ABSTRACT
Enterococcus cecorum lesion strains cause more embryonic mortality after inoculation into the albumen of embryonated eggs compared to cloaca strains. We hypothesized that these strain differences are a result of differences in sensitivity to the antimicrobial effects of the albumen. In this study, the sensitivity of 14 lesion strains and 14 cloaca strains to albumen from 12-day incubated and non-incubated eggs was assessed. A major antimicrobial protein of the albumen is lysozyme and, therefore, the lysozyme resistance of all strains was determined. Albumen from 12-day incubated and non-incubated eggs was inoculated with four cloaca strains and four lesion strains (104 CFU/tube). Based on the results, in a subsequent experiment, all 28 strains were inoculated only into albumen from non-incubated eggs. For all strains, the minimal inhibitory concentrations (MIC) of lysozyme were determined using an agar dilution method and the growth rates in broth with (500 and 2000 µg/ml) or without lysozyme were assessed. Compared to cloaca strains, lesion strains had 320 times higher odds of being reisolated from albumen (95% confidence interval: 55–3962) and had 8.5 times higher reisolation ratios (reisolation dose/inoculation dose) (95% confidence interval: 4.8–15.3). Thirteen cloaca strains had a MIC ranging from 1000 to 8000 µg/ml, while one cloaca strain and all lesion strains were resistant to the lysozyme concentrations tested. Growth rates of cloaca strains were decreased more by lysozyme compared to lesion strains. In conclusion, lesion strains had higher survival rates in egg albumen and were more resistant to lysozyme compared to cloaca strains.
RESEARCH HIGHLIGHTS
Egg albumen inhibits Enterococcus cecorum cloaca strains more than lesion strains.
Enterococcus cecorum lesion strains are resistant to high concentrations of lysozyme.
Lysozyme resistance could enhance survival in albumen and body fluids.
Introduction
Clinical outbreaks of Enterococcus cecorum are characterized by lameness or paralysis of affected birds and the typical macroscopic lesions are pericarditis, arthritis, and osteomyelitis of the femur, tibia, and the free thoracic vertebrae (Herdt et al., Citation2009; Kense & Landman, Citation2011; Borst et al., Citation2012; Robbins et al., Citation2012; Jung & Rautenschlein, Citation2014; Borst et al., Citation2017; Jung, Petersen et al., Citation2017). An outbreak can have a major economic impact as mortality and condemnation rates can be elevated. No specific E. cecorum strains are known to be responsible for outbreaks in chickens, but strains isolated from lesions (pathogenic strains) are genetically less diverse compared to strains isolated from the cloaca (commensal strains) (Kense & Landman, Citation2011; Boerlin et al., Citation2012; Borst et al., Citation2012; Dolka et al., Citation2016; Manders et al., Citation2022; Laurentie et al., Citation2023). This suggests that pathogenic E. cecorum strains have specific traits for virulence, and identification of virulence traits could be used to differentiate pathogenic strains from commensal E. cecorum strains.
In different studies, embryo lethality assays were used to type E. cecorum isolates (Borst et al., Citation2014; Jung, Metzner et al., Citation2017; Dolka et al., Citation2022; Manders et al., Citation2022; Huang et al., Citation2023). Manders et al. (Citation2022) showed that inoculation into the albumen was the best inoculation route for virulotyping E. cecorum. After inoculation into the albumen of 12-day embryonated broiler eggs, mortality rates for lesion strains were much higher compared to cloaca strains (Manders et al., Citation2022). This difference in mortality rates could be explained by the differences in virulence and sensitivity to the antimicrobial effects of the albumen.
Egg albumen is the main source of water and proteins for the growing chicken embryo and, importantly, it also protects the embryo against microbial infection. The hostile environment of the albumen to microbes is a result of antimicrobial proteins and physicochemical properties. During the first half of the incubation period, the antimicrobial activity of the albumen changes (Willems et al., Citation2014; Guyot et al., Citation2016). The pH of the albumen increases from approximately neutral to alkaline during storage (pre-incubation), while, during the first 12 days of incubation, it gradually decreases back to neutral (Guyot et al., Citation2016). During this period, the water in the albumen is distributed to other extra-embryonic compartments and the albumen diminishes to one-third of its original volume, while the amount of solids remains nearly constant (Romanoff & Romanoff, Citation1967). This results in an increased concentration of proteins in the albumen and increased viscosity.
A large number of potential antibacterial proteins are present in the albumen, of which lysozyme is one of the major antimicrobial components. Lysozyme exerts its antibacterial effects through the hydrolysis of cell wall peptidoglycan and cationic antimicrobial peptide activity causing membrane permeabilization, which affects Gram-positive and Gram-negative bacteria (Ragland & Criss, Citation2017). In addition to its role in the albumen, lysozyme also plays a major role in the innate immune system. The protein is excreted in mucus in the oviduct, lungs, and intestines. The Gram-positive pathogen Enterococcus faecalis was shown to be resistant to lysozyme (Benachour et al., Citation2012). The resistance of pathogenic or commensal E. cecorum strains to lysozyme is unknown.
