of challenge and time between challenge and necropsy
The route of challenge differed mostly based on the virulent M. gallisepticum strain used in the trials. However, different routes have been used to deliver the same virulent strain in separate trials (Gates et al., Citation2008; Raviv et al., Citation2008). In most of the trials (31/48), the virulent M. gallisepticum strain was delivered as an aerosol, either by spraying the culture over the bird or by nebulizing a culture containing M. gallisepticum using compressed air in an enclosed environment (Gaunson et al., Citation2006a, Citationb; Shil et al., Citation2011; Tseng et al., Citation2017; Kanci et al., Citation2018; Noormohammadi & Whithear, Citation2019; Kanci Condello, Kulappu Arachchige, et al., Citation2020; Kanci Condello, Underwood, et al., Citation2020; Kulappu Arachchige et al., Citation2021) (). Intratracheal inoculation was used in 12/48 trials and 2/48 trials used the intraocular route to challenge the birds with virulent M. gallisepticum (). While most of the trials used a single route of infection, 3/48 trials delivered the virulent M. gallisepticum strain to each bird via both the intranasal and intraocular routes (Pakpinyo et al., Citation2015) ().
Figure 2. Numbers of trials and the routes used to deliver each virulent Mycoplasma gallisepticum strain used for challenge. Five different strains, Ap3AS, R-strain, SAS strain, Rlow and a Thai strain, were used, with these different challenge strains delivered by aerosol, or intratracheal, intraocular or intranasal instillation. The time between challenge and necropsy varied from 4 days to 6 weeks after challenge.
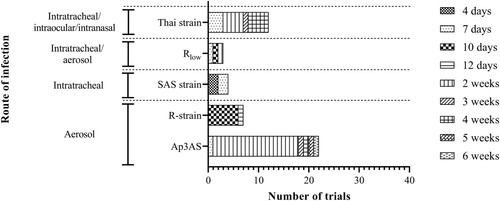
The earliest time-point at which the birds were necropsied was 4 days after challenge (Yagihashi et al., Citation1992), while the latest-time point was 6 weeks after challenge (Gaunson et al., Citation2006a). The other time-points at which birds were necropsied included 7, 10, 12 days, or 2, 3, 4 and 5 weeks after challenge ().
Discussion
The development of efficacious vaccines against M. gallisepticum for poultry is the most effective intervention strategy to control avian mycoplasmosis. In this review, our aim was to evaluate the different experimental designs and parameters applied to assess the efficacy of M. gallisepticum vaccines in previously published vaccine-challenge studies, with the view of identifying the most discriminatory and reproducible parameter for assessing the pathological changes, and hence disease, caused by M. gallisepticum.
The control of infection with and disease caused by M. gallisepticum under experimental conditions has been evaluated by determining the reduction in the prevalence of birds from which virulent M. gallisepticum can be isolated from the respiratory tract (Moura et al., Citation2012), the reduction in the concentration of M. gallisepticum in the respiratory tract (Sayed et al., Citation2018), the reduction in the severity of pathological changes in the trachea and/or air sacs (Bíró et al., Citation2005; Gates et al., Citation2008; Raviv et al., Citation2008; Shil et al., Citation2011; Ferguson-Noel, Laibinis et al., Citation2012; Leigh et al., Citation2012), the reduction in the severity and/or prevalence of clinical signs (Bíró et al., Citation2005), the reduction in horizontal and vertical transmission (Barbour & Newman, Citation1990; Feberwee et al., Citation2006), the prevalence and/or concentration of antibodies against M. gallisepticum in the serum (Kanci et al., Citation2018) and respiratory tract washings (Gates et al., Citation2008), improvements in egg production (Evans et al., Citation2007) and hatchability (Domermuth, Citation1962), and increases in weight gain (Bíró et al., Citation2005). While all of these parameters can indicate the efficacy of vaccines against M. gallisepticum, reductions in the severity of the pathological changes in the respiratory system are most directly correlated with the chronic respiratory disease caused by infection with M. gallisepticum (Nunoya et al., Citation1987). Histological lesions in the trachea and gross lesions in the air sacs have been evaluated in the majority of papers assessing protection induced by vaccination against M. gallisepticum. However, this review has demonstrated that there has been considerable variation between different papers in the parameters and statistical tests used to evaluate lesions in the trachea and air sacs and that this variability between reports on vaccine studies prevents direct comparisons of many of the studies. While there has been similar variation between papers on vaccines against other pathogens of poultry (Chapman et al., Citation2005; Swayne, Citation2009; Coppo et al., Citation2013; Villanueva-Cabezas et al., Citation2017; Soutter et al., Citation2020; Pumtang-on et al., Citation2021), this highlights the importance of developing a more standardized method for assessing the efficacy of vaccines against M. gallisepticum in the future. The European Pharmacopoeia states that the parameters that must be recorded to demonstrate the immunogenicity of killed vaccines against M. gallisepticum are deaths, clinical signs and air sac lesion scores (Monograph, Citation01/Citation2017: Citation1942), but assessment of tracheal lesions may be a more appropriate measure to be used to demonstrate the effectiveness of vaccines against the respiratory disease caused by M. gallisepticum.
