ABSTRACT
Highly pathogenic avian influenza (HPAI) viruses from the Goose/Guangdong/96-lineage emerged in Southeast Asia and subsequently spread to the Middle East, Africa and Europe, infecting a range of birds and mammals (including humans). This lineage of H5 viruses can efficiently establish itself in wild birds after circulating among gallinaceous poultry, facilitating reassortment with low pathogenic avian influenza (LPAI) virus strains, enhancing dispersal over long distances and contributing to endemicity. The detection of HPAI H5N8 virus (clade 2.3.4.4B) in 2017 in the Mpumalanga Province of South Africa marked the beginning of an epidemic that devastated the South African poultry industry. Vaccines were tested to assess protection against the circulating field strain. This article describes the performance of a reverse genetics inactivated H5N1 vaccine from Zoetis (RG-H5N1), with 96.1% identity to the circulating HPAI H5N8 virus. Two locally formulated benchmarks, one containing an H5N8 antigen homologous to the field strain (Benchmark-H5N8), the other containing a heterologous (87.6% identity to field virus) LPAI H5N1 antigen (Benchmark-H5N1), were included for comparison. Efficacy was assessed in specific pathogen-free (SPF) chickens using a prime-boost approach (injections at days 21 and 45), followed by a challenge with a South African HPAI H5N8 isolate (70 days of age). The Zoetis RG-H5N1 vaccine and Benchmark-H5N8 outperformed the Benchmark-H5N1 in terms of humoral response against the H5N8 antigen and reduction of shedding. The Zoetis RG-H5N1 vaccine protected 100% of the chickens against clinical disease and death. This study confirmed that antigenically matched inactivated vaccines could induce robust protection and markedly reduce viral shedding.
Conditionally licensed vaccine protected against HPAI H5N8 (clade 2.3.4.4B).
Complete protection against clinical disease and mortality.
Drastic reduction of viral shedding after challenge.
RESEARCH HIGHLIGHTS
Introduction
The current interpretation of epidemiological trends amongst highly pathogenic avian influenza (HPAI) viruses suggests that most strains of the H5 and H7 subtypes initially circulate as low pathogenic versions within the wild bird reservoir. Once these strains enter gallinaceous poultry, they may undergo genetic mutations and acquire the phenotypic trait of high pathogenicity (Lee et al., Citation2017; Sims et al., Citation2017; Khomenko et al., Citation2018).
The HPAI H5 viruses of the Goose/Guangdong/96-lineage (Gs/GD/96) acquired the ability to efficiently re-infect and establish themselves in the wild bird reservoir after circulating amongst gallinaceous poultry (Vijaykrishna et al., Citation2008; Lee et al., Citation2017). This lineage of viruses may persist within the wild bird population while causing asymptomatic infections and sporadically fatal disease (Lee et al., Citation2017). It is plausible that this ability to re-enter the wild bird reservoir enabled the HPAI Gs/GD/96 viruses to reassort with several of the circulating low pathogenic avian influenza virus (LPAI) strains (Lee et al., Citation2017; Sims et al., Citation2017; Khomenko et al., Citation2018). This exchange of genetic material resulted in a notable spectrum of neuraminidases (and other internal influenza genes) associated with the H5 Gs/GD/96-lineage. It is also believed that the ability of these viruses to efficiently re-infect various groups of migratory birds (especially waterfowl) greatly facilitated their rapid dispersal over vast distances (Global Consortium for H5N8 and Related Influenza Viruses, Citation2016; Lee et al., Citation2017; Sims et al., Citation2017; Khomenko et al., Citation2018; Antigua et al., Citation2019).
The detection of HPAI, subtype H5N8, clade 2.3.4.4B, during June 2017 near the town of Villiers in the Mpumalanga Province of South Africa marked the beginning of an AI epidemic which devastated the South African poultry industry and alarmed avian conservationists in the region (OIE, Citation2017; Khomenko et al., Citation2018). This event also confirmed that the H5 Gs/GD/96-lineage of viruses reached the tip of Africa during the fourth wave of intercontinental dispersal from Asia (Sims et al., Citation2017; OFFLU, Citation2018).
Retrospective and prospective analyses estimated the economic impact of the outbreak at approximately 128 million US$ (1.87 billion ZAR). While only 17% of these losses resulted from the direct costs associated with the culling of ca. 5.4 million domestic chickens, the income forgone associated with sales of poultry products, day-old chicks, pullets and live birds was estimated at ca. 103 million US$. Due to a higher prevalence amongst layer birds during the outbreak, it was estimated that approximately 75% of the losses could be ascribed to the commercial laying industry, while the broiler industry suffered relatively less (BFAP report 2018).