The aim of this study was to examine the sensitivity to the antimicrobial effect of egg albumen and the resistance to lysozyme of E. cecorum strains isolated from the cloaca of healthy broiler reproduction chickens (cloaca strains) and strains originating from typical lesions in broilers (lesion strains). We hypothesized that: (1) cloaca strains are more sensitive to the antimicrobial effect of egg albumen than lesion strains and (2) lesion strains have a higher lysozyme resistance compared to cloaca strains. To test these hypotheses four experiments were performed. (1) Four cloaca strains and four lesion strains were inoculated into albumen from non-incubated and 12-day incubated eggs and the viable bacterial doses were assessed at three different timepoints (0.25, 24, and 48 h post-inoculation). (2) The viable bacterial doses of 14 cloaca strains and 14 lesion strains were determined after inoculation in the albumen from non-incubated eggs at two different timepoints (24 and 48 h post-inoculation). (3) The minimal inhibitory concentrations of lysozyme were determined for 14 cloaca strains and 14 lesion strains using an agar dilution method. (4) Bacterial growth curves of the E. cecorum strains without and with 500 and 2000 µg/ml lysozyme were compared.
Materials and methods
E. cecorum strains
The 28 E. cecorum strains used in this study were previously described by Manders et al. (Citation2022). Briefly, 14 E. cecorum strains were isolated from cloacal swabs taken from clinically healthy broiler reproduction flocks (cloaca strains). Bacterial culture of swabs of typical E. cecorum lesions (pericarditis, femur head necrosis, arthritis, and spondylitis) in broilers was performed, and when E. cecorum was isolated in pure culture these strains were preserved (lesion strains) (). Commensal E. cecorum bacteria vary in their ability to ferment mannitol while pathogenic strains are not able to ferment mannitol (Borst et al., Citation2012; Jung, Metzner et al., Citation2017). Therefore, only E. cecorum strains originating from cloaca swabs which were able to ferment mannitol, and only E. cecorum strains isolated from lesions which were unable to ferment mannitol were included. For both cloaca strains and lesion strains, only one E. cecorum strain per flock was selected. Whole genome sequencing showed that the strains were not clonally related (Manders et al., Citation2022).
Table 1. Characteristics of E. cecorum strains used in the study.
Rationale and design of experiments
The survival or bacterial growth of E. cecorum strains in chicken albumen was determined in two experiments. In experiment 1, albumen originating from non-incubated eggs and 12-day incubated eggs was inoculated with four lesion strains, four cloaca strains, and a placebo. At three different timepoints (0.25, 24, and 48 h post-inoculation), the bacterial dose in the albumen was assessed.
The inoculum did not homogenize properly with the viscous albumen of 12-day incubated eggs. After sampling with an inoculation loop, the fluid inoculum could be over- or under-represented in the sample. Therefore, a timepoint of 0.25 h post-inoculation was included in the experimental design. We hypothesized that the bacterial dose would not be affected by the albumen after 0.25 h and that the same bacterial dose as the inoculation dose should, therefore, be recovered if the albumen and inoculum homogenize properly.
The results of experiment 1 showed that the reisolated dose of bacteria in albumen of non-incubated eggs at timepoint 0.25 h post-inoculation was similar to the inoculation dose. Therefore, for experiment 2 only albumen of non-incubated eggs was used. All 28 E. cecorum strains were inoculated and the viable bacterial dose in the albumen was assessed at 24 and 48 h post-inoculation.
In the last two experiments, the inhibitory capacity of lysozyme, a major antimicrobial component of the albumen, was assessed in a lysozyme MIC assay (experiment 3) and bacterial growth curves were obtained with and without lysozyme (experiment 4).
Inocula preparation
E. cecorum strains were recovered from cryopreservation (15% glycerol stocks at −80°C), plated on Columbia Agar plates supplemented with 5% Sheep Blood (CBA) (PB5039A, Thermo Fisher Scientific Inc., Waltham, MA, USA), and incubated at 37°C in a 5% CO2-enriched atmosphere. After 24 h of incubation, several colonies were scraped off the plate and suspended in Dulbecco’s phosphate-buffered saline (PBS) (GibcoTM, Thermo Fisher Scientific Inc.) to an optical density of 0.5 McFarland (McFarland Densitometer, type DEN-1, Grant instruments Ltd., Shepreth, England), which corresponds to approximately 108 colony-forming units (CFU)/ml. This suspension was serially diluted with peptone physiological saline (PPS) (Biotrading, Mijdrecht, the Netherlands) to a final concentration of approximately 0.5 × 105 CFU/ml for experiments 1 and 2 and 106 CFU/ml for experiment 3. For experiment 4 the suspension was serially diluted with Todd–Hewitt broth (Oxoid Ltd., Basingstoke, UK) with 1% yeast extract (THBY) (Oxoid Ltd) to a final concentration of approximately 106 CFU/ml. The final bacterial concentrations of the inocula were assessed by colony counting of ten-fold serial dilutions on CBA.