At a minimum, the discrimination required for measures for evaluating pathological changes after infection must be that they reliably detect a significant difference between the negative-control group and the positive-control group. Only then is it possible to assess the protective immunity induced by a vaccine, as otherwise it would be impossible to determine whether vaccination has significantly reduced the pathological changes caused by infection. The proportion of trials analysed in this review that reported a significant difference between the negative-control group and the positive-control group in measures of tracheal lesions was significantly greater than the proportion of trials that reported a significant difference in measures of air sac lesions. This suggests that measures of tracheal lesions are more discriminatory and reproducible measures of disease caused by infection with M. gallisepticum than the measures of air sac lesions. The dose of virulent M. gallisepticum (3.3 × 105 colour changing units/dose) used in the only trial that did not report a significant difference in measures of tracheal lesions between the two control groups (Leigh et al., Citation2012) was considerably lower than that used in other trials (1 × 108 colony forming units/dose and 2.51 × 108 colour changing units/dose) that used the same virulent strain of M. gallisepticum (Gates et al., Citation2008; Raviv et al., Citation2008), suggesting that use of a dose comparable with that used in other studies may have resulted in a significant difference, as significant differences in tracheal lesions have been seen after experimental infection with different doses of the same M. gallisepticum strain in chickens and turkeys (Whithear et al., Citation1996; Wijesurendra et al., Citation2015).
The two measures that were used to evaluate histological lesions in the trachea in the trials analysed in this review were tracheal mucosal thicknesses and tracheal lesion scores. Tracheal mucosal thicknesses would seem to be a more appropriate measurement than tracheal lesion scores, as scoring lesions based on qualitative criteria can be quite subjective. There was considerable variation between the studies in the anatomical levels of the trachea used to measure mucosal thicknesses. However, most studies measured the thicknesses at four points transected by vertical and horizontal lines at more than one level of the trachea (upper, middle and lower), and this approach is likely to detect any significant differences in tracheal mucosal thicknesses along the length of the trachea. Similarly, it would be more appropriate to compare the tracheal mucosal thicknesses at different levels separately, as has been done in several studies analysed here (Noormohammadi et al., 2002; Shil et al., Citation2011; Tseng et al., Citation2017), if conclusions are to be drawn about differences in tracheal mucosal thicknesses between different sections of the trachea. In addition, parametric statistical tests can be used to compare the tracheal mucosal thicknesses, as has been the case in the majority of the trials analysed in the review, as tracheal mucosal thicknesses are continuous variables and generally have a normal distribution within a population. However, one study analysed here reported that the mucosal thicknesses were not normally distributed, and a nonparametric test was applied (Gates et al., Citation2008). The lower group size (n = 6) in this study compared to the other studies (n ∼ 10), could have contributed to the failure of the data in this study to pass the test for normality. Data derived from tracheal lesion scoring are categorical and will not be normally distributed, necessitating use of non-parametric statistical methods, which are less powerful and therefore less likely to discriminate between different treatment groups.