Apart from an unprecedented economic impact on the agricultural sector, the H5N8 epidemic was also notable for significant losses amongst captive and free-roaming wild bird populations (FAO report 2018; Ndumu et al., Citation2018). Mass mortality events, reminiscent of the deaths associated with an HPAI H5N3 amongst common terns (Sterna hirundo) during 1961, were observed amongst swift terns (Thalasseus bergii) at multiple breeding and roosting sites (Becker, Citation1966, FAO report 2018). Sporadic deaths amongst species with a high conservation status (e.g. mainland colonies of the African penguin) prompted authorities to temporarily halt research activities at sites of zoological and ecological importance to reduce the risk of viral dissemination through human activity (FAO report 2018; Wild, Citation2018; Cannon, Citation2019). Pressing issues, such as insufficient funding for owner compensation and the appearance of multiple easily spreadable H5Nx strains amongst the Gs/GD/96-lineage of viruses sparked renewed interest in immunoprophylaxis as a control tool (Capua & Alexander, Citation2008; Bertran et al., Citation2017).
In this manuscript, we describe the efficacy of a reverse genetics inactivated avian influenza vaccine (Zoetis RG-H5N1) in comparison to two locally produced benchmark vaccines (Benchmark-H5N8 and Benchmark-H5N1). The Zoetis RG-H5N1 vaccine was conditionally licensed by the US Department of Agriculture (USDA) for stockpile purpose during the 2014/2015 US outbreak. This vaccine contains the haemagglutinin (HA) gene of the HPAI A/gyrfalcon/Washington/41088-6/2014 (H5N8) virus with 96.1% genetic similarity with the HA of the HPAI H5N8 virus circulating in South Africa. One of the locally produced benchmark vaccines (Benchmark-H5N8) contained antigen homologous to the HPAI H5N8 field virus. The Benchmark-H5N1 contained antigen of a heterologous low pathogenic H5N1 strain with 87.6% genetic similarity to the H5N8 field strain. The results are presented and discussed in the context of post-vaccinal serological responses, clinical protection, and the reduction of post-challenge shedding. It is envisaged that this information will assist veterinary authorities, producers and veterinarians in South Africa, and the rest of the world, to make informed decisions about the role of vaccination for controlling avian influenza.
Materials and methods
Ethics statement and regulatory aspects
The study was performed according to the South African National Standard for the Care and Use of Animals for Scientific Purposes (SANS 10386:2008). The ethical aspects of animal experimentation were reviewed and approved by the NHREC-registered Deltamune Ethics Committee. The trial was conducted at biosafety level 3 (BSL3) as approved by the South African Department of Agriculture, Land Reform and Rural Development.
Veterinary investigational products and benchmark items
The Zoetis RG-H5N1 was imported into South Africa under the terms and conditions of Section 21 of the Medicines and Related Substances Act (Act 101 of 1965) and donated to the project. The antigenic fraction of this water-in-oil emulsion comprises recombinant virus, constructed via reverse genetics from the internal genes of the high-yield human vaccine strain A/PR/8/34 (H1N1) and the HA (H5) gene from A/gyrfalcon/Washington/41088-6/2014 H5N8, Clade 2.3.4.4 (GenBank KP307984.1) (Hoffman et al., Citation2002; Bertran et al., Citation2017).
Two inactivated, mineral-oil adjuvanted, autogenous vaccines were produced by Deltamune (Pty) Ltd to serve as benchmarks during the trial. The Benchmark-H5N8 vaccine contained an antigen homologous to the circulating HPAI H5N8 field strain, while the Benchmark-H5N1 vaccine contained an antigen derived from a heterologous local low pathogenicity isolate, A/duck/SA/811/04 (H5N1) (GenBank EF041479).
A placebo was produced for the negative control group, by replacing the antigenic fraction of the vaccine formulation with sterile allantoic fluid.
Challenge material
Stocks of HPAI A/Ck/SA/275401/17 H5N8 clade 2.3.4.4B, Gs/GD/96-lineage (GenBank MH165788) were prepared in the allantoic cavity of specific pathogen-free (SPF) embryonated chicken eggs. The viral concentration in the challenge material was titrated in 11-day-old chicken embryos. This procedure involved the inoculation of six eggs per dilution and daily monitoring for embryo death over a period of 7 days. The presence of haemagglutination activity was confirmed in the allantoic fluid of embryos that died during the monitoring period, using a macroscopic haemagglutination test (5% chicken red blood cells (RBCs)). A 50% egg infective dose (EID50) was calculated using the method of Reed and Muench (Citation1938).
Experimental design
The 160 1-day-old SPF White Leghorn chickens were obtained from a local supplier (Avi-Farms Pty. Ltd., Centurion, South Africa) and randomly allocated to four equally-sized treatment groups (A to D). Each treatment group was further subdivided into three subgroups with 20 birds allocated to Subgroup-1 and 10 birds to Subgroup-2 and Subgroup-3 each (). The birds were individually identified with colour-coded leg bands and quarantined within an environmentally-controlled animal facility operated at BSL3.
Table 1. Summary of the various treatment groups and their respective subdivisions.