Albumen sensitivity tests
Albumen collection
Hatching eggs were collected on the day of lay from a Ross 308 broiler breeder flock when the birds were 34 and 39 weeks of age for experiment 1 and 2, respectively. The eggs were transported to the Faculty of Veterinary Medicine of Utrecht University and stored for 24 h at room temperature. Albumen was harvested from non-incubated eggs or from eggs which were incubated for 12 days at 37.5°C, 53% relative humidity. From all eggs, the albumen was aseptically harvested as follows: eggshells were decontaminated with ethanol 70%, the eggs were broken into two halves and the contents of the eggs were carefully poured into a sterile Petri dish. A minimum of 3 ml of thick albumen was collected with a 5 ml syringe and distributed in 15 ml tubes (62.554.502, Sarstedt, Nümbrecht, Germany) in experiment 1 or 2 ml microtubes in experiment 2. To ensure repeatability of the conditions within strains, albumen originating from a single egg was used for one strain for every timepoint post-inoculation.
Inoculation of the albumen
The albumen from 12-day incubated eggs is very viscous, which made it impossible to standardize the volume of albumen in the tubes. Therefore, approximately 1 ml of albumen was injected into tubes and the exact weight of the albumen was determined. A volume of 200 µl inoculum, containing approximately 5 × 105 CFU/ml, was added to each tube by using a pipette (Thermo Fisher Scientific Inc.), resulting in 104 CFU/tube. To homogenize the albumen with the inoculum, 1 ml of PBS was added to the 15 ml tube in experiment 1. The addition of PBS to the albumen was not included in experiment 2 as only albumen from non-incubated eggs was used, which is less viscous compared to albumen from 12-day incubated eggs. Thereafter, in a pilot study, no differences in results were obtained with and without the addition of PBS (data not shown). Before incubation at 37°C, the tubes were thoroughly vortexed for a minimum of 30 s. The final bacterial doses of the inoculum were assessed, the inoculum volume was standardized (200 µl/tube) and the weight of the albumen per tube was determined; with this information the inoculation doses per gram albumen were calculated.
Reisolation
At 0.25, 24, and 48 h post-inoculation, samples of the albumen were taken by inserting a 10 µl inoculation loop in the central axis of the tubes. The collected albumen was distributed equally on a CBA plate with the inoculation loop and the plates were incubated at 37°C in a 5% CO2-enriched atmosphere. Due to the viscosity of the albumen, pipetting was not possible and samples were collected with inoculation loops. These inoculation loops were weighed on an analytical balance (Sartorius ENTRIS64-1S, Sartorius Lab Instruments GmbH & Co., Goettingen, Germany) before and after the albumen was plated, and the amount of albumen that was plated was noted. After 48 h incubation the number of CFU on the plate was counted and the number of viable bacteria per gram of albumen (bacterial dose) was calculated. Bacterial doses were assessed in quadruplicate per strain and time-point.
Due to the viscosity of the albumen, it was not feasible to standardize the volume of albumen per tube. Although the preparation of the inocula was standardized, the bacterial dose of the inoculum varied slightly between strains and experiments (data not shown). The foregoing resulted in differences in the bacterial dose between tubes inoculated with the same strain and between strains. Therefore, the bacterial dose after reisolation was divided by the bacterial dose directly after inoculation and presented as a ratio (reisolation ratio).
Lysozyme MIC assay
A previously described lysozyme MIC assay was slightly modified and used for this study (Wichgers Schreur et al., Citation2012). Columbia blood agar plates with or without hen eggwhite lysozyme (CBAL) (Sigma-Aldrich, Zwijndrecht, the Netherlands) were made in-house. Two-fold increasing concentrations of lysozyme ranging from 62.5 to 8000 µg/ml were used. MICs were determined by spotting a droplet of 10 µl inoculum on the CBAL plates (approximately 104 CFU/spot). In total, 21 CBAL plates per lysozyme concentration were produced; for each plate, four different strains and negative control (physiological saline) were spotted (seven different plates) and for each strain and lysozyme concentration the MIC assay was performed in triplicate. Bacterial growth was evaluated after incubation at 37°C in a 5% CO2-enriched atmosphere for 24 h. The minimal concentration of lysozyme in which no bacterial growth was observed was designated as the MIC value. In case the MIC values differed between the three replicates, the highest concentration was used for the MIC value.
Assessment of bacterial growth in the presence of lysozyme
The bacterial growth of all 28 E. cecorum strains in THBY and THBY with two different lysozyme concentrations was assessed. Wells of a sterile 96-well flat bottom microplate were filled with 100 µl of THBY with 106 CFU/ml suspended E. cecorum bacteria (inocula) and with 100 µl of THBY without lysozyme or with a lysozyme solution of 1000 or 4000 µg/ml in THBY (final lysozyme concentrations 500 and 2000 µg/ml, respectively).