Assessment of gross air sac lesions is a useful adjunct to measurement of tracheal mucosal thicknesses in M. gallisepticum vaccine-challenge studies, as it is likely to provide more information about the protection the vaccines induce against chronic respiratory disease in vaccinated birds. However, the discrepancies between the scoring systems that are used, and location and number of air sacs used to determine the lesion score, make direct comparisons between studies difficult, as highlighted in this review. Adherence to a standardized evaluation method in future studies would aid comparisons of the efficacies of different vaccines, particularly between novel vaccines and currently available vaccines, facilitating an improved and consistent standard for the assessment of respiratory disease, which requires fewer animals for clinical testing during the development programme for novel vaccines. However, de novo development of standards would involve a considerable amount of time-consuming work, and it may be best to base any future standards on the most representative and discriminatory approaches used in previous studies. The majority of the trials analysed in this review scored gross air sac lesions on a scale of 0-4. A scale of 0-4 seems more appropriate than a scale of 0-3 as there can be a broad range of gross changes in the air sacs after infection with M. gallisepticum (Kulappu Arachchige et al., Citation2021) that are not as well represented using a shorter scale. Most of the trials scored the thoracic and abdominal air sacs. Calculation of the cumulative score for each bird after scoring the two cranial thoracic air sacs, the two caudal thoracic air sacs and the two abdominal air sacs individually would be a more representative measure of gross air sac lesion scores. Scoring the clavicular air sac and the two cervical air sacs is difficult, as they tend to get disrupted during the early stages of necropsies. The resultant gross air sac lesion scores are categorical (ordinal) variables, and thus are most appropriately analysed using non-parametric statistical tests, as was the case for most of the trials analysed in this review. However, even though most of the trials used non-parametric tests to compare the gross air sac lesion scores for different groups, the descriptive statistics reported for many studies were means and standard deviations, which assume a normal distribution, rather than median scores and the range or interquartile range, which better describe categorical data that are not normally distributed.
The analysis of published M. gallisepticum vaccine studies reported here has highlighted the variation in methods used to evaluate and report about vaccines against this important poultry pathogen. We propose that future experimental studies should follow a standardized model for reporting and evaluation. The bird type (e.g. broiler and layer), health status (SPF or commercial) and breed (e.g. Cobb or Ross), the age of the birds, the group sizes, housing type (cage or isolator with mesh floor or deep litter), the type of vaccine tested, the route of vaccination, the dose of the vaccine administered, the method of challenge, the challenge strain, the age at challenge, the dose(s) of the challenge strain and whether another infectious agent has also been administered would be the minimal reporting requirements. For evaluation of efficacy, while various measures could be reported, the primary outcome that needs to be assessed to determine efficacy in controlling chronic respiratory disease in poultry is a reduction in the severity of pathological changes in the respiratory tract, particularly the trachea and air sacs. The analysis we have reported here indicated that assessment of tracheal lesions is more discriminative than assessment of air sac lesions, and that an assessment of tracheal lesions should therefore be the primary outcome variable that is assessed in vaccine efficacy studies, with air sac lesion scores included as secondary variables. A more detailed comparison of raw data, where they are available, from the trials analysed in this review, should enable definitive determination of which outcome variable has the greatest statistical power and thus has the most capacity for assessing differences in efficacy. Such a comparison could also yield recommendations for appropriate thresholds for determining whether a challenge has caused sufficient disease to enable efficacy to be assessed in a trial and for determining whether a vaccine has induced effective protection against this challenge.
Supplemental Material
Download MS Word (350.8 KB)Data availability statement
Only published data were included in this systematic review and their citations are provided in the reference list.
Disclosure statement
Gregory J. Underwood and Daniel M. Andrews are employees of Bioproperties Pty. Ltd. The other authors have no potential conflicts of interest (financial, professional or personal) related to the research reported here.
Additional information
Funding
References
- Abd-El-Motelib, T.Y. & Kleven, S.H. (1993). A comparative study of Mycoplasma gallisepticum vaccines in young chickens. Avian Diseases, 37, 981–987.
- Barbour, E.K. & Newman, J.A. (1990). Preliminary data of efficacy of Mycoplasma gallisepticum vaccines containing different adjuvants in laying hens. Veterinary Immunology and Immunopathology, 26, 115–123.