All the chickens in Subgroup-1 and Subgroup-2 of the four treatment groups were primed at 21 days of age by subcutaneously injecting 0.5 ml of the applicable vaccine or the placebo. A booster dose was given at 45 days of age. The birds in Subgroup-3 were left unvaccinated to serve as fully susceptible sentinels.
All the chickens in Subgroup-1 of the four treatment groups were challenged with 106.0 EID50/100 µl via the oculo-nasal route at 70 days of age (25 days after booster) and transferred in pairs to 10 negatively pressurized isolator units. To avoid inadvertent exposure to the challenge material, the chickens in Subgroup-2 (contacts, vaccinated but not challenged) and Subgroup-3 (contacts, not vaccinated and not challenged) were transferred in pairs to the isolators containing birds of the corresponding subgroup 1 only 2 days after the challenge procedure. This ensured that, from the second day after challenge, each one of the 10 isolator units housed two challenged vaccinees (Subgroup-1) commingled with either two vaccinated in-contact controls (Subgroup-2) or two unvaccinated sentinels (Subgroup-3).
Serological assessment
Serum samples were collected from all birds prior to the first vaccination at 21 days. Serum samples from all Subgroup 1 birds were collected 3 weeks after priming, 3 weeks after booster and 12 days post-challenge. Serum samples from Subgroup-2 and Subgroup-3 birds were collected 3 weeks after placing them in contact with challenged birds (Subgroup-1).
The serological responses were quantified using a haemagglutination inhibition (HI) assay. Twofold serial dilutions of the test sera were assessed using 0.33% chicken RBCs and maintaining the antigen concentration at 4 HAU/25 µl throughout the study. Each serum panel was evaluated in parallel using binary ethylenimine (BEI) inactivated HI antigens equivalent to the vaccine antigens incorporated in Benchmark-H5N8 and Benchmark-H5N1. Central tendency of the results was expressed as the geometric mean (GM), and dispersion around the mean, as the geometric standard deviation (GSD) (Reverberi, Citation2008).
Clinical monitoring
The clinical response to the challenge was monitored by visual inspection twice daily and recorded as clinical scores. Healthy birds were scored as “2”, clinically sick birds as “1” and dead birds as “0”. Moribund birds were scored as “1”, then humanely terminated and recorded as zero from the next day onwards.
Monitoring of shedding
The shedding of challenge virus from the vaccinated and challenged Subgroup-1 birds was monitored by real-time reverse transcriptase PCR. The specimens comprised choanal mucous or cloacal contents collected onto dry swabs. Sampling was done daily for the first 4 days post-challenge, and subsequently, every 48 h until the 10th day.
The swabs were immersed in Bacterial Lysis Buffer (Roche, Midrand, South Africa). Total nucleic acid was extracted with the Roche MagNA Pure 96 DNA and Viral RNA Small Volume Kit. Viral RNA was detected by real-time RT–PCR on the LightCycler® 480 (Roche) using a primer set and hydrolysis probe specific for the influenza matrix gene (Nagy et al., Citation2010). The estimated viral concentration in the specimens was extrapolated from the Cp values using a standard curve which was constructed on the LightCycler 480® from 10-fold serial dilutions of the challenge material, similarly to what has been done by others (Lee & Suarez, Citation2004).
The infectivity of the excreted challenge virus, and its ability to spread, were assessed via placement of SPF sentinels (Subgroup-3) and immunized in-contact controls (Subgroup-2) alongside the challenged vaccinees (Subgroup-1), 48 h after challenge.
Statistical analyses
Prior screening of the applicable data sets by means of Shapiro–Wilk analysis and the construction of box-plots indicated that the data were mostly not normally distributed. Comparisons between groups were therefore made by using non-parametric tests (including the Wilcoxon signed-rank test for paired samples, Mann–Whitney U test for independent samples and Kruskal–Wallis test for analysis of variance between more than two samples). The significance level (α) was kept constant at 0.05 throughout the analyses.
Results
Serology
Humoral response against the A/Ck/SA/275401/17 (H5N8) antigen
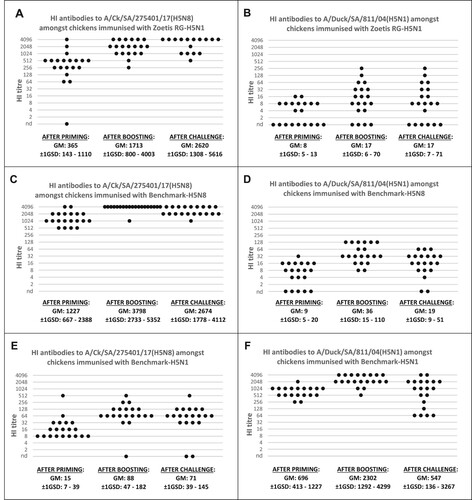
Serological responses against the A/Ck/SA/275401/17 (H5N8) HI antigen were detected in all the vaccinated birds (i.e. Subgroup-1 of Treatment group A, B and C), but not in the corresponding subgroups of Treatment group D (placebo). The group primed with Zoetis RG-H5N1 (Treatment group A) showed an antibody response intermediate to that of the two benchmark vaccines (GM: 365, ±1GSD: 143–1110). This response significantly increased after boosting (GM: 1713, ±1GSD: 800–4003) (A). The vaccinees from Treatment group B (Benchmark-H5N8) had the highest antibody titres after priming (GM: 1227, ±1GSD: 667–2388) and boosting (GM 3798, ±1GSD: 2733–5352) (C). The chickens vaccinated with Benchmark-H5N1 (Treatment group C) showed the lowest titres after priming (GM: 15, ±1GSD: 6–48) and boosting (GM: 88, ±1GSD: 47–182) (E). None of the treatment groups showed statistically significant increases in antibody titres when evaluated 12 days post-challenge.