Microplates were incubated at 37°C in a 5% CO2-enriched atmosphere and the optical density (OD) at 600 nm was measured (Synergy HTX multi-mode microplate reader, BioTek® instruments, Winooski, VT, USA) every 10 min for 16 h. In between measurements, the microplate was shaken at 282 rpm. Bacterial growth was assessed in duplicate for each strain and control/lysozyme concentration. For each strain, the maximum OD was determined and the time to reach an OD of 0.2 was calculated using GraphPad Prism version 9.3.1 (GraphPad Prism, Citation2022).
Statistics
Data analysis was conducted using the statistical software R 4.2.2. in R studio (R Core Team, Citation2022). Reisolation (yes or no) was analysed using logistic regression with strain as a random effect and with a group (lesion or cloaca strains), time (24 or 48 h post-inoculation), and the interaction between group and time as fixed effects. Akaike information criterion (AIC) was used for model reduction, selecting the model with the lowest AIC. Randomized quantile residuals were used to check for abnormalities. For the final model, 95% profile (log-) likelihood confidence intervals were calculated. Of the positive reisolations, the log-ratio was analysed using a linear mixed effect model with strain as a random effect and with group (lesion or cloaca strains), time (24 or 48 h post-inoculation), and all two-way interactions as fixed effects. AIC was used for model reduction, selecting the model with the lowest AIC. Normal probability plots were used to check normality. For the final model, 95% profile (log-) likelihood confidence intervals were calculated.
Maximum OD was analysed using a linear mixed effect model with strain as a random effect and with group (lesion strains or cloaca strains), dose (0, 500, or 2000 µg lysozyme/ml) and the interaction between the group and dose as fixed effects. AIC was used for model reduction. Normal probability plots were used to check normality. For the final model, 95% profile (log-) likelihood confidence intervals were calculated.
The time to reach an OD of 0.2 was modelled with a Weibull model with dose, group, and their interaction as fixed effects and with strains as clusters using robust estimates for the standard errors.
Ethical statement
Under Dutch law (Dutch Animal Procedure Act (Wet op de dierproeven)) embryonated eggs are not classified as experimental birds.
Results
Albumen sensitivity tests
The results of experiment 1 are shown in . The mean reisolation ratios at 0.25 h post-inoculation from the albumen of 12-day incubated eggs were 1.40 and 1.38 and from the albumen of non-incubated eggs 0.92 and 0.91 for cloaca and lesion strains, respectively. The reisolation ratios for the cloaca strains declined with time in albumen from both 12-day incubated eggs and non-incubated eggs. For lesion strains 15 and 16, reisolation ratios were 23.79 and 4.01 in albumen from 12-day incubated eggs 24 h post-inoculation, and after 48 h the ratio declined to 0.43 and 0.85, respectively. For the other two lesion strains lower reisolation ratios (0.70 and 0.17) were observed at 24 h post-inoculation and the rate increased multiple times at 48 h post-inoculation. The foregoing resulted in a wide range in reisolation ratios for lesion strains in albumen of 12-day incubated eggs compared to cloaca strains. The reisolation ratios in the albumen of non-incubated eggs inoculated with lesion strains declined at each timepoint, but bacteria could be reisolated at both timepoints post-inoculation for all lesion strains (reisolation ratio > 0). In contrast, for cloaca strains only two of the four strains could be reisolated after 48 h and the reisolation ratios were about ten-fold lower.
Table 2. Summary of the descriptive data on the reisolation of Enterococcus cecorum bacteria at three different timepoints post-albumen inoculation. Albumen from 12-day incubated eggs or non-incubated eggs was inoculated with four cloaca and four lesion E. cecorum strains.
In , the results of experiment 2 are presented. For the cloaca strains at 24 h post-inoculation the reisolation yielded no bacterial growth in 2/14 strains, while at 48 h no bacterial growth was observed for 10/14 strains in either of the replicates (Online Supplementary Table 1). For all lesion strains bacterial growth was observed at both timepoints post-inoculation. The logistic regression model showed that, for the lesion strains, it was more likely (odds ratio = 320, 95% confidence interval [CI]: 55–3962) to reisolate the E. cecorum bacteria compared to the cloaca strains. For both cloaca strains and lesion strains at 48 h after the inoculation the likelihood to reisolate bacteria was decreased (odds ratio = 0.016 95%CI: 0.002–0.006). The interaction between group (cloaca strain or lesion strain) and time (24 or 48 h post-inoculation) did not improve the fit of the model and was not included in the final model.
Figure 1. Effect of albumen from non-incubated eggs on the bacterial concentration of 14 cloaca and 14 lesion Enterococcus cecorum strains at 24 and 48 h after inoculation. The bacterial concentration is presented as a ratio (reisolation ratio ± SD) of the initial inoculated dose. Bacterial concentrations were assessed in quadruplicate for each strain and time-point.