- Bíró, J., Povazsán, J., Kőrösi, L., Glávits, R., Hufnagel, L. & Stipkovits, L. (2005). Safety and efficacy of Mycoplasma gallisepticum TS-11 vaccine for the protection of layer pullets against challenge with virulent M. gallisepticum R-strain. Avian Pathology, 34, 341–347.
- Chapman, H.D., Roberts, B., Shirley, M.W. & Williams, R.B. (2005). Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathology, 34, 279–290.
- Coppo, M.J.C., Noormohammadi, A.H., Browning, G.F. & Devlin, J.M. (2013). Challenges and recent advancements in infectious laryngotracheitis virus vaccines. Avian Pathology, 42, 195–205.
- Domermuth, C.H. (1962). Vaccination of chickens with Mycoplasma gallisepticum. Avian Diseases, 6, 412–419.
- Evans, J.D., Leigh, S.A., Branton, S.L. & Collier, S.D. (2007). Effects of increased dosages of the Mycoplasma gallisepticum vaccine MYCOVAC-L® in layer chickens subsequently challenged with virulent M. gallisepticum: egg production and serologic response. Avian Diseases, 51, 912–917.
- Evans, R.D. & Hafez, Y.S. (1992). Evaluation of a Mycoplasma gallisepticum strain exhibiting reduced virulence for prevention and control of poultry mycoplasmosis. Avian Diseases, 36, 197–201.
- Feberwee, A., von Banniseht-Wysmuller, T., Vernooij, J.C.M., Gielkens, A.L.J. & Stegeman, J.A. (2006). The effect of vaccination with a bacterin on the horizontal transmission of Mycoplasma grallisepticum. Avian Pathology, 35, 35–37.
- Ferguson, N.M., Leiting, V.A. & Kleven, S.H. (2004). Safety and efficacy of the avirulent Mycoplasma gallisepticum strain K5054 as a live vaccine in poultry. Avian Diseases, 48, 91–99.
- Ferguson-Noel, N., Cookson, K., Laibinis, V.A. & Kleven, S.H. (2012). The efficacy of three commercial Mycoplasma gallisepticum vaccines in laying hens. Avian Diseases, 56, 272–275.
- Ferguson-Noel, N.M., Laibinis, V.A. & Kleven, S.H. (2012). Evaluation of Mycoplasma gallisepticum K-strain as a live vaccine in chickens. Avian Diseases, 56, 44–50.
- Ferguson-Noel, N.M. & Williams, S.M. (2015). The efficacy of Mycoplasma gallisepticum K-strain live vaccine in broiler and layer chickens. Avian Pathology, 44, 75–80.
- Gates, A.E., Frasca, S., Nyaoke, A., Gorton, T.S., Silbart, L.K. & Geary, S.J. (2008). Comparative assessment of a metabolically attenuated Mycoplasma gallisepticum mutant as a live vaccine for the prevention of avian respiratory mycoplasmosis. Vaccine, 26, 2010–2019.
- Gaunson, J.E., Philip, C.J., Whithear, K.G. & Browning, G.F. (2006a). Age related differences in the immune response to vaccination and infection with Mycoplasma gallisepticum. Vaccine, 24, 1687–1692.
- Gaunson, J.E., Philip, C.J., Whithear, K.G. & Browning, G.F. (2006b). The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease. Vaccine, 24, 2627–2633.
- Glisson, J.R. & Kleven, S.H. (1984). Mycoplasma gallisepticum vaccination – effects on egg transmission and egg-production. Avian Diseases, 28, 406–415.
- Kanci, A., Wijesurendra, D.S., Wawegama, N.K., Underwood, G.J., Noormohammadi, A.H., Markham, P.F. & Browning, G.F. (2018). Evaluation of Mycoplasma gallisepticum (MG) ts-304 vaccine as a live attenuated vaccine in turkeys. Vaccine, 36, 2487–2493.