Figure 1. Density dot plots illustrating the HI responses of the chickens immunised with the Zoetis RG-H5N1, Benchmark-H5N8 and Benchmark-H5N1 vaccines. The three dot plots in graph (A) illustrate the serological response of birds immunised with Zoetis RG-H5N1 after priming (left), boosting (middle) and post-challenge (right) when evaluated with the H5N8 HI antigen. Graph (B) illustrates the HI results obtained using the H5N1 HI antigen to evaluate the sera of the birds immunised with Zoetis RG-H5N1. Graphs (C) and (D) show the corresponding results obtained with sera from chickens immunised with Benchmark-H5N8. Graphs (E) and (F) demonstrate the corresponding results with sera from birds immunised with Benchmark-H5N1. GM = Geometrical mean, GSD = Geometrical standard deviation.
Humoral response against the A/duck/SA/811/04 (H5N1) antigen
The HI titres of the birds primed with Zoetis RG-H5N1 (GM: 8, ±1GSD: 5–13) (B) and Benchmark-H5N8 (GM: 9, ±1GSD: 5–20), (D) were significantly lower (Kruskal–Wallis test: P = 1.7E-09, α = 0.05) than the titres of birds immunized with Benchmark-H5N1 (GM: 696, ±1GSD: 413–1227), (F). The antibody titres against A/duck/SA/811/04 (H5N1) also increased significantly after booster vaccination in all the vaccinated subgroups.
No increase in antibody concentration could be demonstrated after challenge.
The sham-vaccinated negative control group remained serologically naïve until challenge. Both sham-vaccinated individuals which survived exposure to the challenge virus showed clear evidence of seroconversion at the termination of the experiment (data not shown).
Protective immunity
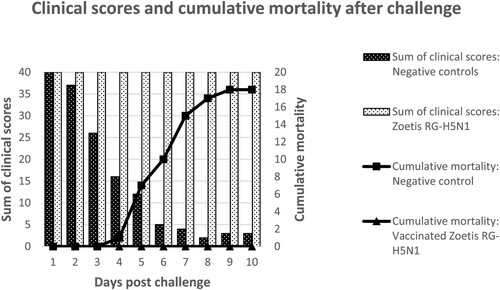
While all the chickens vaccinated with Zoetis RG-H5N1 or the benchmark vaccines were clinically protected against the challenge virus, the sham-vaccinated negative controls developed obvious clinical disease 3–4 days after challenge (). The clinical signs progressed rapidly from lethargy and laboured breathing to prostration and dyspnoea. Eighteen of the 20 placebo birds succumbed within 9 days after challenge, either due to fatal infection or because of termination for humane reasons. Two birds survived until the termination of the study. All the chickens vaccinated with the benchmark vaccines were clinically protected against the challenge virus. Unexpectedly, in all treatment groups, the contact vaccinated or non-vaccinated controls remained clinically sound throughout the study. No seroconversion was detected in the unvaccinated sentinels at the end of the study (data not shown).
Figure 2. A visual summary of the clinical scores and mortality rates among birds vaccinated with Zoetis RG-H5N1 or the negative controls which received a placebo. The clinical scores were obtained by awarding a score of 2 for each healthy bird per observation, 1 for each sick bird, and 0 for each dead bird. Therefore, the healthiest group obtained the highest clinical scores.
Shedding
Four out of the 20 (20%) birds vaccinated with the Zoetis RG-H5N1 vaccine excreted low levels of virus after challenge. The viral RNA was demonstrable on the choanal swabs (), but not on the cloacal swabs (), of the affected birds and the levels ranged from an equivalent viral concentration of 102.73 EID50/ml to 103.08 EID50/ml, with a mean of 102.94 EID50/ml. The shedding occurred only during the first 3 days post-challenge, with none of the affected birds testing positive for more than 1 d. In contrast to the vaccinated groups, all the sham-vaccinated negative controls shed high concentrations of viral RNA through both respiratory and gastro-intestinal secretions (up to an equivalent viral concentration of 107.34 and 106.72 EID50/ml, respectively). Viral RNA could be detected from the first day post-challenge and shedding generally continued until death or euthanasia of the birds. The two chickens which did not succumb during the clinical phase remained PCR-positive on choanal swabs until termination of the study. In most birds, the concentration of viral RNA on cloacal specimens peaked around 2 days post-challenge. On choanal specimens, the peak of shedding was less synchronized (occurring mostly around 3–4 days post-challenge, but in some individuals as late as 6 days) ( and ).