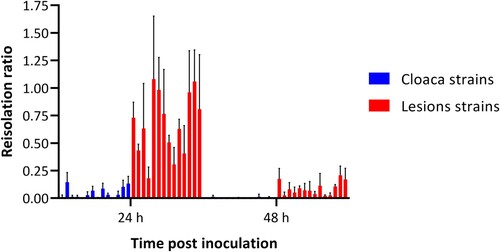
The mean reisolation ratio for the cloaca strains was 0.06 (95% CI: 0.04–0.10). The reisolation ratio of the lesion strains was 8.54 (95% CI: 4.82–15.25) times higher than the ratio for the cloaca group. After 48 h incubation the reisolation ratio declined with a factor of 0.129 (95% CI: 0.102–0.165). The interaction between group and time did not improve the fit of the model and was not included in the final model.
Lysozyme MIC assay
In the agar dilution MIC assay (Online Supplementary Table 1), all lesion strains (n = 14) were resistant to the lysozyme concentrations tested (MIC > 8000 µg/ml), while 13 cloaca strains had a MIC ranging from 1000 to 8000 µg/ml and one cloaca strain was resistant (MIC > 8000 µg/ml). One cloaca strain had a MIC of 1000 µg/ml, seven cloaca strains had a MIC of 4000 µg/ml, and five cloaca strains had a MIC of 8000 µg/ml. In all cases, the MIC values were the same on the three different plates per lysozyme concentrations tested, except for one cloaca strain (strain 2). For this strain, no bacterial growth was observed on one plate with 4000 µg/ml, while, on the other two plates, one and five colonies were present and the MIC was defined as 8000 µg/ml.
Assessment of bacterial growth in the presence of lysozyme
The mean times to reach an OD of 0.2 at 600 nm and the mean maximum OD at 600 nm of the cloaca strains and lesion strains grown in THBY with or without the addition of lysozyme are given in . For all lesion strains an OD of 0.2 was reached within 16 h of incubation, unlike for 10 cloaca strains. The maximum OD of these 10 cloaca strains at a lysozyme concentration of 500 and 2000 µg/ml ranged from 0.116 to 0.199 and 0.025 to 0.189, respectively. Only one cloaca strain (strain 12) did not reach an OD of 0.2 in both concentrations. Therefore, the time to reach an OD of 0.2 could not be determined for these cloaca strains (Online Supplementary Table 1).
Table 3. Bacterial growth of 14 cloaca and 14 lesion Enterococcus cecorum strains in the presence of three different lysozyme concentrations (0, 500, and 2000 µg/ml).
Because there was an interaction between group and dose in the linear mixed effect and Weibull models, dose-effect per group and their confidence intervals were calculated by using the nested version of the model. Compared to cloaca strains grown without the addition of lysozyme, the median times to reach an OD of 0.2 were 3.54 (95% CI: 2.83–4.44) and 3.76 (95% CI: 3.10–4.56) times longer for cloaca strains grown with 500 or 2000 µg lysozyme/ml, respectively. Although an inhibitory effect of lysozyme on lesion strains was observed, this effect was smaller than for cloaca strains. The median times to reach an OD of 0.2 were 1.06 (95% CI: 1.01–1.12) and 1.32 (95% CI: 1.14–1.53) times longer for lesion strains grown with 500 and 2000 µg lysozyme/ml, respectively, compared to the growth of lesion strains without lysozyme.
The observed maximum ODs for the cloaca strains were lower when lysozyme was added to the growth medium. Without lysozyme, the maximum OD for cloaca strains was 0.44 (95% CI: 0.40–0.48), but it was 0.19 (95% CI: 0.14–0.24) and 0.22 (95% CI: 0.17–0.27) lower when these strains were grown with 500 or 2000 µg lysozyme/ml, respectively. The maximum OD for the lesion strains without lysozyme was 0.51 (95% CI: 0.45–0.56). No effect on the maximum OD was observed between lesion strains grown without lysozyme and with 500 µg lysozyme/ml. But when lesion strains were grown with 2000 µg lysozyme/ml the maximum OD was 0.11 (95% CI: 0.06–0.16) lower compared to the lesion strains without the addition of lysozyme.
Discussion
A previous study by our group showed that virulotyping of different E. cecorum isolates can be done using an embryo lethality assay (ELA) where bacteria are inoculated in the albumen of 12-day incubated eggs (Manders et al., Citation2022). Differences in embryo mortality for eggs inoculated with cloaca or lesion strains could be caused by differences in virulence and sensitivity to the antimicrobial effects of albumen. The aim of this study was to test the sensitivity of cloaca-associated and lesion-associated E. cecorum strains to the antimicrobial effect of albumen. Therefore, the albumen from 12-day incubated eggs (day of incubation in the ELA) and albumen from non-incubated eggs were inoculated. Thereafter, the resistance to lysozyme, one of the main antimicrobial proteins of the albumen, was determined. For all experiments, the same set of E. cecorum strains, i.e. strains isolated from cloacas of healthy broiler reproduction chickens or typical lesions, were used ().