- Kanci Condello, A., Kulappu Arachchige, S.N., Shil, P.K., Underwood, G.J., Noormohammadi, A.H., Markham, P.F., Wawegama, N.K. & Browning, G.F. (2020). Duration of protective immunity induced by Mycoplasma gallisepticum strain ts-304 vaccine in chickens. Veterinary Microbiology, 251, 108883.
- Kanci Condello, A., Underwood, G.J., Shil, P.K., Noormohammadi, A.H., Markham, P.F., Wawegama, N.K. & Browning, G.F. (2020). Mycoplasma gallisepticum strain ts-304 is a safe and effective live attenuated vaccine for use in chickens. Veterinary Microbiology, 244, 108654.
- Kleven, S. (2008). Control of avian mycoplasma infections in commercial poultry. Avian Diseases, 52, 367–374.
- Kleven, S.H., King, D.D. & Anderson, D.P. (1972). Airsacculitis in broilers from Mycoplasma synoviae: effect on air-sac lesions of vaccinating with infectious bronchitis and Newcastle virus. Avian Diseases, 16, 915–924.
- Kulappu Arachchige, S.N., Kanci Condello, A., Zhu, L., Shil, P.K., Tivendale, K.A., Underwood, G.J., Noormohammadi, A.H., Browning, G.F. & Wawegama, N.K. (2021). Effects of immunosuppression on the efficacy of vaccination against Mycoplasma gallisepticum infection in chickens. Veterinary Microbiology, 260, 109182.
- Leigh, S.A., Branton, S.L., Evans, J.D. & Collier, S.D. (2012). Effect of infection route and concurrent infectious bronchitis virus vaccination on Mycoplasma gallisepticum disease pathology in an experimental model. Avian Pathology, 41, 497–503.
- Ley, D.H. (2008). Mycoplasma gallisepticum infection. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nalon & D.E. Swayne (Eds.), Diseases of poultry 12th edn (pp. 807–834). Ames, IA: Blackwell Publishing.
- Limsatanun, A., Sasipreeyajan, J. & Pakpinyo, S. (2016). The efficacy of chitosan-adjuvanted, Mycoplasma gallisepticum bacterin in chickens. Avian Diseases, 60, 799–804.
- Limsatanun, A., Sasipreeyajan, J. & Pakpinyo, S. (2018). Chitosan-adjuvanted Mycoplasma gallisepticum bacterin via intraocular administration enhances Mycoplasma gallisepticum protection in commercial layers. Poultry Science, 97, 1934–1940.
- Lin, M. & Kleven, S. (1982). Pathogenicity of two strains of Mycoplasma gallisepticum in turkeys. Avian Diseases, 26, 360–364.
- Luginbuhl, R., Tourtellotte, M. & Frazier, M. (1967). Mycoplasma gallisepticum-control by immunization. Annals of the New York Academy of Sciences, 143, 234–238.
- Monograph. (01/2017: 1942). European pharmacopoeia 10.0. European Directorate for the Quality of Medicine & Health Care of the Council of Europe (EDQM), 1129–1130.
- Moura, L., Dohms, J., Almeida, J.M., Ferreira, P.S., Biffi, C.P. & Backes, R.G. (2012). Development and evaluation of a novel subunit vaccine for Mycoplasma gallisepticum. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 64, 1569–1576.
- Noormohammadi, A.H., Browning, G.F., Cowling, P.J., O’Rourke, D., Whithear, K.G. & Markham, P.F. (2002). Detection of antibodies to Mycoplasma gallisepticum vaccine ts-11 by an autologous pMGA enzyme-linked immunosorbent assay. Avian Diseases, 46, 405–411.
- Noormohammadi, A.H., Jones, J.F., Underwood, G. & Whithear, K.G. (2002). Poor systemic antibody response after vaccination of commercial broiler breeders with Mycoplasma gallisepticum vaccine ts-11 not associated with susceptibility to challenge. Avian Diseases, 46, 623–628.
- Noormohammadi, A.H. & Whithear, K.G. (2019). Comparison of the short-term and long-term efficacies of the Mycoplasma gallisepticum vaccines ts-11 and 6/85. Avian Pathology, 48, 238–244.