Figure 3. Summary of the quantitative PCR results obtained from choanal swabs during the 10-d monitoring period after challenge. The Cp-values recorded in the assay were converted to equivalent viral concentration using a standard graph constructed from 10-fold dilutions of the challenge material. Results from the Zoetis RG-H5N1 group are represented by grey circles, the Benchmark-H5N8 group by white squares, the Benchmark-H5N1 group by white triangles and those of the placebo group by black diamonds.
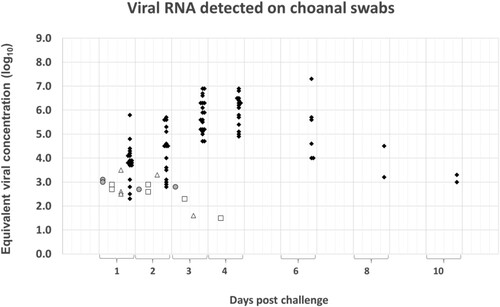
Figure 4. Summary of the quantitative PCR results obtained on cloacal swabs during the 10-d monitoring period after challenge. The Cp-values recorded in the assay were converted to equivalent viral concentration using a standard graph constructed from 10-fold dilutions of the challenge material. Results from the Zoetis RG-H5N1 group are represented by white circles, the Benchmark-H5N8 group by white squares, the Benchmark-H5N1 group by white triangles and those of the placebo group by black diamonds.
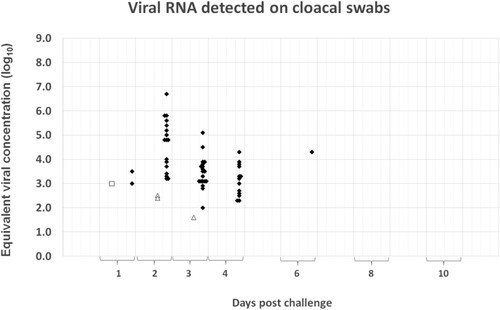
The shedding pattern of the birds immunized with the homologous Benchmark-H5N8 vaccine corresponded closely to that of the Zoetis RG-H5N1 group ( and ). Viral RNA with an equivalent viral concentration ranging from 101.46 EID50/ml to 102.90 EID50/ml (mean: 102.64 EID50/ml) was detected on the choanal swabs of six of the 20 birds in the group. Additionally, one bird tested positive on a cloacal swab (concentration: 102.99 EID50/ml) but yielded a negative result on the corresponding choanal specimen. The shedding period was also short since none of the shedders tested positive for more than 1 d. No positive specimens were detected beyond the fourth day of sampling.
Low concentrations of viral RNA were demonstrated on both, choanal and cloacal swabs of six of the 20 birds immunized with the heterologous Benchmark-H5N1 ( and ). Of the six shedding birds, three tested positive on the choanal specimens only, two on the cloacal specimens only and one bird tested positive on both. The equivalent viral concentration on the choanal specimens ranged from 101.60 EID50/ml to 103.50 EID50/ml (mean: 103.06 EID50/ml), and on the cloacal swabs from 101.60 and 102.51 EID50/ml. Like the shedding birds in the Zoetis RG-H5N1 group and the Benchmark-H5N8 group, most of the shedders in this group tested positive on a single day only. The exception was the bird that tested positive on both the choanal and cloacal specimens. No positive specimens were detected beyond the fourth day of sampling.
Discussion
Like all avian influenza viruses in the Gs/GD/96-lineage, the South African HPAI strain A/Ck/SA/275401/17 (H5N8) is highly contagious in chickens and causes severe clinical disease with high mortality (Antigua et al., Citation2019; Sims et al., Citation2017; Bureau for Food and Agricultural Policy., Citation2018). In this study, the virus infected all the fully-susceptible negative controls, caused prostration and respiratory distress within 3–4 days after experimental exposure and resulted in 90% mortality (if including the birds humanely euthanised).
The use of vaccination in the control and prevention of HPAI is a contentious issue. While some experts believe that vaccination may be conducive to the endemicity of HPAI in poultry populations and/or increase the pandemic potential for humans, others firmly believe that vaccination constitutes a crucial element of effective control and, if used correctly, will ensure long-term benefits for bird and human health (Butler, Citation2005; Capua & Alexander, Citation2008; Ellis et al., Citation2004; Lee et al., Citation2004; Savill et al., Citation2006; Kumar et al., Citation2007; Read et al., Citation2015; Webster et al., Citation2006). Control efforts in most developed countries still rely on stamping out campaigns with compensation and strict biosecurity. Epidemiological changes associated with Gs/GD/96-lineage HPAI viruses, including endemicity in wild birds and infection of mammals, are causing increased dissemination of HPAI viruses globally and challenging current disease control practices. Strategic vaccination as a complementary tool could reinforce current control measures, especially in developing countries and resource-poor communities (Alexander, Citation2007; Sims et al., Citation2016).