In the embryonated egg, the albumen is transferred to the amniotic cavity via the sero-amniotic cavity from embryo day 12 onwards (Deeming, Citation1991; Manders et al., Citation2021). When E. cecorum isolates survive the hostile environment of the albumen upon transfer to the amniotic cavity and can cause embryo mortality, these strains can be classified as virulent in an ELA. In this study, the sensitivity to albumen was tested by inoculating E. cecorum into albumen and reisolating and quantifying bacteria after incubation. For lesion strains, reisolations were always successful. Conversely, the reisolation failed for some cloaca strains at 24 h (2/14) and most cloaca strains (10/14) at 48 h ( and ). Additionally, reisolation ratios were generally higher for lesion strains compared to cloaca strains (). These results showed that lesion strains are less sensitive to the antimicrobial effect of albumen compared to cloaca strains.
Albumen can be divided into thick, inner, and outer thin albumen; approximately 60% of the total volume of albumen consists of thick albumen (Heath, Citation1978). The antimicrobial protein concentrations in thick and thin albumen do not differ; however, the antimicrobial effect of thick albumen is stronger compared to thin albumen (Fang et al., Citation2012).
Lysozyme is a key component of the innate immune system and is present in high concentrations (>500 µg/ml) in different body fluids of mammals (Benachour et al., Citation2012; Ragland & Criss, Citation2017). Three different types of lysozyme have been identified: c-type (chicken type), g-type (goose type), and i-type (invertebrate type). C-type and g-type lysozymes are found in vertebrates and both types have highly similar antimicrobial properties (Nile et al., Citation2004). In chickens, c-type lysozyme is predominantly found in the oviduct and the egg. In the intestines of young chickens up to 8 days old, the expression of c-type lysozyme was found, while in the intestines of older birds, as well as in lungs and bone marrow, the g-type lysozyme gene was expressed (Nakano & Graf, Citation1991; Nile et al., Citation2004). In the present study, the resistance of E. cecorum strains to lysozyme was tested with c-type lysozyme from hen eggwhite.
A crucial step in the pathogenesis of E. cecorum infections is the colonization of the intestines. In broiler flocks experiencing an E. cecorum outbreak, this colonization occurs in the first week of life (Borst et al., Citation2017; Jung, Petersen et al., Citation2017). As lysozyme is present in several body fluids such as the intestines (Nakano & Graf, Citation1991), resistance to lysozyme or other antimicrobial peptides may give pathogenic E. cecorum isolates an advantage to colonize the intestines.
Based on a lysozyme MIC assay using blood agar plates and an assessment of bacterial growth in THBY in the presence of lysozyme, we conclude that lesion strains are more resistant to lysozyme compared to cloaca strains. All lesion strains were resistant to 8000 µg/ml lysozyme in the MIC agar assay and only the highest lysozyme concentration tested (2000 µg/ml) inhibited the growth rates of lesion strains. For most cloaca strains (13/14) a MIC for lysozyme could be determined and 500 µg/ml lysozyme already inhibited bacterial growth ( and Online Supplementary Table 1).
Higher tolerance to lysozyme (up to 50 mg/ml) has been found in pathogenic E. faecalis and Staphylococcus aureus, when compared to other Gram-positive bacteria (Benachour et al., Citation2012). Lysozyme resistance mechanisms in bacteria are based on either: (1) modifying the peptidoglycan to protect against hydrolysis by lysozyme, (2) alternating bacterial envelope charge, or (3) expressing lysozyme inhibitors (Ragland & Criss, Citation2017). These mechanisms have not been described for E. cecorum and would be of interest for further research. The full genomes of the strains used may unveil potential genes that explain the differences in lysozyme resistance between cloaca and lesion strains.
All lesion strains were resistant to lysozyme and had higher reisolation ratios after albumen inoculation compared to the cloaca strains. Only one cloaca strain (strain 4) was resistant to lysozyme but did not cause embryo mortality in the ELA (Online Supplementary Table 1). After 48 h this strain could not be reisolated from albumen from 12-day and non-incubated eggs. Although this strain was resistant to high concentrations of lysozyme in the MIC assay, it may have been killed by other antimicrobial proteins in the albumen or the physiochemical properties of the albumen.
Generation time for many common bacteria is quite short under optimal conditions and is longer under the influence of several environmental factors, such as pH, temperature, and the presence of nutrients (Nagaraja, Citation2022). Under optimal conditions, Enterococcus faecalis doubles in approximately 26 min (Powell, Citation1955). In experiment 1, an interval of 15 min between inoculation and reisolation was chosen to ensure no bacterial multiplication had taken place before sampling. With an accurate sampling method, a bacterial dose close to the inoculation dose should be recovered, which would result in a reisolation ratio close to 1.00. Reisolation ratios of 1.40 and 1.38 were obtained for albumen originating from 12-day incubated eggs for cloaca and lesion strains, respectively. For albumen from non-incubated eggs, ratios were closer to 1.00, with 0.92 and 0.91 for cloaca strains and lesion strains, respectively (). A possible explanation for reisolation ratios above 1 was the difficulty to homogenize the inoculum with the viscous albumen from 12-day incubated eggs. The unhomogenized more fluid inocula may have been sampled more effectively with the inoculation loop compared to the viscous albumen. Therefore, albumen from non-incubated eggs was used in experiment 2.