- Nunoya, T., Tajima, M., Yagihashi, T. & Sannai, S. (1987). Evaluation of respiratory lesions in chikens induced by Mycoplsma gallisepticum. The Japanese Journal of Veterinary Science, 49, 621–629.
- Pakpinyo, S., Limsatanun, A., Sangthongdang, K., Paniago, M. & Soares, R. (2015). Protection against Mycoplasma gallisepticum in layers immunized with recombinant fowl poxvirus vaccine followed by live F strain vaccine. Thai Journal of Veterinary Medicine, 45, 197–204.
- Pakpinyo, S., Wanaratana, S., Sangthongdang, K. & Paniago, M. (2013). Efficacy and safety of different live Mycoplasma gallisepticum vaccines in layer chickens. Thai Journal of Veterinary Medicine, 43, 533–540.
- Pumtang-on, P., Mahony, T.J., Hill, R.A. & Vanniasinkam, T. (2021). A systematic review of Campylobacter jejuni vaccine candidates for chickens. Microorganisms, 9, 397.
- Raviv, Z., Callison, S.A., Ferguson-Noel, N. & Kleven, S.H. (2008). Strain differentiating real-time PCR for Mycoplasma gallisepticum live vaccine evaluation studies. Veterinary Microbiology, 129, 179–187.
- Rodriguez, R. & Kleven, S. (1980). Pathogenicity of two strains of Mycoplasma gallisepticum in broilers. Avian Diseases, 24, 800–807.
- Sayed, R.H., Ahmed, H.A., Shasha, F.A. & Ali, A.M. (2018). Real time PCR quantification and differentiation of both challenge and vaccinal Mycoplasma gallisepticum strains used in vaccine quality control. Journal of World’s Poultry Research, 8, 50–58.
- Shil, P.K., Kanci, A., Browning, G.F., Marenda, M.S., Noormohammadi, A.H. & Markham, P.F. (2011). GapA+ Mycoplasma gallisepticum ts-11 has improved vaccine characteristics. Microbiology, 157, 1740–1749.
- Soutter, F., Werling, D., Tomley, F.M. & Blake, D.P. (2020). Poultry coccidiosis: design and interpretation of vaccine studies. Frontiers in Veterinary Science, 7, 101.
- Swayne, D.E. (2009). Avian influenza vaccines and therapies for poultry. Comparative Immunology, Microbiology and Infectious Diseases, 32, 351–363.
- Tseng, C.-W., Chiu, C.-J., Kanci, A., Noormohammadi, A.H., Browning, G.F. & Markham, P.F. (2017). Safety and efficacy of a Mycoplasma gallisepticum oppD knockout mutant as a vaccine candidate. Vaccine, 35, 6248–6253.
- Villanueva-Cabezas, J.P., Coppo, M.J.C., Durr, P.A. & McVernon, J. (2017). Vaccine efficacy against Indonesian highly pathogenic avian influenza H5N1: systematic review and meta-analysis. Vaccine, 35, 4859–4869.
- Whithear, K. (1993). Avian mycoplasmosis. In L.A. Corner & T.J. Bagust (Eds.), Australian standard diagnostic techniques for animal diseases (pp. 1–12). East Melbourne: CSIRO for the Standing Committee on Agriculture and Resource Management.
- Whithear, K. (1996). Control of avian mycoplasmoses by vaccination. Revue Scientifique et Technique de l’OIE, 15, 1527–1553.
- Whithear, K.G., Harrigan, K.E. & Kleven, S.H. (1996). Standardized method of aerosol challenge for testing the efficacy of Mycoplasma gallisepticum vaccines. Avian Diseases, 40, 654–660.
- Wijesurendra, D.S., Kanci, A., Tivendale, K.A., Bacci, B., Noormohammadi, A.H., Browning, G.F. & Markham, P.F. (2015). Development of a Mycoplasma gallisepticum infection model in turkeys. Avian Pathology, 44, 35–42.
- Yagihashi, T., Nunoya, T., Sannai, S. & Tajima, M. (1992). Comparison of immunity induced with a Mycoplasma gallisepticum bacterin between high- and low-responder lines of chickens. Avian Diseases, 36, 125–133.