Proper vaccine selection requires not only verification of protection against clinical disease but also protection against infection and shedding. For optimal efficacy, it is imperative that the vaccine antigen matches the antigenic profile of the circulating field strain as closely as possible, especially in terms of the HA protein. Since HA proteins of different subtypes do not cross-protect, vaccine antigens need to be of a homologous HA subtype. Due to antigenic drift, the antigenicity of HA can, however, vary within a given subtype. While immunization with a HA of an appropriate subtype usually confers protection against clinical disease and death, significant levels of infection and viral transmission may still occur if the antigenic profile of the infecting field strain has drifted (Criado et al., Citation2020; Lee et al., Citation2004; Swayne, Citation2009; Swayne et al., Citation2013). This study demonstrated that the Zoetis RG-H5N1 vaccine, a product containing the HA of the genetically related HPAI virus that caused the 2014/2015 US outbreak (A/gyrfalcon/Washington/41088-6/2014 H5N8, clade 2.3.4.4) (96.1% genetic similarity) induced robust protection against the South African A/Ck/SA/275401/17 (H5N8) virus. SPF chickens immunized with this vaccine developed strong humoral responses and resisted a potentially lethal dose of the South African HPAI isolate A/Ck/SA/275401/17 (H5N8), without developing clinical disease. In addition, virus shedding was prevented in 80% of the vaccinated chickens or reduced by several orders of magnitude. While, for most vaccinees, the level of shedding was below the detection threshold, the four birds which tested positive excreted mere traces of viral RNA for a limited period (<48 h).
The serological assessment corroborated a close antigenic relationship between the Zoetis RG-H5N1 vaccine antigen, and the South African HPAI H5N8 strain. Birds immunized with Zoetis RG-H5N1 developed high HI titres against the A/Ck/SA/275401/17 (H5N8) antigen and much lower titres against the antigenically-divergent A/duck/SA/811/04 (H5N1). The chickens vaccinated with Benchmark-H5N8 had slightly higher, but comparable, levels of antibodies against homologous H5N8 and heterologous H5N1 antigens. While it is tempting to speculate that the slightly stronger serological response from the Benchmark-H5N8 group reflected the contribution of a matching neuraminidase, it should be mentioned that the antigen concentration, method of antigen inactivation, choice of adjuvant and the method of vaccine formulation were not standardized between Zoetis RG-H5N1 and the two benchmark vaccines.
A minor limitation in the serological assessment involved the use 0.33% chicken RBCs during HI testing. While this approach improved the clarity of the RBC pellets and offered an easier way to distinguish between complete and partial inhibition, this approach is different from the widely used 1% red blood cell concentration, as recommended by the WOAH (OIE, Citation2022). Although this approach facilitated the serological assessment in our laboratory, it may complicate the comparison of these results with the results from other studies.
The phylogenetically more distant Benchmark-H5N1 HA (87.6% genetic similarity) differed by one to several amino acids in each of the five major epitope regions of the protein and in some of the known glycosylation sites (Abolnik et al., Citation2006; Citation2019; Duvvuri et al., Citation2009). Considering the dissimilarity of the HA antigen in Benchmark-H5N1, it was surprising that this vaccine reduced post-vaccinal shedding to levels comparable to that observed amongst the groups vaccinated with Benchmark-H5N8 and Zoetis RG-H5N1 (Kruskal–Wallis test, P = 0.330, α = 0.05). Interestingly, viral RNA shedding via gastro-intestinal secretions appeared to be more prevalent amongst the Benchmark H5N1 birds, albeit at very low concentrations.
While quantitative PCR can detect and quantify viral excretion by infected hosts, it does not qualify the infectivity or transmissibility of the agent in the sample. To overcome this limitation, unvaccinated sentinel birds were placed in contact with vaccinated and challenged and sham-vaccinated and challenged birds to monitor the ability of the vaccine to control shed and spread of the challenge virus. Surprisingly, in this study, the virus did not spread to the sentinels co-housed with unvaccinated and challenged control birds; therefore, disruption of virus shedding and/or transmission in the vaccinated groups could unfortunately not be validated. The reason why A/Ck/SA/275401/17 (H5N8) failed to be transmitted in this study remains unknown. It can be speculated that the environmental conditions in the well-ventilated negatively pressurized isolators, with a downward afferent air current and flooring that restricted access to manure, might have been unconducive to aerosol transmission and/or transmission via the faecal-oral route. Considering the high levels of viral RNA that were demonstrated on the choanal and cloacal swabs of the fatally-infected control birds, and the close contact observed between the challenged birds and the sentinels, this study raises new questions about the critical elements required in transmission studies with HPAI.