The antibacterial activity of proteins in the albumen decreases during incubation, but the increased concentration of these proteins may partly counterbalance this loss of activity. Furthermore, the pH of the albumen goes up from neutral to alkaline during pre-incubation and from alkaline back to neutral during incubation. High pH has a negative influence on bacterial growth and also alters the antibacterial properties of proteins, especially lysozyme (Guyot et al., Citation2016). Therefore, we expected to see higher reisolation ratios in albumen from 12-day incubated eggs compared to albumen from non-incubated eggs for cloaca and lesion strains. Indeed, in this study, only high reisolation ratios were obtained after inoculation with lesion strains in the albumen of 12-day incubated eggs at 24 or 48 h post-inoculation, while, in the albumen from non-incubated eggs, the reisolation ratios progressively decreased. Decreasing isolation ratios were also observed for cloaca strains for both types of albumen.
To reduce the number of embryos used for ELA, an alternative assay to virulotype E. cecorum strains is desirable. The results of the lysozyme MIC assay corresponded with the results of the ELA. Most cloaca strains (13/14) were not resistant to lysozyme and also caused no or limited embryonic mortality in an ELA. For lesion strains the opposite was true; these strains were resistant to lysozyme and caused high mortality rates in the ELA. The lysozyme MIC assay could be a replacement for the ELA as a quick virulence screening test for E. cecorum isolates. However, the validation of the lysozyme MIC assay with more E. cecorum strains is required.
In conclusion, we showed that E. cecorum strains isolated from lesions were less sensitive to the hostile environment of the albumen from incubated (12 days) and non-incubated eggs and were more resistant to lysozyme compared to strains isolated from cloacas. A lysozyme MIC assay could be a quick animal-free screening test for virulotyping of E. cecorum isolates.
Supplemental Material
Download MS Word (30.1 KB)Acknowledgements
We thank Dr W.J.M. Landman and Dr J.H.H. van Eck for their bright minds and thoughts on the experimental design and Dr J. van den Broek for his comprehensive help with the statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Benachour, A., Ladjouzi, R., Le Jeune, A., Hebert, L., Thorpe, S., Courtin, P., Chapot-Chartier, M.P., Prajsnar, T.K., Foster, S.J. & Mesnage, S. (2012). The lysozyme-induced peptidoglycan N-acetylglucosamine deacetylase PgdA (EF1843) is required for Enterococcus faecalis virulence. Journal of Bacteriology, 194, 6066–6073.
- Boerlin, P., Nicholson, V., Brash, M., Slavic, D., Boyen, F., Sanei, B. & Butaye, P. (2012). Diversity of Enterococcus cecorum from chickens. Veterinary Microbiology, 157, 405–411.
- Borst, L.B., Suyemoto, M.M., Keelara, S., Dunningan, S.E., Guy, J.S. & Barnes, H.J. (2014). A chicken embryo lethality assay for pathogenic Enterococcus cecorum. Avian Diseases, 58, 244–248.
- Borst, L.B., Suyemoto, M.M., Robbins, K.M., Lyman, R.L., Martin, M.P. & Barnes, H.J. (2012). Molecular epidemiology of Enterococcus cecorum isolates recovered from enterococcal spondylitis outbreaks in the southeastern United States. Avian Pathology, 41, 479–485.
- Borst, L.B., Suyemoto, M.M., Sarsour, A.H., Harris, M.C., Martin, M.P., Strickland, J.D., Oviedo, E.O. & Barnes, H.J. (2017). Pathogenesis of enterococcal spondylitis caused by Enterococcus cecorum in broiler chickens. Veterinary Pathology, 54, 61–73.
- Deeming, D.C. (1991). Reasons for the dichotomy in egg turning in birds and reptiles. In D.C. Deeming & M.W.J. Ferguson (Eds.), Egg incubation: its effects on embryonic development in birds and reptiles (pp. 307–324). Cambridge: Cambridge University Press.
- Dolka, B., Chrobak-Chmiel, D., Makrai, L. & Szeleszczuk, P. (2016). Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Veterinary Research, 12, 129.
- Dolka, B., Czopowicz, M., Dolka, I. & Szeleszczuk, P. (2022). Chicken embryo lethality assay for determining the lethal dose, tissue distribution and pathogenicity of clinical Enterococcus cecorum isolates from poultry. Scientific Reports, 12, 10675.
- Fang, J., Ma, M., Jin, Y., Qiu, N., Huang, Q., Sun, S., Geng, F. & Guo, L. (2012). Liquefaction of albumen during the early incubational stages of the avian embryo and its impact on the antimicrobial activity of albumen. Journal of Food, Agriculture & Environment, 10, 423–427.
- Guyot, N., Réhault-Godbert, S., Slugocki, C., Harichaux, G., Labas, V., Helloin, E. & Nys, Y. (2016). Characterization of egg white antibacterial properties during the first half of incubation: a comparative study between embryonated and unfertilized eggs. Poultry Science, 95, 2956–2970.