Acknowledgements
The authors would like to thank Dr Hannes Swart from Avi-Farms for the donations made to the project. The authors gratefully acknowledge Marlé Grobler, Shirley Luhwareni, Xing Stander, of Roche Diagnostics for the training and assistance of Deltamune personal during the preparatory phase of the trial, as well as the technical members of Roche Diagnostics for the support and assistance during the installations and use of the LightCycler® 480 and MagNa Pure 96.
Disclosure statement
The authors L. Maartens and B. Erasmus declare that they have no conflict of interest. Zoetis, the registration holder of the vaccine Avian Influenza Vaccine H5N1-RG, contributed financially to this study through the Zoetis A.L.P.H.A. initiative. The authors S. Dawson and L. Frizzo da Silva are employed by Zoetis. N. Love was employed by Zoetis at the time of this study.
Additional information
Funding
References
- Abolnik, C., Cornelius, E., Bisschop, S.P., Romito, M. & Verwoerd, D. (2006). Phylogenetic analyses of genes from South African LPAI viruses isolated in 2004 from wild aquatic birds suggests introduction by Eurasian migrants. Developmental Biology, 124, 189–199.
- Abolnik, C., Pieterse, R., Peyrot, B.M., Choma, P., Phiri, T.P., Ebersohn, K., van Heerden, C.J., Vorster, A.A., van der Zel, G., Geertsma, P.J., Laleye, A.T., Govindasamy, K. & Rauff, D.L. (2019). The incursion and spread of highly pathogenic avian influenza H5N8 clade 2.3.4.4 within South Africa. Avian Diseases, 63, 149–156.
- Alexander, D. J. (2007). An overview of the epidemiology of avian influenza. Vaccine, 25, 5637–5644.
- Antigua, K.J.C., Choi, W.S., Baek, Y.H. & Song, M.S. (2019). The emergence and decennary distribution of clade 2.3.4.4 HPAI H5Nx. Microorganisms, 7, 1–16.
- Avian Influenza Community Reference Laboratory. Detection of influenza A matrix gene by real time Taqman® RT-PCR. SOP VI 493 Edition 2. https://science.vla.gov.uk/flu-lab-net/docs/pub-protocol-ai-vi493.pdf.
- Becker, W.B. (1966). The isolation and classification of tern virus: influenza A/tern/South Africa/1961. Journal of Hygiene, 64, 309–320.
- Bertran, K., Balzli, C., Lee, D., Suarez, D.L., Kapczynski, D.R. & Swayne, D.E. (2017). Protection of White Leghorn chickens by U.S. emergency H5 vaccination against clade 2.3.4.4 H5N2 high pathogenicity avian influenza virus. Vaccine, 35, 6336–6344.
- Bureau for Food and Agricultural Policy (BFAP). (2018). Report: Economic impact of the 2017 Highly Pathogenic Avian Influenza outbreak in South Africa.
- Butler, D. (2005). Vaccination will work better than culling, say bird flu experts. Nature, 434, 810–810.
- Cannon, J.C. (2019). Bird flu in Namibia’s penguins wanes, after killing nearly 500. https://news.mongabay.com/.
- Capua, I. & Alexander, D.J. (2008). Avian influenza vaccines and vaccination in birds. Vaccine, 26, D70–D73.
- Criado, M.F., Sá e Silva, M., Lee, D.-H., Salge, C.A.L., Spackman, E., Donis, R., Wan, X.-F. & Swayne, D.E. (2020). Cross-protection by inactivated H5 pre-pandemic vaccine seed strains against diverse Goose/Guangdong lineage H5N1 highly pathogenic avian influenza viruses. Journal of Virology, 94, e00720–20.
- Duvvuri, V.R.S.K., Duvvuri, B., Cuff, W.R., Wu, G.E. & Wu, J. (2009). Role of positive selection pressure on the evolution of H5N1 hemagglutinin. Genomics, Proteomics & Bioinformatics, 7, 47–56.
- Ellis, T.M., Leung, C.Y.H.C., Chow, M.K.W., Bissett, L.A., Wong, W., Guan, Y. & Peiris, J.S.M. (2004). Vaccination of chickens against H5N1 avian influenza in the face of an outbreak interrupts virus transmission. Avian Pathology, 33, 405–412.
- Global Consortium for H5N8 and Related Influenza Viruses. (2016). Role for migratory wild birds in the global spread of avian influenza H5N8. Science, 354, 213–217.
- Hoffman, E., Krauss, S., Perez, D., Webby, R. & Webster, R.G. (2002). Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine, 20, 3165–3170.
- Khomenko, S., Abolnik, C., Roberts, L., Waller, L., Shaw, K., Monne, I., Taylor, J., Dhingra, M., Pittiglio, C., Mugyeom, M., Roche, X., Fredrick, K., Kamata, A., Okuthe, S., Kone, P., Wiersma, L., Von Dobschuetz, S., Soumare, B., Makonnen, Y., Morzaria, S. & Lubroth, J. (2018). 2016–2018 Spread of H5N8 highly pathogenic avian influenza (HPAI) in sub-Saharan Africa: Epidemiological and ecological observations, Food and Agriculture Organization of the United Nations. FOCUS ON, No. 12, August 2018, Rome.
- Kumar, M., Chu, H.-J., Rodenberg, J., Krauss, S. & Webster, R.G. (2007). Association of serologic and protective responses of avian influenza vaccines in chickens. Avian Diseases, 51, 481–483.
- Lee, C.-W., Senne, D.A. & Suarez, D.L. (2004). Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. Journal of Virology, 78, 8372–8381.
- Lee, C.-W. & Suarez, D.L. (2004). Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. Journal of Virological Methods, 119, 151–158.
- Lee, D.-H., Bertran, K., Kwon, J.-H. & Swayne, D.E. (2017). Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. Journal of Veterinary Science, 18, 269–280.
- Nagy, A., Vostinakova, V., Pirchanova, Z., Cernikova, L., Dirbakova, Z., Mojzis, M., Jirincova, H., Havlickova, M., Dan, A., Ursu, K., Vilcek, S. & Hornickova, J. (2010). Development and evaluation of a one-step real-time RT-PCR assay for universal detection of influenza A viruses from avian and mammal species. Archives of Virology, 155, 665–673.
- Ndumu, D., Zecchin, B., Fusaro, A., Arinaitwe, E., Erechu, R., Kidega, E., Kayiwa, J., Muwanga, E., Kirumira, M., Kirembe, G., Lutwama, J. & Monne, I. (2018). Highly pathogenic avian influenza H5N8 clade 2.3.4.4B virus in Uganda, 2017. Infection, Genetics and Evolution, 66, 269–271.
- OFFLU. (2018). OFFLU Avian Influenza Post VCM Report. Avian Influenza Events for the period September 2017 to February 2018.
- OIE. (2017). ‘Immediate notification report, AI_H5N8_2017’, Ref OIE: 24127, 22/06/2017, South Africa.
- OIE. (2022). Chapter 3.3.4. Avian Influenza (including infection with high pathogenicity avian influenza viruses). In: Manual for Diagnostic Tests and Vaccines for Terrestrial Animals 2022. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/.
- Read, A.F., Baigent, S.J., Powers, C., Kgosana, L.B., Blackwell, L., Smith, L.P., Kennedy, D.A., Walkden-Brown, S.W. & Nair, V.K. (2015). Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biology, 13(7). DOI:10.1371/journal.pbio.1002198
- Reed, L.J. & Muench, H. (1938). A simple method of estimating fifty percent endpoints. American Journal of Epidemiology, 27, 493–497.
- Reverberi, R. (2008). The statistical analysis of immunohaematological data. Blood Transfusion, 6, 37–45.
- Savill, N.J., St Rose, S.G., Keeling, M.J. & Woolhouse, M.E.J. (2006). Silent spread of H5N1 in vaccinated poultry. Nature, 442, 757–757. PMID: 16915278.
- Sims, L., Harder, T., Brown, I., Gaidet, N., Belot, G., von Dobschuetz, S., Kamata, A., Kivaria, F., Palamara, E., Bruni, M., Raizman, E. & Lubroth, J. (2017). Highly pathogenic H5 avian influenza in 2016 and 2017 – observations and future perspectives, Food and Agriculture Organization of the United Nations. FOCUS ON, No. 11, November 2017, Rome.
- Sims, L., Tripodi, A., von Dobschuetz, S., Gardner, E. & Aguanno, R. (2016). Rational use of vaccination for control and prevention of H5 highly pathogenic avian influenza, Food and Agriculture Organization of the United Nations, FOCUS ON, No. 10, May 2016, Rome.
- Swayne, D.E. (2009). Avian influenza vaccines and therapies for poultry. Comparative Immunology, Microbiology and Infectious Diseases, 32, 351–363.
- Swayne, D.E., Suarez, D.L. & Sims, L.D. (2013). Influenza. In D.E. Swayne (Ed.), Diseases of poultry 13th Ed. (pp. 181–218). Wiley & Sons Inc.
- Vijaykrishna, D., Bahl, J., Riley, S., Duan, L., Zhang, J.X., Chen, H., Peiris, J.S.M., Smith, G.J.D. & Guan, Y. (2008). Evolutionary dynamics and emergence of panzootic H5N1 influenza viruses. PLoS Pathogens, 4, e1000161.
- Webster, R.G., Webby, R.J., Hoffman, E., Rodenberg, J., Kumar, M., Chu, H.-J., Seiler, P., Krauss, S. & Songserm, T. (2006). The immunogenicity and efficacy against H5N1 challenge of reverse genetics-derived H5N3 influenza vaccine in ducks and chickens. Virology, 351, 303–311.
- Wild, S. (2018). Avian flu freezes coastal bird research in South Africa. Nature News, 1 28 March 2018.