- Heath, J.L. (1978). The effect of egg size on the relative percentages of thick, inner thin and outer thin albumen. Poultry Science, 57, 312–313.
- Herdt, P., Defoort, P., Steelant, J., Swam, H., Tanghe, L., Goethem, S.V. & Vanrobaeys, M. (2009). Enterococcus cecorum osteomyelitis and arthritis in broiler chickens. Vlaams Diergeneeskundig Tijdschrift, 78, 44–48.
- Huang, Y., Eeckhaut, V., Goossens, E., Rasschaert, G., Van Erum, J., Roovers, G., Ducatelle, R., Antonissen, G. & Van Immerseel, F. (2023). Bacterial chondronecrosis with osteomyelitis related Enterococcus cecorum isolates are genetically distinct from the commensal population and are more virulent in an embryo mortality model. Veterinary Research, 54, 13.
- Jung, A., Metzner, M. & Ryll, M. (2017). Comparison of pathogenic and non-pathogenic Enterococcus cecorum strains from different animal species. BMC Microbiology, 17, 33.
- Jung, A., Petersen, H., Teske, L. & Rautenschlein, S. (2017). Colonization patterns of Enterococcus cecorum in two different broiler production cycles detected with a newly developed quantitative real-time PCR. BMC Microbiology, 17, 106.
- Jung, A. & Rautenschlein, S. (2014). Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Veterinary Research, 10, 311.
- Kense, M.J. & Landman, W.J.M. (2011). Enterococcus cecorum infections in broiler breeders and their offspring: molecular epidemiology. Avian Pathology, 40, 603–612.
- Laurentie, J., Loux, V., Hennequet-Antier, C., Chambellon, E., Deschamps, J., Trotereau, A., Furlan, S., Darrigo, C., Kempf, F., Lao, J., Milhes, M., Roques, C., Quinquis, B., Vandecasteele, C., Boyer, R., Bouchez, O., Repoila, F., Le Guennec, J., Chiapello, H., Briandet, R., Helloin, E., Schouler, C., Kempf, I. & Serror, P. (2023). Comparative genome analysis of Enterococcus cecorum reveals intercontinental spread of a lineage of clinical poultry isolates. mSphere, 8, e0049522.
- Manders, T.T.M., Matthijs, M.G.R., Veraa, S., Van Eck, J.H.H. & Landman, W.J.M. (2021). Success rates of inoculation of the various compartments of embryonated chicken eggs at different incubation days. Avian Pathology, 50, 61–77.
- Manders, T.T.M., van Eck, J.H.H., Buter, G.J. & Landman, W.J.M. (2022). Assessment of the best inoculation route for virulotyping Enterococcus cecorum strains in a chicken embryo lethality assay. Avian Pathology, 51, 613–625.
- Nagaraja, T.G. (2022). Basic bacteriology. In D.S. McVey, M. Kennedy, M.M. Chengappa, & R. Wilkes (Eds.), Veterinary microbiology (pp. 11–28). Hoboken: John Wiley & Sons, Inc.
- Nakano, T. & Graf, T. (1991). Goose-type lysozyme gene of the chicken: sequence, genomic organization and expression reveals major differences to chicken-type lysozyme gene. Biochimica et Biophysica Acta, 1090, 273–276.
- Nile, C.J., Townes, C.L., Michailidis, G., Hirst, B.H. & Hall, J. (2004). Identification of chicken lysozyme g2 and its expression in the intestine. Cellular and Molecular Life Sciences, 61, 2760–2766.
- Powell, E.O. (1955). Some features of the generation times of individual bacteria. Biometrika, 42, 16–44.
- Prism, G. (2022). GraphPad Prism version 9.3.1. for windows, GraphPad software. www.graphpad.com.
- Ragland, S.A. & Criss, A.K. (2017). From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathogens, 13, e1006512.
- R Core Team. (2022). R: A Language and Environment for Statistical Computing. https://www.R-project.org/.
- Robbins, K.M., Suyemoto, M.M., Lyman, R.L., Martin, M.P., Barnes, H.J. & Borst, L.B. (2012). An outbreak and source investigation of enterococcal spondylitis in broilers caused by Enterococcus cecorum. Avian Diseases, 56, 768–773.
- Romanoff, A.L. & Romanoff, A.J. (1967). Chemistry of the nonembryonic portions of the egg. In A.L. Romanoff (Ed.), Biochemistry of the avian embryo – A quantitative analysis of prenatal development (pp. 177–232). New York: Interscience Publishers a division of John Wiley & Sons.
- Wichgers Schreur, P.J., van Weeghel, C., Rebel, J.M., Smits, M.A., van Putten, J.P. & Smith, H.E. (2012). Lysozyme resistance in Streptococcus suis is highly variable and multifactorial. PLoS One, 7, e36281.
- Willems, E., Decuypere, E., Buyse, J. & Everaert, N. (2014). Importance of albumen during embryonic development in avian species, with emphasis on domestic chicken. World’s Poultry Science Journal, 70, 503–518.
